Open Journal of Orthopedics and Rheumatology
Total vertebrectomy through posterior approach for thoracic tumors
Jefferson Soares Leal*, Rogério Lúcio Chaves de Resende, Daniel Ferreira Ghedini, Leandro Vinícius Vital, Haroldo Oliveira de Freitas Júnior and Luiz Eduardo Moreira Teixeira
Cite this as
Leal JS, Chaves de Resende RL, Ghedini DF, Vital LV, De Freitas Júnior HO, et al. (2021) Total vertebrectomy through posterior approach for thoracic tumors. Open J Orthop Rheumatol 6(1): 030-036. DOI: 10.17352/ojor.000035Objective: To demonstrate the benefits and complications of the single posterior approach through bilateral costotransversectomy in the treatment of neoplastic disease of the thoracic spine.
Methods: Twelve consecutive patients with thoracic spine tumors, who underwent single posterior approach with bilateral costotransversectomies were reviewed. Through posterior extrapleural access, total vertebrectomy and reconstruction were performed. In reconstruction, a cage was used anteriorly, and a pedicular screw fixation was used posteriorly. The minimum follow-up was sixteen months. The parameters analyzed were pain, neurological and functional capacity, survival time, fixation stability, and complications.
Results: All patients had improvement in their pain or in their functional capability. Among those with a preoperative neurological deficit, 71.4% showed improvement of at least one degree at postoperative evaluation. There was no functional or neurological decline in any patient. The observed complications were: one adult respiratory stress syndrome, one excessive bleeding, one pneumothorax, one infection and one local recurrence. All but one of these complications was reversed with appropriate treatment.
Conclusion: The posterolateral approach through costotransversectomy was safe and secure method for the resection and reconstruction of thoracic vertebrae affected by neoplastic disease.
Introduction
The best surgical treatment for localized malignant bone tumors is the wide surgical margins resection. Nevertheless, the resection of malignant tumors of the spine is incomparably more difficult than those in limbs. The topographic proximity of vital structures to the vertebral column, such as the spinal cord, nerve roots, aorta artery and vena cava, limits the feasibility and complicates the performance of wide resection margins for malignant vertebral tumors. Additionally, extra-compartmental tumor growth with dural extension or anterior invasion around greater vascular structures does not allow for wide resection. Thus, in many instances, a technique that provides good decompression and stability with less morbidity can be the best for certain patients. In our series, all patients were not eligible for a total en bloc spondylectomy.
It is generally accepted that when the compressive lesion is anterior, the surgical approach should be anterior, followed by decompression and stabilization. In the thoracic spine, this usually requires thoracotomy or thoracoscopy [1]. However, in the upper thoracic spine the anterior approach can be difficult to perform because of the kyphosis and the proximity of the heart, lungs, and greater vessels [2]. These special features are important barriers for resection and stable reconstruction of the vertebral body through cervicosternotomy or transcavitary approaches.
Costotransversectomy was first described for abscess drainage in Pott’s Disease in 1894. In this technique, a medial segment of the rib is removed in a manner which allows extrapleural access to the vertebral body, without the necessity of opening the thoracic cavity. Anterior extrapleural approach through costotransversectomy for vertebral body tumor extirpation allows circumferential decompression of the spinal cord and has been described by several authors [3]. However, in most of these studies, the results were not very encouraging, mainly due to the lack of a reliable reconstruction technique following resection [4]. More recently, with the dissemination of more stable spine fixation systems [5], this approach has been more commonly used, and has again aroused interest in the treatment of thoracic spine tumors, especially when the en bloc resection is not feasible, as it may be considered less risky [6].
The extrapleural costotransversectomy approach offers the potential advantage of avoiding tumor dissemination to the pleural space and worsening of a pre-existing pulmonary condition that may occur with a transcavitary approach. Lesions which infiltrate the vertebral arch posteriorly may also be resected through the same single approach.
A modified costotransversectomy technique with posterior midline longitudinal incision and bilateral partial rib resection gives wide extrapleural access to the whole circumference of the thoracic vertebrae, allowing extensive resections and stable reconstructions.
The objective of this paper is to present the results of a series of patients treated in a similar manner by using the costotransversectomy approach for decompression, reconstruction and stabilization of neoplastic lesions of the thoracic spine.
Material and methods
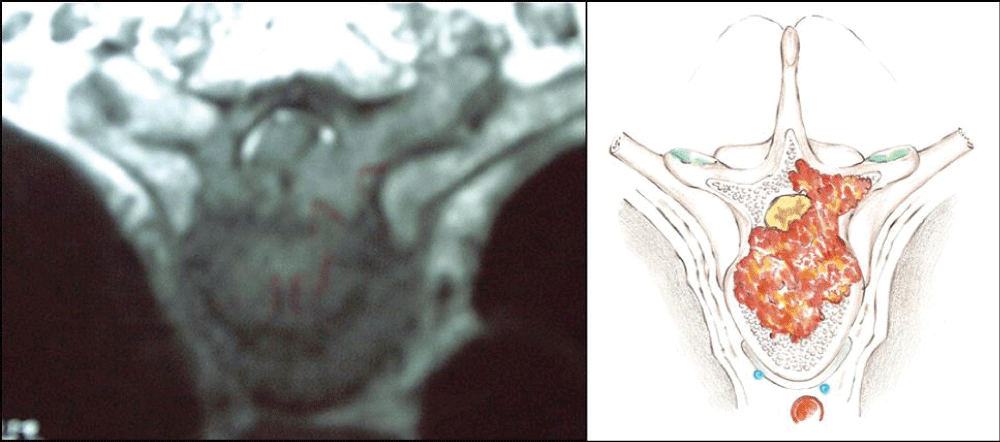
A cohort of 12 consecutive patients with thoracic spine tumors, who were submitted to single-stage vertebrectomy through a bilateral costotransversectomy followed by anterior and posterior reconstruction, was reviewed. All patients presented extra-compartmental vertebral infiltration and dural extension. Specific indications for the costotransversectomy approach were motivated by one or more of the following reasons: (1) infiltrative lesion in the high thoracic spine, (2) involvement of the vertebral body and arch (Figure 1), (3) patient’s clinical conditions restricting transcavitary or combined approaches. Other general indications for the treatment were: (4) neurological deficit, (5) unstable lesion with pain and impending neurological deficit and (6) absence of definitive diagnosis in an unstable infiltrative lesion of the spine. All patients had an estimated prognosis of at least three months survival as a precondition to surgical treatment.
The following variables were analyzed: gender, age, type of primary neoplasm, levels compromised by the tumor, neurological classification, functional classification, pain, adjuvant radiotherapy, preoperative tumor embolization, length of surgery, blood transfusions, hospital stay, length of survival, stability of the fixation and complications. The parameters of pain, neurological classification [7] and functional classification [3] were compared pre and postoperatively. Pain intensity was obtained through a Visual Analog Scale (VAS). The determination of the subject profiles was made with the distribution of frequencies of the categorical variables and the measurements of the central tendency for the continuous variables.
The surgical technique aimed to resect the compromised vertebra, followed by reconstruction through an extrapleural approach. The resection of the vertebral body was done through two costovertebral corridors created by bilateral costotransversectomy. The reconstruction of the anterior column was accomplished with a titanium cage. The posterior tension band was rebuilt with the segment pedicle fixation system using pedicle screws only.
Surgical technique
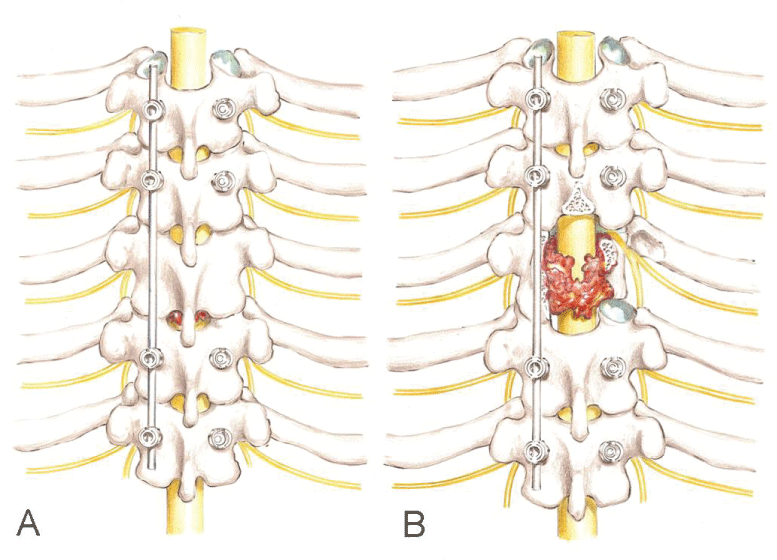
With the spine exposed posteriorly, pedicle screws were inserted segmentally in two segments above and two below the lesion. Intraoperative posteroanterior and lateral fluoroscopy was used for localization of the level of the lesion and insertion of the pedicle screws. The costovertebral joint, and the three medial centimeters of the ribs on each side of the compromised level were exposed by subperiosteal dissection. The screws were connected on one side with a temporary rod to provide safe stabilization followed by resection of the lamina, transverse process and facet joints (Figure 2). The following step was the resection of rib (three centimeters medially), costovertebral joint and pedicle of the affected vertebra on the side opposed to the connected rod to create the costovertebral corridor for access to the vertebral body on one side.
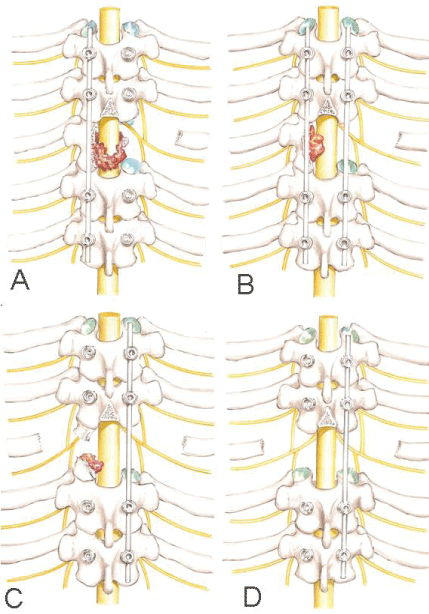
Subperiosteal dissection was performed down to the lateral wall of the vertebral body until the anterior surface of the body. Electric cauterization and Surgicel ® (Johnson& Johnson, New Brunswick, NJ) controlled the bleeding if segmental vessels were injured during dissection. The intervertebral discs were removed in piecemeal fashion. Resection of the vertebra then proceeded from lateral to medial until approximately the midline, working through the costovertebral corridor created. Following this step, another rod was inserted into this side and securely locked to the screws with slight distraction. The temporary rod on the opposite side was then removed to allow resection of the remaining vertebra, repeating the same sequences described previously. It should be emphasized that when changing the rod side, one should first connect the second rod on the working side before removing the temporary one. This precaution keeps the spine stabilized during the whole resection procedure, avoiding acute bending of the spinal cord after removing the temporary rod (Figure 3). At completion of the resection, the spinal cord was checked for a circumferential decompression.
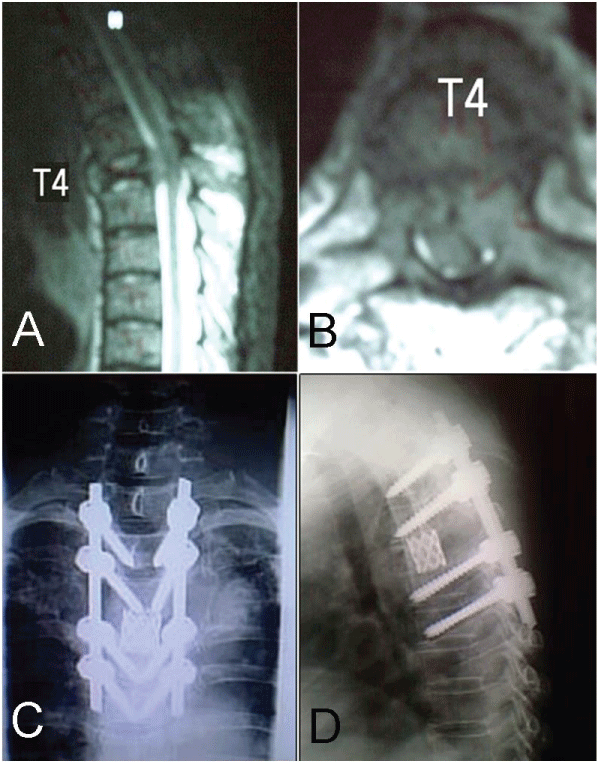
The reconstruction stage began with the insertion of the titanium cage (Figure 4). The reconstruction procedure finished with reinsertion of the second rod, and using the posterior pedicle fixation system to apply a light compression on the cage bilaterally. The integrity of the pleura was verified, and then the fascia, subcutaneous and skin layers were sutured separately. After 24 hours postoperative, the patient was encouraged to sit after drain removal and a radiological control exam was taken (Figure 5). After 48 hours, the patient was encouraged to walk, if his or her condition permitted (Figure 6).
Results
Patient population
In this study, there were six women and six men. The average age was 58.2 years (range, 40–69). All patients presented extra-compartmental infiltrative lesions involving one pedicle and at least 50% of the vertebral body, and non-traumatic vertebral instability characterized by spontaneous pathological fracture of the vertebral body and progressive worsening of pain. All presented only one level of vertebral involvement in the thoracic region, varying from T2 to T12. Five patients presented no neurological deficit (Frankel E), five presented incomplete deficit (Frankel B or C) and two presented complete deficit (Frankel A). Functionally, according to Akeyson and McCutcheon [3], five patients ambulated without assistance (class I) and seven were confined to bed or wheelchair (class III or IV). Five patients (41.6%) underwent preoperative embolization of tumor. Only one patient, who was misdiagnosed as having plasmacytoma, had received preoperative radiotherapy at tenth month before the surgical treatment (Table 1).
Postoperative results
The final anatomopathological diagnoses were four myeloma, three lung adenocarcinomas, two renal cell carcinomas, one plasmacytoma and one prostate carcinoma. In one patient, the diagnosis was inconclusive postoperatively (Table 2). The average of the duration of surgery was 412.5 minutes (range, 320-500) with a CI 95% (confidence interval to 95%) = 374.7 – 450.2. Stratified between the first and second halves of the study group series, the surgery time was 458.3 minutes for the first half, and 366.6 minutes for the second one (p=0.001). The average of red blood cell transfusion was 425ml (range, 0.0 – 1,800). The average hospital stay after surgery was 6 days (range, 4 – 14). A total of 96 screws were inserted. All thoracic levels were instrumented. Pedicle screws were inserted also in C7, L1 and L2. Anterior reconstruction was done in all patients with a titanium cage filled with polymethylmethacrylate (PMMA) in eleven cases and autologous bone graft in one case (case 10). Nine patients (66.7%) had subsequent postoperative chemotherapy and radiotherapy. In these cases, the chemo and radiotherapy was delayed for an average of 21 days postoperatively to allow for appropriate wound healing.
After surgery, the majority of patients experienced an important relief of their pain and improved neurological status. The preoperative average for pain was 6.2 (CI 95% = 4.79 – 7.60) while the postoperative average was 2.6 (CI 95% = 1.74 – 3.45) (p=0.026). Ten patients experienced improvement of pain postoperatively; one remained unchanged and one died at second month after the surgery before of the first evaluation. One patient (case 1) developed dorsal thoracic pain 16 months after surgery that led to the discovery of a recurrent tumor. According to Frankel classification, five patients improved at least one functional grade and seven remained unchanged postoperatively.
The functional status of all patients was improved or unchanged. Four patients who were confined to bed or chair prior to surgery were able to ambulate postoperatively. No patients experienced a decline in their functional or neurological grade as a result of the surgery.
Intraoperative complications were one excessive bleeding in small vessels peripheral to the tumor, and one inadvertent pneumothorax. Postoperative complications were one adult respiratory distress syndrome, one infection with consequent dehiscence of surgical wound, and one local recurrence. No patient presented failure of the anterior or posterior instrumentation, despite that in one case, osteolysis had been seen around the two screws at the distal extremities without compromise to general alignment or the anterior reconstruction (case 1).
In the long term, there were no patients lost at follow-up. At the most recent evaluation, three (25.0%) patients were alive with an average 30 months (range, 16–38) of follow-up. All of those who were alive continued to maintain their ability to ambulate and normal bowel and bladder function. For the nine patients who died, the average life span after surgical decompression and stabilization was 14.2 months (range, 2–37). There was no death reported during 30 days postoperative.
Discussion
The technique presented in this study describes a simultaneous 360-degree tumor resection and immediate spinal column reconstruction through a single midline approach involving bilateral costotransversectomy in all patients of this series. The main advantages of this technique include: (1) avoidance of thoracotomy, resulting in less risk of damage to the thoracic contents and worsening of pulmonary dysfunction, (2) wide field for resection and reconstruction of the anterior column, and (3) ability to address pedicles or posterior elements infiltrated without a second approach.
If the anterior approach is chosen for an anterior lesion with neoplastic infiltration into the pedicle or posterior elements, a second posterior approach is often necessary [8,9]. Moreover, if there is a tumor invasion superior to 50% of the vertebral body, even if an adequate decompression is performed from an anterior approach, supplemental posterior fixation is recommended [10,11].
Pedicular fixation utilized in this technique can be considered an additional advantage. The distraction permitted by the pedicular fixation allows an increase in the surgical field for resection and reconstruction, while maintaining stability in the spinal column. A filled titanium cage inserted anteriorly acts as a support or pivotal point for the posterior fixation when tightened in compression and results in excellent biomechanical stability [12]. The firmly stabilized titanium cage also prevents the spinal cord from being shortened excessively after tumor resection.
Wang, et al. [13] have suggested that the posterolateral approach is limited in not providing direct vision for decompression of the anterior dura. However, other studies have shown that this approach allows complete access to anterior dura [14,15]. Xu, et al. [16] compared the results of vertebrectomy performed using the anterior, posterior and combined approach. In their experience, the three approaches had good functional clinical results, but the posterior approach had the worst performance in terms of infection and deep vein thrombosis. The authors considered the anterior approach to be advantageous over the posterior or combined approach. However, the groups compared were different in relation to some important characteristics. In the group submitted to the posterior approach, the patients were older, the number of patients was twice as high, the number of instrumented levels was significantly higher, and some patients underwent posterior en bloc spondylectomy via Tomita [17], which is a highly complex surgery. Therefore, the results of this study may be distorted due to a possible bias related to the heterogeneity of the groups.
Minimally invasive techniques using the anterior approach are an inexorable trend [1]. However, the learning curve is long and the possibility of conversion to thoracotomy is still high. The conversion to thoracotomy in lesions that affect the upper thoracic spine can make the procedure extremely complex and at increased risk, especially when it involves major resections and the need for reconstruction.
The results obtained from this study support the use of the single-stage posterolateral vertebrectomy through a costotransversectomy in the surgical management of neoplastic disease of the thoracic spine in patients that do not meet the pre-requisites for wide resection for malignant vertebral tumors. Our data demonstrates that all patients had some improvement in their functional condition. Neurologically, all of them either remained stable or improved after the surgery. Patients with complete deficit (Frankel A) or without deficit (Frankel E) remained stable. Among the five patients with incomplete neurological deficit preoperatively, two improved one degree, and three improved two degrees in Frankel classification. Of the seven non-ambulatory patients (class III or IV), four recovered their ability to ambulate (class I or II). Akeyson and McCutcheon [3], using a similar technique to resection, though without stable fixation, related that 24% of their series improved at least one functional grade postoperatively. Three patients who were bedridden prior to surgery were able to ambulate with assistance postoperatively, representing a two-grade improvement in the functional scale (Akeyson & McCutcheon, 1996) [3]. Shen, et al. [14], in their series using also a similar technique, but with an expandable cage, had 47% of partial motor grade improvement, but one patient had suffered a neurologic decline in the early postoperative phase, remaining ambulatory thereafter.
In relation to pain, all patients but one of our series reported improvement. The average reduction in pain intensity was 58% in the VAS. Akeyson and McCutcheon [3] reported that the majority of their patients experienced full or partial relief of pain. Street, et al. showed that the mean of the VAS scores had an improvement of 45.5% after surgery.
The complications observed in four of five distinct patients were effectively reverted with appropriate treatment. In one patient, with local recurrence, no treatment was instituted.
The patient who had excessive intraoperative bleeding was controlled through local compression, cauterization, and homeostatic sealant. This patient was among those who had not been submitted to preoperative embolization. One pneumothorax occurred in case 10 during surgical divulsion between the pleura and the infiltrated vertebral body. The pneumothorax was resolved through widening the thoracic cavity and draining with a chest tube at the end of the procedure.
One adult respiratory distress syndrome occurred in case 5, and was treated in the Intensive Care Unit (ICU) for four days. The postoperative infection with wound dehiscence occurred in case 12. The infection was resolved using surgical debridement and antibiotics, without removal of the fixation material. Five weeks after the initial surgery, the patient was admitted again for plastic surgery with muscle flap coverage. This patient was the only case of the series submitted to preoperative radiotherapy. According to Ghogawala, et al. [18], prior radiotherapy may create a three-fold increase in the risk of infection and dehiscence.
Local recurrence was diagnosed in case 1 at the 16th month of follow-up. This patient reported an increase in pain in the thoracic region, and during the course of investigation of this symptom was found out a local recurrence without neurological compression. The recurrence was not treated due to the worsened condition of the terminal patient. This patient died in the 18th month and continued to maintain her ability to ambulate and retain bowel and bladder function.
The majority of complications in our study were considered to be minor. The overall patient complication rate in our series was of 41.6%. Although it may appear high, this rate should be considered acceptable, given the special condition of the patients and the complexity of the surgical treatment needed. Street, et al. [15], using a very similar technique, reported an overall patient complication rate of 47% with 26% of major complication. Twenty one percent of patients in their series required early reoperation.
We conclude that palliative surgery, when well indicated, results in an improvement in quality of life in patients who are ineligible for wide resection with margin. The technique presented was a safe alternative for resection and reconstruction of tumors localized in the thoracic spine.
- Court C, Boulate D, Missenard G, Mercier O, Fadel E, Bouthors C (2021) Video-Assisted Thoracoscopic En Bloc Vertebrectomy for Spine Tumors: Technique and Outcomes in a Series of 33 Patients. J Bone Joint Surg Am. Link: https://bit.ly/3byGA0V
- Knöller SM, Brethner L (2002) Surgical treatment of the spine at the cervicothoracic junction: an illustrated review of a modified sternotomy approach with the description of tricks and pitfalls. Arch Orthop Trauma Surg 122: 365-368. Link: https://bit.ly/3eTt36g
- Akeyson EW, McCutcheon IE (1996) Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg 85: 211-220. Link: https://bit.ly/3tR74Rt
- McAfee PC, Bohlman HH, Ducker T, Eismont FJ (1986) Failure of stabilization of the spine with methylmethacrylate. A retrospective analysis of twenty-four cases. J Bone Joint Surg Am 68: 1145-1157. Link: https://bit.ly/3uTQsdf
- Tedesco G, Gasbarrini A, Bandiera S, Ghermandi R, Boriani S (2017) Composite PEEK/Carbon fiber implants can increase the effectiveness of radiotherapy in the management of spine tumors. J Spine Surg 3: 323-329. Link: https://bit.ly/3fpz6OU
- Dang L, Liu Z, Liu X, Jiang L, Yu M, et al. (2020) Sagittal en bloc resection of primary tumors in the thoracic and lumbar spine: feasibility, safety and outcome. Scientific Reports 10. Link: https://bit.ly/33RXCTr
- Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, et al. (1969) The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 7: 179-192. Link: https://bit.ly/3uZheB6
- Ernstberger T, Brüning T, König F (2005) Vertebrectomy and anterior reconstruction for the treatment of spinal metastases. Acta Orthop Belg 71: 459-466. Link: https://bit.ly/342m8kZ
- Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, et al. (2001) Surgical strategy for spinal metastases. Spine 26: 298-306. Link: https://bit.ly/3tTz5HW
- Gezercan Y, Çavuş G, Ökten AI, Menekşe G, Çıkılı M, et al. (2016) Single-Stage Posterolateral Transpedicular Approach With 360-Degree Stabilization and Vertebrectomy in Primary and Metastatic Tumors of the Spine. World Neurosurg 95: 214-221. Link: https://bit.ly/3foggHR
- Metcalfe S, Gbejuade H, Patel NR (2012) The posterior transpedicular approach for circumferential decompression and instrumented stabilization with titanium cage vertebrectomy reconstruction for spinal tumors: consecutive case series of 50 patients. Spine 37: 1375-1383. Link: https://bit.ly/3fnXRuH
- Melcher RP, Harms J (2009) Biomechanics and materials of reconstruction after tumor resection in the spinal column. Orthop Clin North Am 40: 65-74. Link: https://bit.ly/3fl2dmp
- Wang JC, Boland P, Mitra N, Yamada Y, Lis E, et al. (2004) Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 1: 287-298. Link: https://bit.ly/3eRVO31
- Shen FH, Marks I, Shaffrey C, Ouellet J, Arlet V (2008) The use of an expandable cage for corpectomy reconstruction of vertebral body tumors through a posterior extracavitary approach: a multicenter consecutive case series of prospectively followed patients. Spine J 8: 329-339. Link: https://bit.ly/3yeft4R
- Street J, Fisher C, Sparkes J, Boyd M, Kwon B, et al. (2007) Single-stage posterolateral vertebrectomy for the management of metastatic disease of the thoracic and lumbar spine: a prospective study of an evolving surgical technique. J Spinal Disord Tech 20: 509-520. Link: https://bit.ly/3fogHlt
- Xu R, Garcés-Ambrossi GL, McGirt MJ, Witham TF, Wolinsky JP, et al. (2009) Thoracic vertebrectomy and spinal reconstruction via anterior, posterior, or combined approaches: clinical outcomes in 91 consecutive patients with metastatic spinal tumors. J Neurosurg Spine 11: 272-284. Link: https://bit.ly/3eP2G10
- Tomita K, Kawahara N, Baba H, Tsuchiya H, Fujita T, et al. (1997) Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine 22: 324-333. Link: https://bit.ly/3fliYxT
- Ghogawala Z, Mansfield FL, Borges LF (2001) Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine 26: 818-824. Link: https://bit.ly/2SMDu2E
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
