Journal of HIV for Clinical and Scientific Research
Trends in HIV Clinical Trials Worldwide over the Last Two Decades – Equity at stake?
Melvin George1, Sree Harinadh K1* and Subramaniyan K2
2Department of General Medicine, SRM Medical College Hospital, Kancheepuram, Tamil Nadu, India
Cite this as
George M, Sree Harinadh K, Subramaniyan K (2020) Trends in HIV Clinical Trials Worldwide over the Last Two Decades – Equity at stake? J HIV Clin Sci Res 7(1): 001-005. DOI: 10.17352/jhcsr.000029Background: The study aims to determine the secular trends in HIV trials among the developed nations as well as regions with a high burden of HIV infection in the last two decades.
Methods: Using the clinical trials.gov database, we identified the number of trials registered in the years 2000 to 2019 in developed nations as well as countries with a high burden of HIV. We used the search word “HIV infection” under Advanced Search option. We also searched for country specific no.of trials from the developed nations as well as the most populated regions in Africa and those with a high burden of HIV infection.
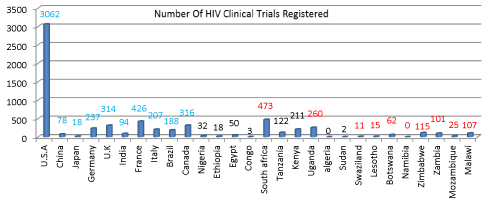
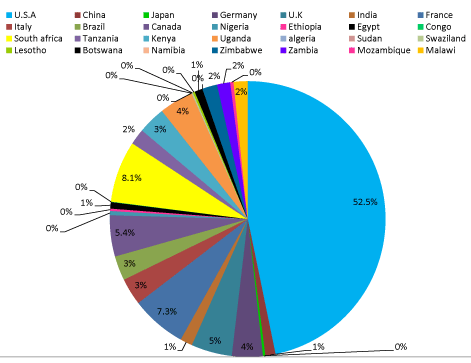
Results: The total number of trials registered worldwide during the study period in HIV infection was 6236 during the period. Among these, 5825 studies fitted within our study criteria. In these studies, 52.5% of studies were done in the USA. The countries reporting maximum no of trials apart from USA, included France (7.3 %), Canada (5.4%) and UK (3%). Among African nations the countries with the most number of trails included South Africa (8.1%), Kenya (3%), Uganda (4%), Tanzania (2%), and Egypt (1%). Although countries such as Swaziland, Lesotho, and Botswana have a high burden of HIV infection, the number of trials with HIV from these countries is less than 1 % of the total HIV trials. All nations also showed a steady increase in the number of trials during the period.
Conclusion: Although African nations have a high burden of HIV infection, most of the clinical trials in HIV were done predominantly in the USA and Western European countries. There does not appear to be a proportionate distribution of HIV trials based on disease burden which largely reflects the care taken by sponsors to avoid using African nations as “guinea pigs” for clinical trials.
Introduction
This briefing paper provides an overview of HIV [Human Immunodeficiency Virus] clinical trials that had happened in the past two decades in Developed countries as well as African nations with high populated and high burden of HIV infection. Clinical trials are conducted to determine whether new medical approaches are safe and effective in people. The past two decades have seen extraordinary advances in our understanding of Human Immunodeficiency Virus (HIV). However, the prevalence of HIV continues to grow in alarming proportions especially in African nations. HIV/AIDS clinical trials help researchers find better ways to prevent, detect, or treat HIV/AIDS. HIV/AIDS clinical trials include studies of new HIV medicines, studies of vaccines to prevent or treat HIV, and studies of medicines to treat infections related to HIV. The benefits and possible risks of participating in an HIV/AIDS clinical trial are explained to study volunteers before they decide whether to participate in a study [1].
In conduction of clinical trials the ethical issues primarily involves protection of rights, safety, and well-being of the research participants. Although all the guidelines are based on the cardinal principles of autonomy, non-maleficence, beneficence, and justice, the ethical issues to be tackled are increasing day by day with the advancement of new technologies, wide range of research activities, and globalization of clinical research [2].
A number of unethical HIV trials have been carried out in developing countries such as Africa during the 1990s that were funded by the US Government. For instance, as many as 9 trials were carried out which randomized HIV mothers to receive placebo and depriving them of zidovudine even though it was well known by then that zidovudine could prevent transmission from mother to offspring. At the same point of time, none of the HIV mothers in the US were provided placebo [3]. Dr. Chester Southam injected live cancer cells into 22 elderly patients at Jewish Chronic Disease Hospital in Brooklyn in the year 1962. After being rebuffed by his institution, Sloan-Kettering, he convinced Dr. Emanuel Mandel at Jewish Chronic Disease. He sought to learn whether people who were debilitated by cancer could reject cancer cells. None of the patients were informed about the risks and were never informed that the experiment involved injecting live cancer cells. Several doctors told Southam they did not want their patients experimented on but he used them any way [4]. In 1996 Pfizer sent a team to Kano in the north of Nigeria during an epidemic of meningococcal meningitis. To test the efficacy of its new antibiotic trovafloxacin (Trovan) they carried out an open label trial in 200 children, half of whom were given trovafloxacin and half the gold standard treatment for meningitis, ceftriaxone. Five of the children given trovafloxacin died, together with six who were given ceftriaxone. Pfizer said that 15000 people died during the epidemic [5].
The first U.S. Federal law to require trial registration was the Food and Drug Administration Modernization Act of 1997 (FDAMA). Section 113 of FDAMA (FDAMA 113) required the National Institutes of Health (NIH) to create a public information resource on certain clinical trials regulated by the Food and Drug Administration (FDA). Specifically, FDAMA 113 required that the registry include information about federally or privately funded clinical trials conducted under investigational new drug applications to test the effectiveness of experimental drugs for patients with serious or life-threatening diseases or conditions [6].
The information in the registry was intended for a wide audience, including individuals with serious or life-threatening diseases or conditions, members of the public, health care providers, and researchers.
2000: NIH Releases ClinicalTrials.gov Web Site
With input from FDA and others, the NIH National Library of Medicine (NLM) developed ClinicalTrials.gov. The first version of ClinicalTrials.gov was made available to the public on February 29, 2000. At the time, ClinicalTrials.gov primarily included NIH-funded studies. The World Medical Association (WMA) has developed the Declaration of Helsinki as a statement of ethical principles for medical research involving human subjects, including research on identifiable human material and data in the year 1964 [7].
Vulnerable Groups and Individuals
Clinical individuals whose willingness to volunteer in a clinical trial may be unduly influenced the expectation, whether justified or not, of benefits associated with participation, or of a retaliatory response from senior members of a hierarchy in case of refusal to participate [8]. Some groups and individuals are particularly vulnerable and may have an increased likelihood of being wronged or of incurring additional harm. All vulnerable groups and individuals should receive specifically considered protection.
Medical research with a vulnerable group is only justified if the research is responsive to the health needs or priorities of this group and the research cannot be carried out in a non-vulnerable group. In addition, this group should stand to benefit from the knowledge, practices or interventions that result from the research. One of the cardinal tenets of bioethics is justice and equality. We sought to explore if there was an increased preponderance of HIV trials being carried out in developing countries to verify if the principle of justice and equality was violated [2].
Aim
To determine the secular trends in HIV trials among the developed nations as well as regions with a high burden of HIV infection in the last two decades.
Objectives
1. To compare the clinical trials happened between developed countries and most populated African nation.
2. To compare the clinical trials happened between developed countries and with high burden of HIV infection.
Methodology
We have the countries mainly as three categories i.e., developed nations, Populated African countries, African Countries with high burden of HIV. And, we used the clinical trials.gov database. We identified the number of trials registered in the years January 1st 2000 to October 31st 2019. We used the search word “HIV infection” under Advanced Search option. We had explored for number of HIV trials in each country. We had analyzed the data which has been obtained and make over some patterns to get a conclusion on over all trails happened in the last two decades regarding the HIV.
Inclusion critetria
1. The trials which have been registered in the clinical trial.gov database.
2. The total number of trials that has been registered in between January 1st 2000 to October 31st 2019.
3. The number of trials came with search word “HIV infection”.
4. The total number of trials that had categorized under selected developed countries and African nations with high burden of HIV and high populated countries.
Exclusion critetria
1. The trials which were not registered in the clinical trial.gov database.
2. The trials which were not included in inclusion criteria.
Results
The total number of trials registered worldwide during the study period in HIV infection was 6236 during the period. Among these, 5825 studies fulfilled our study criteria. We performed the search with in the three divisions of countries, and made the data interpretation to know whether clinical trials for HIV were more widely carried out among developing countries.
Developed countries which were selected randomly and they are U.S.A, China, Japan, Germany, U.K, India, France, Italy, Brazil, and Canada. We prepared table for the data which has been obtained Table 1.
African nations with high burden of HIV were Swaziland, Lesotho, Botswana, South Africa, Namibia, Zimbabwe, Zambia, Mozambique, Malawi, and Uganda [9]. We prepared the table with obtained information from database Table 2.
Highly Populated African nations include Nigeria, Ethiopia, Egypt, Congo, South Africa, Tanzania, Kenya, Uganda, Algeria, and Sudan [10]. and table was prepared with obtained data Table 3.
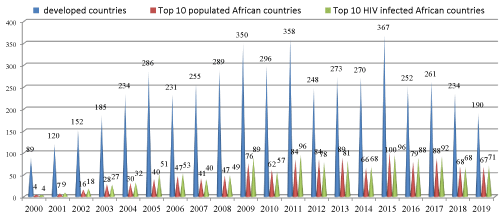
By using the data which has been obtained from database we compared and made trends in HIV clinical trials. We had compared the cumulative sum of each country and made the graphs with their numbers and percentages. And we also compared the data according to year wise distribution in last two decades Figures 1-3.
Discussion
In these studies, 52.5% of studies were done in the USA. The countries reporting maximum no of trials apart from USA, included France (7.3 %), Canada (5.4%) and UK (3%). Among African nations the countries with the most number of trails included South Africa (8.1%), Kenya (3%), Uganda (4%), Tanzania (2%), and Egypt (1%). Although countries such as Swaziland, Lesotho, and Botswana have a high burden of HIV infection, the number of trials with HIV from these countries is less than 1 % of the total HIV trials. All nations also showed a steady increase in the number of trials during the period.
Conclusion
Although African nations have a high burden of HIV infection, most of the clinical trials in HIV were done predominantly in the USA and Western European countries. There does not appear to be a proportionate distribution of HIV trials based on disease burden which largely reflects the care taken by sponsors to avoid using African nations as “guinea pigs” for clinical trials.
It is pleasure to convey gratitude to Dr. Melvin George MD DM sir, working as Assistant Professor in SRM Medical College Hospital, Kancheepuram, Tamil Nadu. Who has given these idea and helped for progressing the work.
- Fact sheet (2017) - global HIV statistics. UNAIDS. Link: https://bit.ly/35fQPms
- Muthuswamy V (2013) Ethical issues in clinical research, Perspect Clin Res 4: 9-13. Link: https://bit.ly/3aI7HDn
- Shalala D (1997) Letter Concerning Funding of Unethical Trials Which Administer Placebos to HIV-infected Pregnant Women. Link: https://bit.ly/3eTnh2m
- Chester (1962) Southam injected live cancer cells into 22 elderly patients, Alliance for human research protection.
- Wise J (2001) Pfizer accused of testing new drug without ethical approval. BMJ 322: 194. Link: https://bit.ly/3aCDYMd
- Clinical Trails.gov. Link: https://bit.ly/2SbLFT8
- WMA declaration of Helsinki (2019) Ethical principles for medical research involving human subjects.
- Vulnerable Subjects. Link: https://bit.ly/3cRl4CJ
- Countries With the Highest Rates of HIV/AIDs. Link: https://bit.ly/2KDrF7C
- The 10 Most Populated Countries in Africa. Link: https://bit.ly/2VEzFeO

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
