Open Journal of Hepatology
The effect of physical load of varying intensity on the activity of liver enzymes and hepatocytes’ proliferation
II Malyshev1*, LP Romanova2 and OV Vorobeva2
2FSBEI of HE “Chuvash State University named after I. N. Ulyanova Medical Faculty”, Cheboksary, Russia
Cite this as
Malyshev II, Romanova LP, Vorobeva OV (2022) The effect of physical load of varying intensity on the activity of liver enzymes and hepatocytes’ proliferation. Open J Hepatol 4(1): 008-010. DOI: 10.17352/ojh.000008Copyright License
©2022 Malyshev II, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The paper establishes the relationship between enzymatic activity and the proliferation of hepatocytes. Low-intensity physical activity is shown not to influence the activity of hepatocytes’ enzymes and their proliferation. Hard work results in decreased activity of redox enzymes; at the same time, the proliferation of hepatocytes is inhibited for a long time. The most favorable is moderate load, which leads to the activation of oxidative-reducing enzymes and increases the number of binucleated hepatocytes, which persist for a long time after the end of the experiment.
Currently, the structure of mortality and morbidity in developed countries has fundamentally changed. Infectious pathology, with the exception of several viral diseases, has faded into the background, and somatic diseases have taken the main place: coronary heart disease, hypertension, gastric ulcer, mental illness, diabetes, etc. With the diversity of all these diseases in their etiology and pathogenesis often an overly intense and prolonged stress response is common. The study of the influence of repeated stressful effects (including excessive ones) on various body systems has been undertaken repeatedly [1-4], however, studies on the effect of physical activity on the morphological state of various organs and tissues have not been studied enough. In this regard, it can be considered appropriate to study the significance of physical activity of varying severity on the proliferative activity of some animal organs. Since the proliferation of functionally active elements is an indicator of structural and physiological well-being [5-14]. Also little studied is the question of the influence of the intensity of physical activity on the enzymatic activity of the functionally active elements of the organ. Meanwhile, enzymatic activity is of exceptional importance for the implementation of various processes in the organ, including the proliferation of organ elements. The purpose of this work was to study the influence of physical work on the activity of hepatocytes’ enzymes and their proliferation.
Materials and methods
White sexually mature outbred male rats in the amount of 64 individuals weighing 250-265 grams were used as experimental animals. The animals were divided into 3 groups (series) (Table 1).
The first series of rats were given light physical activity, for which they were placed in a bath with a water temperature of 29-32 degrees Celsius in which the animals were swimming for 15 minutes [9-11]. The rats of the second series spent 30 minutes in the bath. We regarded this physical load on animals as a moderate load. Animals of the first and second series after their removal from the bath were active, mobile; signs of fatigue were not visually detected in them. To reproduce heavy physical activity (the third series), the animals swam in the bath until they began to lose strength and to drown. This usually occurred in 55-59 minutes after the animals’ being in the water. After removing from the bath, the animals of this group were sluggish and stayed down for some time. Animals of all series underwent 10 sessions of water loading, after which they were taken out of the experiment immediately after the last session (8 animals per series) and 30 days after the end of the experiment (8 animals per series) using zoletil anesthesia calculated as 5 mg per 100 g in accordance with International Rules for Working with Experimental Animals.
The experimental rats were kept in vivarium conditions in accordance with GOST 33216-2014 “Rules for Working with Laboratory Rodents and Rabbits”.
After the liver was extracted, 1x1cm pieces were cut out of it, which were fixed in 10% neutral formalin and embedded in paraffin; the resulting serial sections were stained with hematoxylin and eosin and using van Gieson’s staining. The tetrazolium method was used to determine SDH, NADH, and NADPH. Acid phosphatase was identified by simultaneous combination with naphthol phosphates AS and stable diazonium salts. Alkaline phosphatase was determined by the Burston method [15]. Histological preparations were studied using a Leica DFC320 camera and a Leica DM1000 microscope (Leica Microsystems, Germany). The binuclear cells in the liver were counted by 500 nuclei.
The quantitative assessment of hepatocytes’ enzymatic activity in dynamics was carried out using photometry. Photometry was carried out in transmitted light on a “Micromed” microscope with a camera adapter FMEL-1 and FEU-79 and an amplifier output voltage of 1200 V. To obtain a monochromatic beam in the red region of the spectrum passing through the preparation, an interference light filter with a maximum light transmission at a wavelength of 620 nm was used. Light transmission was recorded using a digital voltmeter SCH 4300, after which, taking a negative decimal logarithm, the light transmission level was transformed into light absorption, which was expressed in conventional units (c.u.) of optical density. According to Beer–Lambert–Bouguer law, the optical density of the preparation is proportional to the amount of dye. The described method meets the requirements of proportionality of dye concentration and enzyme activity. The optical density was calculated using the formula OD = Lg Ui/100. Statistical processing of digital data was carried out in the program “Statistica” involving Microsoft Office software packages (Word and Excel). Statistical processing was carried out using the program “Statistica 6.0” applying the methods of variation statistics (determination of the arithmetic mean and its error M ± m). Comparison of results subject to normal distribution was carried out using Student’s t-test.
Results
According to literature data, hydrolytic enzymes - Alkaline Phosphatase, Acid Phosphatase are involved in the processes of apoptosis, phagocytosis, pinocytosis, and cell lysis. The enzymes - SDH NADH and NADPH - play a crucial role in the production of ATP by oxidative phosphorylation in mitochondria. Our study revealed that heavy physical exertion leads to the inhibition of redox enzymes and an increase in hydrolytic ones. For this reason, it was these enzymes that were taken.
Table 2 provides information on the activity of liver enzymes after physical load (in c.u.).
It follows from Table 2 that the activity of liver enzymes practically did not change in animals of the first series and insignificantly deviated from normal values in rats of the second series. In animals of the third series, there was a sharp increase in lysosomal enzymes and a statistically significant decrease in the activity of redox enzymes.
Table 3 shows the results of studying the enzymes’ activity 30 days after the end of the physical load test (in c.u.). It can be seen that in rats of series 1 and 2 both lysosomal and redox enzymes approached the control values. At the same time, the control values of enzymes did not restore in group 3 rats.
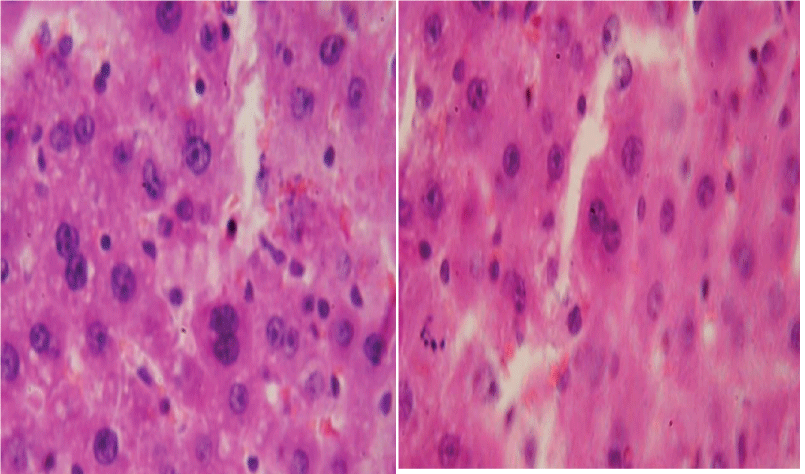
Binucleate hepatocytes were found in the liver of animals of all series. Table 3 provides findings on the number of binucleate hepatocytes in rats immediately / and 30 days after the end of the experiment Table 4.
Attention is drawn to the fact that in rats of the third series, the percentage of binuclear hepatocytes did not reach the level of indicators in control animals and remained at the level of indicators observed immediately after 10 sessions of water load. Binuclear hepatocytes are shown in the figure (Figure 1).
Discussion
A comparative analysis of liver enzyme condition and the proliferative activity of hepatocytes shows that there is a direct relationship between them. Physical activity of low intensity (1 series) practically does not affect the enzyme activity and proliferation of hepatocytes. Physical activity of moderate intensity (series 2) slightly increases the activity of lysosomal enzymes and simultaneously promotes the activation of redox enzymes; at this, the proliferation of hepatocytes is stimulated. In 30 days, liver enzymes return to the original values, but the number of binucleated hepatocytes in the liver remains high. Heavy physical activity (series 3) negatively affects both indicators: the activity of lysosomal enzymes increases and the activity of redox enzymes decreases, which is accompanied by a decrease in the number of binucleated hepatocytes. Recovery of these indicators in 30 days in animals does not take place.
Сlinical and practical significance of this study: аccording to statistics, 80% of cases of sudden death occur in athletes due to unknown causes. Taking into account the fact that in high-performance sports, the sudden death of very young athletes (< 35 years old) has become more common, the main focus in studying the problem of sudden death is directed precisely at this category of persons. Even though all professional athletes undergo pre-competition screening twice a year, still there are death cases among them. That is why, the practical significance of our work is that when studying the state of the internal organs after physical load, in this particular case – that of the liver, it is possible to assess their regenerative potential using available classical methods and histochemistry to get a multidimensional idea of the processes taking place in the body. The data obtained can be used in research works to find methods to influence this cell system, to identify the mechanisms for controlling the processes that develop both locally and at the level of a holistic organism.
- Brodsky VYa, Uryvaeva IV. Cellular polyploidy. Proliferation and differentiation. Science. 259.
- Gorizontova MP. Microcirculation under stress. Pathological physiology and experimental therapy. 1986; 3: 79-84.
- Epifanov VA, Suvorova SS. Capacity and resistance parameters of the athlete's cardiovascular system and their dynamics in regular training. Vopr Kurortol Fizioter Lech Fiz Kult. 2001 Jan-Feb;(1):12-5. Russian. PMID: 11530399.
- Kogan OS. Occupational medicine and prom. Ecology. The state of health of high-class athletes in various sports. 2006; 5: 40-44.
- Anatskaya OV, Vinogradov AE. Genome multiplication as adaptation to tissue survival: evidence from gene expression in mammalian heart and liver. Genomics. 2007 Jan;89(1):70-80. doi: 10.1016/j.ygeno.2006.08.014. Epub 2006 Oct 6. PMID: 17029690.
- Celton-Morizur S, Desdouets C. Polyploidization of liver cells. Adv Exp Med Biol. 2010;676:123-35. doi: 10.1007/978-1-4419-6199-0_8. PMID: 20687473.
- Gupta S. Hepatic polyploidy and liver growth control. Semin Cancer Biol. 2000 Jun;10(3):161-71. doi: 10.1006/scbi.2000.0317. PMID: 10936066.
- Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR Jr, Reid LM, Gupta S. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol. 1999 May;276(5):G1260-72. doi: 10.1152/ajpgi.1999.276.5.G1260. PMID: 10330018.
- Ermolaeva EN, Krivokhizhina LV. Dyslipidemia during chronic physical exertion of varying intensity. Fundamental research. 2015; 1147-1151.
- Krasnova AF, Samodanova GI, Usik SV, Iakovlev NN. Blood uric acid levels as an indicator of the response to physical loads. 1978 Apr;64(4):538-42. Russian. PMID: 658523.
- Gennadievna PI, Kozin SV, Bulanov DV. Histomorphological assessment of the hepatoprotective effect of phytoadaptogens in toxic damage to the liver of mice by carbon tetrachloride against the background of intense physical activity. Bulletin of VolGMU. 2014; No. 2 (50). URL: https://cyberleninka.ru/article/n/gistomorfologicheskaya-otsenka-gepatoprotektornogo-deystviya-fitoadaptogenov-pri-toksicheskom-porazhenii-pecheni-myshey (date of access: 09/29/2022).
- Andreev VP, Tsyrkunov VM, Kravchuk RI. Сlinical morphology of the liver: Нepatocyte nuclear apparatus. Hepatology and gastroenterology. 2020. №2. URL: https://cyberleninka.ru/article/n/klinicheskaya-morfologiya-pecheni-yadernyy-apparat-gepatotsitov (date of access: 09/29/2022).
- Umbetov TZh, Berdalinova AK, Zharilkasinov KE, Chizmanidi L, Baldakov N. Structure of the liver at the combined impact of chemicals on the organism. Bulletin of Medical Internet Conferences (ISSN 2224-6150) 2016; 6.
- Ermolaeva EN. Reactive changes in the blood system and oxidative stress during physical activity of varying intensity. Abstract of the dissertation for the degree of Doctor of Medical Sciences. 2020; 42.
- Loida Z, Gossrau R, Schibler T. Histochemistry of enzymes. Laboratory methods. Moscow: Mir Publ. 1982; 272.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
