International Journal of Agricultural Science and Food Technology
Urea triazone fertilizers-A slow-release nitrogen fertilizer
Michael M Hojjatie*
Cite this as
Hojjatie MM (2021) Urea triazone fertilizers-A slow-release nitrogen fertilizer. J Agric Sc Food Technol 7(3): 272-276. DOI: 10.17352/2455-815X.000119Copyright
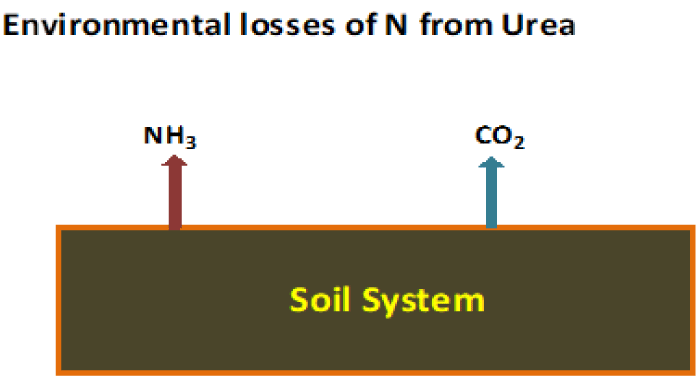
© 2021 Hojjatie MM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Urea is one of the most widely used nitrogen fertilizers worldwide. More than 90% of the world’s production of urea is used as fertilizer [1a]. Soil bacteria containing urease enzyme, catalyze the conversion of urea to ammonia and carbon dioxide via ammonium carbonate formation and decomposition. Nitrogen from urea can be lost to the atmosphere as a gas if fertilizer urea remains on the soil surface for an extended time period during warm weather. Urea breakdown can begin as soon as it is applied to the soil. In the presence of the urease enzyme and a small amount of soil moisture, urea hydrolysis occurs and nitrogen is lost due to ammonia volatilization [1-5].
Several techniques have been applied to prevent the loss of nitrogen from urea due to ammonia volatilization. One approach is to add additives such as urease and nitrification chemicals or inhibitors to the urea containing fertilizers to prevent and/or slow down the hydrolysis of urea [6] Figure 1.
Sulfur coated urea and polymer coated urea have been used to prevent hydrolysis of urea and to slow the release of nitrogen from urea to the soil. Since different coating materials and thicknesses can “control” the release of nitrogen, the term Controlled-Release N (CRN) fertilizer has been introduced.
Slow-Release Nitrogen (SRN) is a measurable quantity and is defined as that portion of nitrogen in a fertilizer that slowly releases to the soil. SRN is dependent upon one or more factors including soil pH, soil moisture and soil temperature.
Many SRN fertilizers are formed by incorporating urea into chemical reactions forming bonded urea products that slowly breakdown in the soil releasing its nitrogen content. These fertilizers can be either solid or liquid products. Triazone Slow-Release Nitrogen Fertilizer is an example of a liquid fertilizer formed by the chemical reaction and modification of urea.
Reaction of aldehydes, including formaldehyde, acetaldehyde, and crotonaldehyde, is well established in organic chemistry. Condensation products of urea and different aldehydes (formaldehyde, isobutyraldehyde, crotonaldehyde) are used in large amounts (more than 300,000 tons per year) in controlled-release and slow-release nitrogen fertilizer for many crops, lawns, trees or shrubs [6].
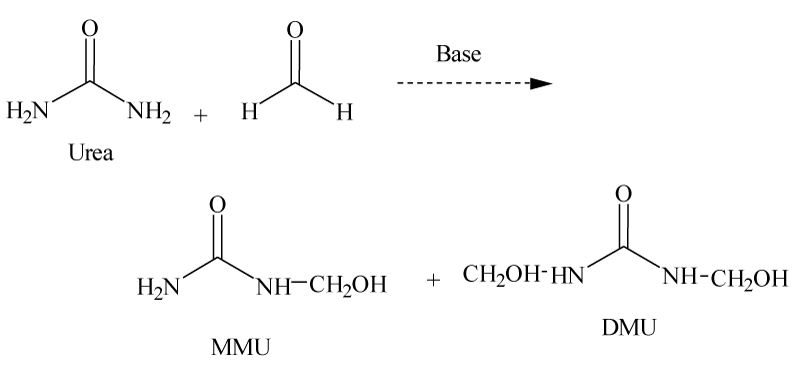
Urea and formaldehyde react together under acidic or basic conditions to form ureaform products, including Dimethylolurea (DMU), methylenediurea (DMU), and Dimethyltriurea (DMTU). Figure 2 demonstrates a based catalyzed reaction of urea and formaldehyde is shown in the following reaction.
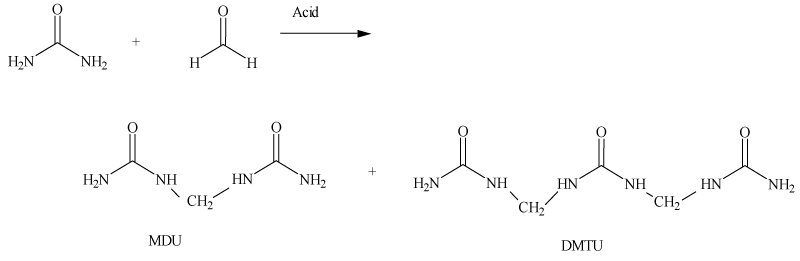
Under acidic conditions, urea and formaldehyde react according to the following chemical reaction as demonstrated in Figure 3.
Ureaforms have been used as Slow-Release Nitrogen Fertilizers, typically as solid products.
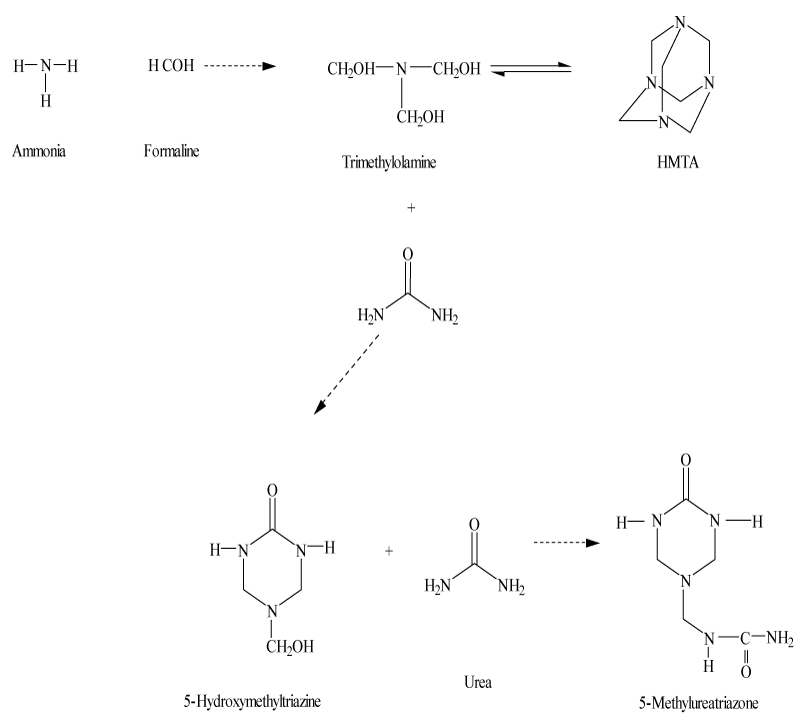
A controlled reaction of urea, formaldehyde, and ammonia forms a six-member ring-slow- release nitrogen fertilizer that contains a high percentage of SRN. The reaction is shown in Figure 4.
According to the definition accepted by the Association of American Plant Food Controlled Officials (AAPFCO, Official Publication, No. 74, 2021), “Urea-Triazone Solution is a stable solution resulting from controlled reaction in aqueous medium of urea, formaldehyde, and ammonia which contains at least 25% total nitrogen. The solution shall contain no more than 40% nor less than 5% of total nitrogen from unreacted urea and not less than 40% from triazone. All other nitrogen shall be derived from water-soluble, dissolved reaction products of the above reactants. It is a source of slowly available nitrogen (Official 1990). The following graph shows the nitrogen release pattern from urea and Triazone.”
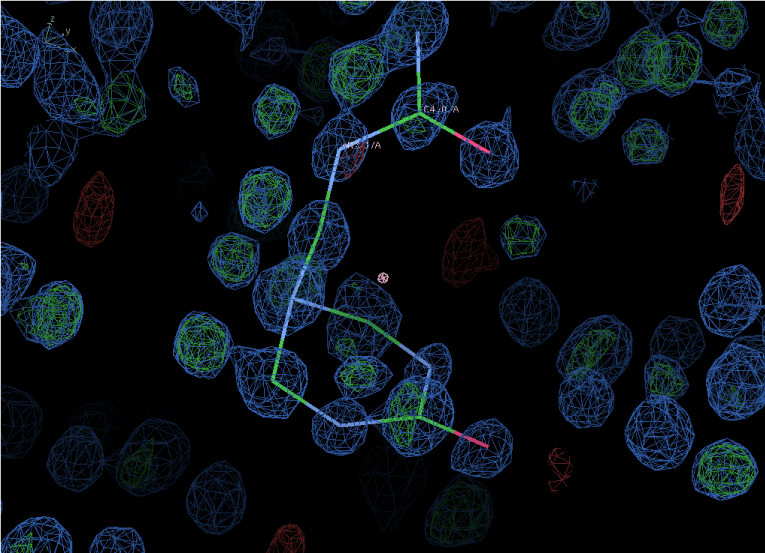
The structure of the six-membered ring triazone has been established by well known laboratory tools such as NMR, and X-Ray. The X-Ray crystal structure of this moiety is shown below Figure 5.
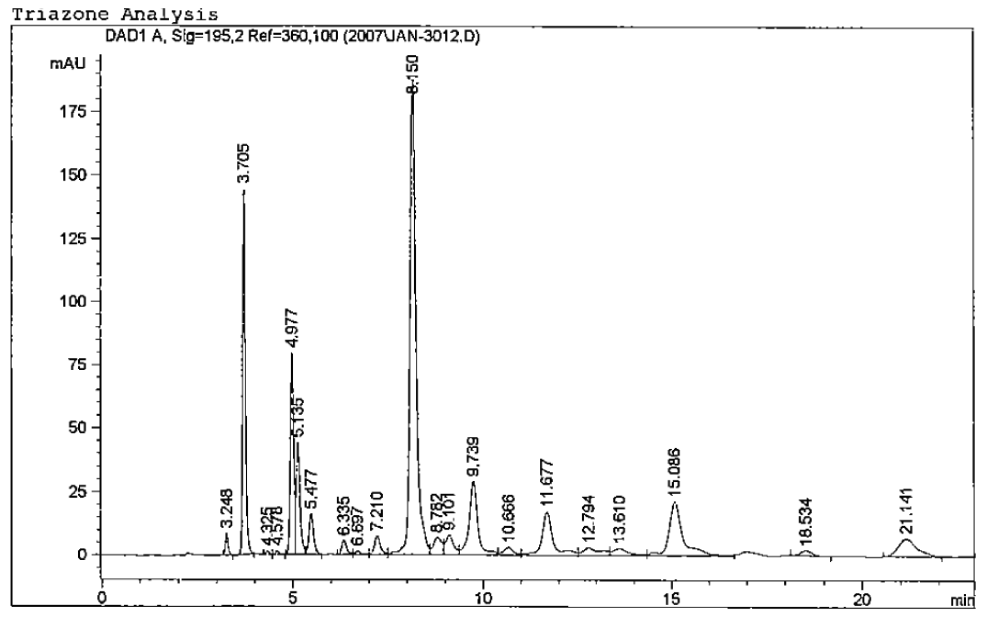
The composition of this liquid fertilizer has been established by High Performance Liquid Chromatography (HPLC), and the use of known and available standards of urea, biuret, and methylene urea products (7-8) . The analyses show the presence of 72-76% triazone as a six-membered ring, 8-12% free urea, and 4-5% methylene urea components, and about 0.3-0.5% biuret. This low level of biuret makes these fertilizers non-burning, with no appreciable phytotoxicity with respect to agricultural and industrial grade urea. No free formaldehyde has been detected in these solutions using chemical analyses and formaldehyde sensors. A HPLC chromatogram of a Triazone solution is provided in Figure 6 showing the presence of the major component triazone (the largest peak):
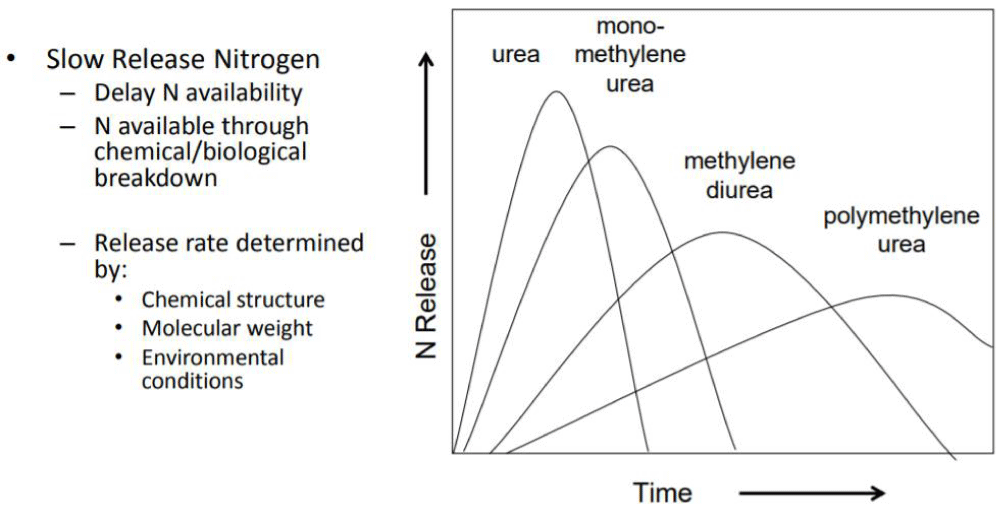
Numerous research projects including both laboratory and field work have shown the effectiveness of Triazone products as slow-release nitrogen fertilizers (sources?). These fertilizer products help protect the environment from the release of ammonia and carbon dioxide to the atmosphere, while saving farmers money as compared with nitrate and banded urea on one hand and feeding the crops and plants with the necessary nitrogen nutrient for better yields on the other. Release curves for various methylene urea chains and their comparisons with surface applied urea is shown in Figure 7. When surface applied, urea nitrogen is released within two weeks while the N-protected urea from urea formaldehyde (UF) products is released slowly up to 90 days.
Release of nitrogen from Triazone as compared with that of urea is shown in Figure 8.
The six-membered ring triazone slow-release N fertilizer takes up to 90 days for the nitrogen to release in the soil as compared to 14 to 30-days for the banded urea [9,10].
Triazone slow-release fertilizer is a clear, water-soluble fertilizer which can be made in different grades ranging from 19% to 32% N of which 72-76% of the nitrogen content is contained as slow release. It is a unique fertilizer product that does not separate, does not settle, and has excellent storage life and a low salt out temperature of less than zero-degree F. Whether it is cold or hot outside, Triazone fertilizers stay clear inside the farmer’s storage tanks. In early spring, late fall and in winter, liquid fertilizers made with urea will salt out when the temperature dips below 65o F. But even at sub-zero temperatures, Triazone fertilizers and their custom blends will not separate, settle out or salt out. They remain in a true solution, easy to handle and convenient to apply all season long. It saves time and money for the farmers. Furthermore, because of its low phytotoxicity and low burning characteristics, they allow for less water usage and low-volume application. Because of the high concentration of non-burning slow release ‘triazone” nitrogen, it can be applied at the standard N rates and spray volumes, without any risk of foliar burn. Moreover, in comparisons with nitrate fertilizers such as Urea Ammonium Nitrate (UAN), Triazone fertilizers reduce the potential of leaching and contamination of surface and ground water.
Leaching occurs when irrigation or rainwater moves nitrogen out of the root zone and down further into the soil profile. This places the N beyond the reach of the roots so that it is never used by the plants, crops, or turf. A large portion of the leached N can find its way into surface water and ground water supplies. The effect this contamination some fertilizers have on the environment has generated a great deal of concern worldwide. In fact, Untied States and European countries have passed laws designed to prevent nitrate contamination by controlling when, how and how much nitrogen fertilizers are applied.
Triazone N fertilizer delivers excellent stability of slow-release nitrogen to a variety of field crops such as corn, soybeans, alfalfa, cotton, hops, sugar beets, rice, peanuts, sunflowers, potatoes and vegetables such asparagus, carrots, beans, celery, sweet corn, lettuce, peas, and pepper. These fertilizers can be used safely on shrubs, trees, and fruit trees. They are also excellent slow-release nitrogen sources for turf grass and lawns. These fertilizers may be soil applied as a band or side-dress, or they can be injected into sprinkler, drip, or center pivot irrigation systems or they can be applied as concentrated or diluted solutions using aerial or ground application. These fertilizers contain three pounds of nitrogen per gallon and are compatible with many other NPK fertilizers and insecticides.
Field research results has shown that these fertilizers improve yields and increase net profits [9-14]. Conversion rates from the Triazone fertilizer to plant available inorganic nitrogen were 30.0%, 52.2%, and 60.0% at 7, 24, and 40 days, respectively [12]. Using the soluble, slow-release nitrogen fertilizer effectively increased yields and nitrogen use efficiency, and reduced the fertilizer production costs; hence, it has great potential for widespread use in agriculture.
Several field studies have shown that Triazone fertilizers are mainly degraded in the soil by microbes, and it decomposes to urea and formaldehyde [12-15]. Urea is used as a nitrogen source via the mineralization of urea to ammonium as the initial plant nutrient source, while formaldehyde is generally incorporated immediately into the microbial C1 metabolic pathways in soil [9]. Initial release of nitrogen from ureaform fertilizers under field conditions has been studied by Hadas, et al. [12]. An increase in soil microbial population was also observed. This degradation pathways and the incorporation of formaldehyde into soil microbe metabolites has been confirmed by many other scientists. No formaldehyde was released into the environment and no adverse effects were observed in the soil microbe populations. In fact, the soil microbes were increased. The impact of these urea-based fertilizers on bacterial diversity in onion bulbs and the main roots of sugar beets were examined by S. Ikeda, et al. [13]. These fertilizers markedly increased bacterial diversity in both plants.
Guo, et.al. have shown that although these liquid fertilizers compared to unreacted urea increased the cost per unit of nitrogen provided to plants, when combined with urea, the fertilizers improved the yields of rape plants and increased net profits [14].
Foliar application of urea-formaldehyde/triazone, showed a significant increase in yield in soybean [15].
In conclusion, slow-release fertilizers have been gaining strong attention and have been thoroughly evaluated due to the recognition of their beneficial and pleiotropic properties. The use of a slow-release fertilizer offers economic and environmental benefits to farmers by saving time and reducing the labor involved with handling fertilizers, reduction of water usage, and prevents the loss of nitrogen volatilization and leaching.
This work was supported by China Agriculture Research System of Ministry of Finance, Ministry of Agriculture and Rural Affairs of the People’s Republic of China (CARS-44- KX J15).
- a) Mikkelsen R (1990) Nut Cycl Agroecosys 26: 311-318.
b) Mattson N, Leatherwood R, Peters C (2009) Nitrogen: All Forms Are Not Equal. Cornell University Cooperative Extension. Link: https://bit.ly/2Xc4CuM - Liu JG, You L, Amini M, Obersteiner M, Herrero M, et al. (2010) A high-resolution assessment on global nitrogen flows in cropland. Proc Natl Acad Sci USA 107: 8035–8040. Link: https://bit.ly/3k54MfM
- Erisman JW, Galloway J, Seitzinger S, Bleeker A, Butterbach-Bahl K (2011) Reactive nitrogen in the environment and its effect on climate change. Curr Opin Environ Sustain 3: 281-290. Link: https://bit.ly/3EeLOLN
- Li SX, Wang ZH, Stewart BA (2013) Responses of crop plants to ammonium and nitrate N. Adv Agron 118: 205–397. Link: https://bit.ly/3k3LE1H
- Akiyama H, Yan X, Yagi K (2010) Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis. Global Change Biol 16: 1837–1846. Link: Link: https://bit.ly/2VC5OXr
- Trenkel ME (2010) Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture-IFA Publication. Link: https://bit.ly/3tDrPBv
- Hojjatie MM, Abrams D, Parham TM (2004) Liquid Chromatographic Determination of Urea in Water-Soluble Urea-Formaldehyde Fertilizer Products and in Aqueous Urea Solutions: Collaborative Study. J AOAC International 87: 346-351. Link: https://bit.ly/2XdVR3k
- Hojjatie MM, Abrams D (2014) Determination of Kjeldahl Nitrogen in Fertilizers by AOAC Official MethodSM 978.02: Effect of Copper Sulfate as a Catalyst. J AOAC International 97: 764. Link: https://bit.ly/2Xa7yre
- Garcia R, Hernandez JD (2005) North Central Soil Fertility Extension Industry. 2: 138-143.
- Clapp JG (2001) Urea-Triazone N Characteristics and Uses. Proceeding of the 2nd International Nitrogen Conference on Science and Policy. Link: https://bit.ly/3lhhOGG
- Jahns T, Ewen H, Kaltwasser H (2003) Biodegradability of Urea-Aldehyde Condensation Products. J of Polymer and the Environmental 11: 155-159. Link: https://bit.ly/3hrPSOZ
- Hadas A, Kafkafi U, Peled A (1975) Initial Release of Nitrogen from Ureaform Under Field Conditions. Soil Science Society of America Journal 39: 1103-1105. Link: https://bit.ly/3C5Xjn8
- Okubo T, Ikeda S, Sasaki K, Oshima K, Hattori M, et al. (2014) An Assessment of Urea-Formaldehyde Fertilizer on the Diversity of Bacterial Communities in Onion and Sugar Beet. Microbes Environ. 29: 231-234. Link: https://bit.ly/3hnAvqU
- Guo Y, Zhang M, Liu Z, Zhao C, Lu H, et al. (2020) Applying and Optimizing Water-Soluble, Slow-Release Nitrogen Fertilizers for Water-Saving Agriculture. ACS Omega 5: 11342-11351. Link: https://bit.ly/3z0iPrq
- Cassim BM, Machado A, Fortune D, Moreira F, De Oliveira Zampar E, et al. (2020) Effects of Foliar Application of Urea and Urea-Formaldehyde/Triazone on Soybean and Corn Crops. Agronomy 10: 1549. Link: https://bit.ly/2XjKuGX
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
