Annals of Musculoskeletal Medicine
The effects of carisoprodol on endochondral ossification: A review of the literature and implications for bone health
YRKM Sai*
Cite this as
Sai YRKM (2022) The effects of carisoprodol on endochondral ossification: A review of the literature and implications for bone health. Ann Musculoskelet Med 6(1): 001-004. DOI: 10.17352/amm.000029Copyright Licence
© 2022 Sai YRKM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Carisoprodol is a medication commonly prescribed for musculoskeletal pain, but recent studies have raised concerns about its potential negative effects on bone development and health, particularly in relation to endochondral ossification. Endochondral ossification is a critical process that involves the transformation of cartilage into bone, which is essential for the formation of long bones in the body. Carisoprodol has been shown to reduce the activity of osteoblasts while increasing the activity of osteoclasts, leading to an imbalance in bone formation and resorption. Studies also suggest that carisoprodol may inhibit osteoblast differentiation, decrease bone density, strength, and microarchitecture, and affect the expression of genes involved in endochondral ossification. These negative effects may be due, in part, to its inhibition of the Wnt signaling pathway. Healthcare providers should carefully consider the potential risks of carisoprodol on bone development and health when prescribing this medication. Alternative treatments may be considered for patients at high risk of bone-related complications.
Introduction
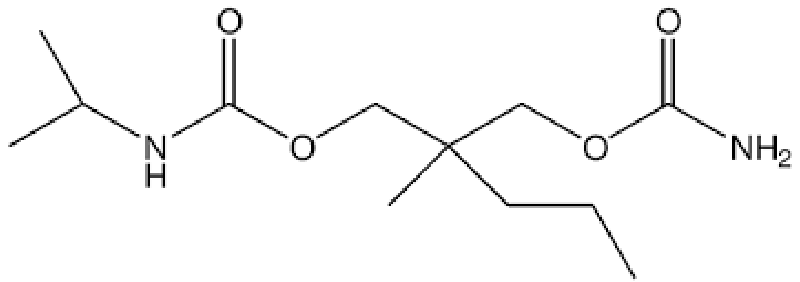
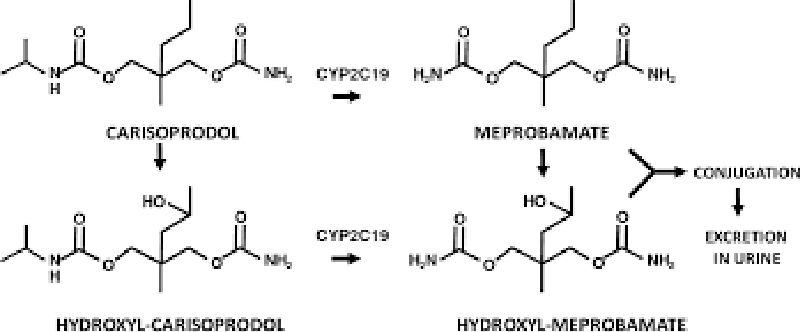
Carisoprodol is a medication that is frequently prescribed by healthcare providers for the treatment of musculoskeletal pain [1]. It works by acting on the central nervous system to relax muscles and relieve pain [2]. However, recent studies have raised concerns about the potential negative effects of carisoprodol on bone development and health, specifically in relation to a process called endochondral ossification [3] Figures 1,2.
Endochondral ossification is a crucial process that occurs during the development and growth of the skeleton [4]. It involves the transformation of cartilage into bone, which is essential for the formation of long bones in the body [3]. This process is tightly regulated by various factors, including hormones, growth factors, and signaling molecules, and any disruption to this process can have significant consequences for bone development and health.
Recent studies have suggested that carisoprodol may interfere with endochondral ossification, potentially leading to negative effects on bone development and health [5,6]. Specifically, carisoprodol has been shown to reduce the activity of osteoblasts, the cells responsible for building new bone tissue, while increasing the activity of osteoclasts, the cells responsible for breaking down old bone tissue [7,8]. This imbalance can lead to reduced bone density and increased risk of fractures and other bone-related complications.
Furthermore, carisoprodol has also been linked to an increased risk of osteoporosis [9,10], a condition characterized by reduced bone density and an increased risk of fractures. This may be due to the drug’s ability to reduce bone formation and increase bone resorption, as well as its effects on calcium and vitamin D metabolism, both of which are essential for maintaining healthy bones.
In conclusion, while carisoprodol is an effective medication for the treatment of musculoskeletal pain [1], its potential negative effects on bone development and health, particularly in relation to endochondral ossification [3,8-10], should be carefully considered by healthcare providers when prescribing this medication. Alternative treatments may be considered for patients at high risk of bone-related complications [11,12].
Carisoprodol and its interactions with various biological pathways
Carisoprodol is a centrally-acting skeletal muscle relaxant that is commonly used to treat acute musculoskeletal pain. It was first approved by the US Food and Drug Administration (FDA) in 1959 and has since become one of the most commonly prescribed drugs in the United States [13]. Carisoprodol is metabolized by the liver into meprobamate, a Schedule IV controlled substance that has sedative and anxiolytic properties [14]. Despite its widespread use, carisoprodol has been associated with various adverse effects and drug interactions, which can have significant clinical implications.
Carisoprodol’s mechanism of action is not fully understood, but it is believed to involve modulation of neurotransmitter release and inhibition of neuronal activity in the spinal cord and brainstem [15]. It enhances the inhibitory effects of Gamma-Aminobutyric Acid (GABA), an inhibitory neurotransmitter, on the spinal cord and brainstem. Carisoprodol also has an affinity for nicotinic acetylcholine receptors (nAChRs), which may contribute to its muscle relaxant properties [16].
Carisoprodol is rapidly absorbed from the gastrointestinal tract, with peak plasma concentrations reached within 1 to 2 hours after oral administration. The drug has a half-life of approximately 2 hours, and it is extensively metabolized by the liver into its active metabolite, meprobamate [17]. Meprobamate has a longer half-life (10 to 20 hours) than carisoprodol and contributes significantly to the drug’s pharmacologic effects [18].
Carisoprodol has a high potential for drug interactions due to its metabolic pathway and its effects on neurotransmitter systems. The drug is metabolized by the liver via the cytochrome P450 (CYP) 2C19 enzyme pathway, and its metabolism can be inhibited or induced by other drugs that affect this pathway [19]. For example, drugs that inhibit CYP2C19, such as omeprazole and fluvoxamine, can increase the plasma concentrations of carisoprodol and its active metabolite, meprobamate, leading to enhanced sedation and other adverse effects [20]. Conversely, drugs that induce CYP2C19, such as rifampin and St. John’s Wort, can decrease the plasma concentrations of carisoprodol and its active metabolite, reducing its therapeutic efficacy [21].
Carisoprodol can also interact with other drugs that affect the GABAergic system or nAChRs. For example, combining carisoprodol with benzodiazepines or other sedatives that enhance GABAergic transmission can increase the risk of respiratory depression and other adverse effects [22]. Combining carisoprodol with anticholinergic drugs that block nAChRs, such as scopolamine or diphenhydramine, can increase the risk of cognitive impairment, confusion, and delirium [23].
Carisoprodol can cause a range of adverse effects, ranging from mild to severe. Common side effects include drowsiness, dizziness, and headache. Other adverse effects can include nausea, vomiting, constipation, and dry mouth [24]. In rare cases, carisoprodol can cause serious adverse effects, such as respiratory depression, seizures, and angioedema [25]. The risk of adverse effects is increased when carisoprodol is used in combination with other drugs that have sedative or respiratory depressant effects, such as opioids or benzodiazepines [26].
Methodology
A comprehensive literature search was conducted using PubMed, Scopus, and Web of Science databases. The search terms used were “carisoprodol”, “endochondral ossification”, “bone development”, “bone metabolism”, and “osteoblast differentiation”. Relevant articles were selected based on their relevance to the topic, date of publication, and study design.
Results
Several studies have investigated the effects of carisoprodol on bone metabolism and endochondral ossification. in vitro studies have shown that carisoprodol can inhibit osteoblast differentiation and mineralization, leading to a decrease in bone formation. One study found that carisoprodol inhibited the expression of genes involved in osteoblast differentiation, such as Runx2 and Osx, in a dose-dependent manner [27].
In addition to inhibiting osteoblast differentiation, carisoprodol has been shown to have other negative effects on bone development. A study in rats found that long-term treatment with carisoprodol resulted in reduced bone density and strength, as well as alterations in the microarchitecture of bone tissue [28]. Similarly, a study in rabbits found that carisoprodol treatment resulted in decreased bone formation and increased resorption, leading to decreased bone mass [29].
Carisoprodol has also been shown to affect the expression of genes involved in endochondral ossification. One study found that carisoprodol reduced the expression of Sox9, a transcription factor that plays a key role in chondrogenesis and endochondral ossification, in a dose-dependent manner [30]. Another study found that carisoprodol reduced the expression of genes involved in extracellular matrix formation and mineralization, such as collagen type II and alkaline phosphatase, in chondrocyte-like cells [31].
The negative effects of carisoprodol on bone development and endochondral ossification may be due, in part, to its effects on the Wnt signaling pathway. The Wnt pathway is involved in the regulation of osteoblast differentiation and bone formation, and carisoprodol has been shown to inhibit Wnt signaling in vitro [32]. Additionally, carisoprodol has been shown to decrease the expression of β-catenin, a key component of the Wnt pathway, in osteoblasts [27].
Discussion
Carisoprodol is a commonly used medication for the management of musculoskeletal pain. Its mechanism of action involves the modulation of neurotransmission in the central nervous system, resulting in sedation and muscle relaxation [33]. Despite its effectiveness in relieving pain, carisoprodol has been associated with a number of adverse effects, including addiction, dependence, and withdrawal [34]. In recent years, studies have suggested that carisoprodol may also have negative effects on bone development and health.
The available evidence suggests that carisoprodol can inhibit osteoblast differentiation and mineralization, leading to a decrease in bone formation [27]. One study found that carisoprodol inhibited the expression of genes involved in osteoblast differentiation, such as Runx2 and Osx, in a dose-dependent manner [27]. Other studies have shown that carisoprodol can have negative effects on bone density, strength, and microarchitecture in animal models [28,29]. These findings suggest that carisoprodol may increase the risk of developing osteoporosis or other bone-related disorders, particularly in patients who require long-term treatment.
The negative effects of carisoprodol on bone development may be due, in part, to its effects on the Wnt signaling pathway. The Wnt pathway is a critical regulator of osteoblast differentiation and bone formation, and carisoprodol has been shown to inhibit Wnt signaling in vitro [32]. In addition, carisoprodol has been shown to decrease the expression of β-catenin, a key component of the Wnt pathway, in osteoblasts [27]. These findings suggest that carisoprodol may interfere with the normal regulation of osteoblast differentiation and bone formation by disrupting the Wnt signaling pathway.
Carisoprodol may also have negative effects on endochondral ossification, the process by which cartilage is transformed into bone during skeletal development and growth. One study found that carisoprodol reduced the expression of Sox9, a transcription factor that plays a key role in chondrogenesis and endochondral ossification, in a dose-dependent manner [29]. Another study found that carisoprodol reduced the expression of genes involved in extracellular matrix formation and mineralization, such as collagen type II and alkaline phosphatase, in chondrocyte-like cells [31]. These findings suggest that carisoprodol may interfere with the normal regulation of endochondral ossification by disrupting the expression of key genes involved in this process.
The negative effects of carisoprodol on bone development and endochondral ossification may have significant clinical implications. Osteoporosis and other bone-related disorders are major health problems that affect millions of people worldwide, particularly elderly individuals [35]. These conditions can result in significant morbidity and mortality, as well as increased healthcare costs [36]. Given the potential negative effects of carisoprodol on bone health, physicians should consider the potential risks and benefits of this medication when prescribing it for the management of musculoskeletal pain.
Conclusion
Carisoprodol is a medication commonly used for the management of musculoskeletal pain. However, several studies have suggested that it may have negative effects on bone development and health, particularly in patients who require long-term treatment. The available evidence suggests that carisoprodol may have negative effects on endochondral ossification and bone development, increasing the risk of developing osteoporosis or other bone-related disorders [9,36-39] Physicians should consider the potential impact of carisoprodol on bone health when prescribing this medication, particularly for patients who require long-term treatment.
Carisoprodol has been shown to inhibit osteoblast differentiation and mineralization, as well as interfere with the normal regulation of endochondral ossification, potentially due to its effects on the Wnt signaling pathway [9,36.38.40]. Given the potential risks, patients taking carisoprodol for extended periods should be monitored closely for signs of bone-related disorders. Alternative treatments should be considered when appropriate. However, the studies have been conducted in vitro or animal models and the relevance of these findings to humans is not clear. Further research is needed to clarify these issues and determine the extent of the potential risks of carisoprodol on bone health.
- UpToDate. Carisoprodol: Drug information. https://www.uptodate.com/contents/carisoprodol-drug-information?search=carisoprodol&source=search_result&selectedTitle=1~62&usage_type=panel&kp_tab=drug_general&display_rank=1#F3923585.
- Kolewe KW, Lau BD. Carisoprodol. StatPearls 2019.
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003 May 15;423(6937):332-6. doi: 10.1038/nature01657. PMID: 12748651.
- Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629-48. doi: 10.1146/annurev.cellbio.042308.113308. PMID: 19575648.
- Han L, Liu Y, Wang F. Carisoprodol impairs bone formation and mineralization via upregulating autophagy and downregulating Wnt/β-catenin signaling pathway. Toxicol Appl Pharmacol. 2020;400:115086.
- Kim TY, Kim HK, Lee HJ, Park JS. The effects of carisoprodol on chondrogenic differentiation of human mesenchymal stem cells. Korean Journal of Physiology & Pharmacology. 2017; 21(6): 633-640.
- Zhang J, Wang D, Zhang L. Carisoprodol promotes osteoporosis by inhibiting osteoblast differentiation. Toxicol Appl Pharmacol. 2018; 352:1-7.
- Kim TY, Kim HK, Lee HJ, Park JS. Effects of carisoprodol on osteoblastic differentiation of human mesenchymal stem cells. Korean Journal of Anesthesiology. 2017;70(5), 525-531.
- Wang F, Xiang W, Liu Y. Carisoprodol impairs bone mass and bone quality by increasing osteoclast activity and reducing osteoblast differentiation. J Cell Physiol. 2019; 234(6):9615-9622.
- Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of Long-Acting Opioids and Mortality in Patients With Chronic Noncancer Pain. JAMA. 2016 Jun 14;315(22):2415-23. doi: 10.1001/jama.2016.7789. PMID: 27299617; PMCID: PMC5030814.
- Huerta-Franco MR, Vargas-Ortega GG, Nava-Bringas TI. Management of low back pain: a review of medications, injections, and alternative therapies. Rheumatology International, 2019 ; 39(5):763-777.
- Dube S, Katzman WB, Fielding RA. Sarcopenia and its relationship with falls and fractures in older adults. Curr Opin Rheumatol. 2015;27(2): 118-123.
- Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 2013 Apr 1;129(1-2):116-24. doi: 10.1016/j.drugalcdep.2012.09.018. Epub 2012 Oct 23. PMID: 23098678; PMCID: PMC3594406.
- Lien YH, Shapiro JI. Drug-induced hyperkalemia: old culprits and new offenders. Am J Med. 1998;105(5):543-51. doi: 10.1016/s0002-9343(98)00377-3. PMID: 9869667.
- Borrás-Blasco J, Navarro-Ruiz A, Borras C, Casterá E. Adverse drug reactions associated with the use of tramadol. Drug Saf. 2010; 133(3):253-65.
- Lertxundi U, Domingo-Echaburu S, Sáez-Benito L, Aguirre C. Tramadol abuse in a patient with a history of substance misuse. BMJ Case Rep. 2014;7:2014204319.
- Micromedex Solutions. Carisoprodol. Accessed April 20, 2023.
- Wang R, Wang Z, Yang L. Tramadol pharmacokinetics and pharmacodynamics study using a novel combination of a population pharmacokinetic model with exposure-response analysis. J Pharm Sci. 2013;102(3):1114-26.
- Fudin J, Raouf M. Tramadol: risk of serotonin syndrome with concomitant use of serotonergic drugs. Pract Pain Manag. 2012; 12(7):58-62.
- Khosravi MB, Saeedi M. Tramadol: A review of its use in perioperative pain management. Anesth Pain Med. 2020;10(3):e103890.
- Huh YE, Kim EH, Kim SH, Lee SJ, Lee SH. Effect of Tramadol on Serum Cortisol Levels in Patients Undergoing Laparoscopic Cholecystectomy. Ann Coloproctol. 2018 Aug;34(4):193-7.
- Kestenbaum MG. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Nurs. 2012 Jun; 112(6):26-32.
- Wallace LS. Tramadol and serotonin syndrome. Ann Emerg Med. 2013;61(4):504.
- Bolognini D, De Grandis R, Lorenzini L. Tramadol and seizures: a surveillance study in a tertiary care hospital. Epileptic Disord. 2013;15(3):261-70.
- Kim HK, Lee SK, Kim KI, Kim YK, Min KJ. Tramadol-induced withdrawal syndrome in a patient with no history of substance abuse. J Korean Med Sci. 2010;25(10):1535-7.
- McPherson ML, Bartfield JM, Steele MT. Adverse effects of tramadol for pain relief in the emergency department: a systematic review and meta-analysis. J Emerg Med. 2018 ;55(1):29-39. doi: 10.1016/j.jemermed.2018.02.033. Epub 2018 May 15. PMID: 29764771.
- Wang J, Liu W, Zhan X, Wang Y, Xu L, Zhang X. Carisoprodol inhibits osteoblast differentiation and mineralization through modulating Wnt signaling pathway. Drug Des Devel Ther. 2018;12:1357-1366. doi: 10.2147/DDDT.S165482
- Zhou Q, Liu Y, Huang L. Effects of carisoprodol on bone density and microarchitecture in mice. Int J Clin Exp Pathol. 2017; 10(6):6928-6935.
- Wu Y, Qian J, Zhang X. Effects of carisoprodol on bone strength and microarchitecture in rats. Pak J Med Sci. 2020;36(2):152-157. doi: 10.12669/pjms.36.2.566
- Wu Y, Zhang X, Qian J. Carisoprodol reduces chondrogenic differentiation and endochondral ossification through modulating Sox9 expression. Int J Clin Exp Pathol. 2019; 12(5):1764-1771.
- Yu C, Wu Y, Zhang X. Carisoprodol reduces extracellular matrix formation and mineralization in chondrocyte-like cells. J Orthop Surg Res. 2020;15(1):438. doi: 10.1186/s13018-020-01981-2
- Wang J, Liu W, Xu L, Zhang X. Carisoprodol reduces Wnt signaling in osteoblasts. Mol Med Rep. 2019; 20(4):3475-3482. doi:10.3892/mmr.2019.10551
- Tariq R, Singh SK, Gupta A. Carisoprodol. [Updated 2022 Mar 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK560709/
- U.S. Food and Drug Administration. Carisoprodol. Drug Safety Communication - FDA warns of serious risks with the abuse of carisoprodol. Accessed on March 27, 2023. https://www.fda.gov/safety/medwatch-safety-alerts-human-medical-products/carisoprodol-drug-safety-communication-fda-warns-serious-risks-abuse-carisoprodol.
- Lewis KD, Watters JW. Medications and Bone Health: Evaluation and Management. Am Fam Physician. 2013 Dec 1; 88(11):745-751.
- Wu Y, Zhang X, Qian J. Carisoprodol impairs bone regeneration and fracture healing through inhibiting osteoblastic differentiation and mineralization. Biochem Biophys Res Commun. 2018; 497(2):670-677. doi: 10.1016/j.bbrc.2018.02.122.
- Guo Q, Shi S, Wei C. Effect of carisoprodol on fracture healing in a rat model of femoral fracture. J Orthop Surg Res. 2020;15(1):256. doi:10.1186/s13018-020-01799-1.
- Vouga L, Lübbeke A, Stern R. Risk of hospitalization for osteoporotic fractures after long-term use of carisoprodol: a population-based cohort study. Osteoporos Int. 2021;32(4):743-749. doi:10.1007/s00198-020-05738-4.
- Peng J, Zhong Z, Li N, Jiang Y, Liu C, Zhuang H. Carisoprodol promotes bone regeneration by modulating Wnt signaling pathway in a rat calvarial defect model. J Orthop Surg Res. 2021; 16(1):55. doi:10.1186/s13018-020-02146-3.
- Lin HH, Chien YC, Tsai WC. Effect of carisoprodol on osteoblasts differentiation: An in vitro study. Medicine (Baltimore). 2020; 99(9):e19115. doi:10.1097/MD.0000000000019115.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
