Open Journal of Chemistry
Synthesis of optically pure calix[4]arenes derived from Evans oxazolidinone and/or pyranose
Claude Bauder and David Sémeril*
Cite this as
Bauder C, Sémeril D (2022) Synthesis of optically pure calix[4]arenes derived from Evans oxazolidinone and/or pyranose. Open Journal of Chemistry 8(1): 001-007. DOI: 10.17352/ojc.000028Copyright
© 2022 Bauder C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Eight new optically pure calixarene derivatives, in which their lower rims were substituted with Evans oxazolidinone or pyranose moieties, are described. All macrocycles were fully characterized by NMR spectroscopy, optical rotation, and elemental analysis. The introduction of chiral auxiliaries reduced the symmetry of the macrocycle as observed by NMR. Stereospecific alkylation on the Evans oxazolidinone moiety allowed the asymmetric introduction of a methyl substituent near a phenolic position of the macrocycle.
Introduction
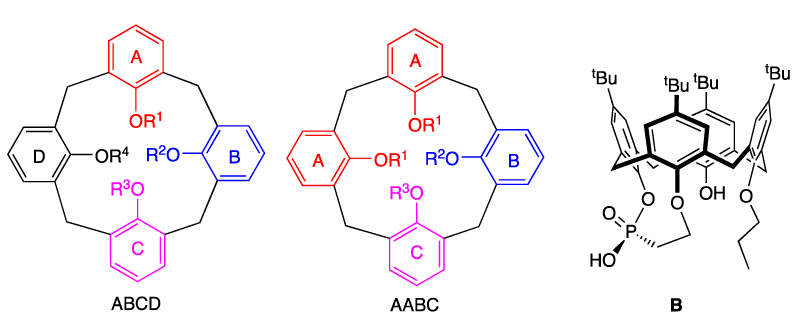
Calix[n]arenes are a well-established family of polyphenolic compounds widely used as building blocks for applications in host-guest chemistry, coordination chemistry, homogeneous catalysis, and materials science (Figure 1) [1-9]. Interest in these macrocycles has developed continuously and in a spectacular fashion since rational methods for the preparation of their parent versions became available ca. 35 years ago. It is interesting to note that these macrocyclic skeletons may adopt various flexible conformations, which can be rigidified through appropriate chemical modifications.
Combined with the rigidity of the calix[4]arene, this macrocycle provides a useful platform for the attachment of various functions, which is interesting from a chirality point of view. The functionalization at particular positions aims to destroy the C4v symmetry of the generic calix[4]arene and therefore afford lower C4, C2, or C1 symmetry molecules. The synthesis of optically active calixarene derivatives is well documented [10-13]. The preparation of these nonracemic compounds can be envisaged under two ways of development:
i) The chirality can be obtained by the attachment of an optically active group either to the hydroxyl function of the phenolic groups or to its para position at the upper rim of the calix[4]arene. This way represents the more popular method to prepare an enantiomerically pure macrocyclic species avoiding a resolution step. Thus, group Matt reported the synthesis of chiral calixarene A, which was employed as a ligand in the allylic substitution of 1,3-diphenylprop-2-enyl acetate leading to low enantiomeric induction [14] (Figure 1).
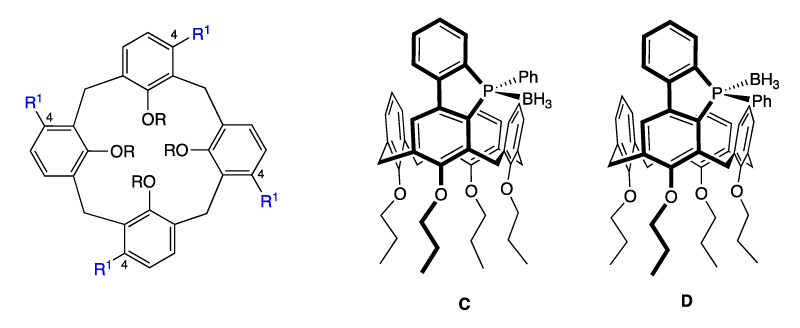
ii) The chirality can be obtained from the functionalization of the calix[4]arene inducing globally an asymmetric system, often named “inherent chirality”. This methodology can follow two strategies:
- macromolecules having no mirror planes or symmetry axes (greater than C1). For that, three adjacent aromatic units must be different leading to a macrocycle of ABCD or AABC type (Figure 2). An exemple of calixarene of ABCD type was reported by the group of Manoury. The phosphonic acid B was employed as an organocatalyst in the aza-Diels-Alder reaction of imines with Danishefsky’s diene with enantiomeric excess up to 21 % [15].
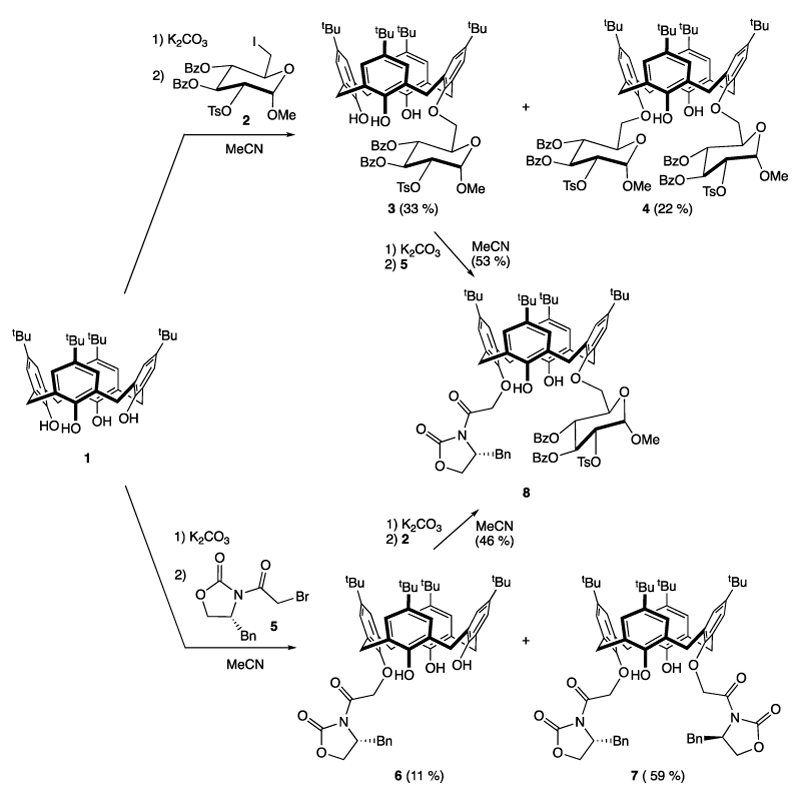
- functionalization of aromatic rings at carbon number 4, which destroys the vertical mirror planes of the C4v macrocycle. The “chirality axis” (coincident with the Cn symmetry axis) (Figure 3). With this aim our group published recently the synthesis of two calix[4]arene-fused benzophospholes C and D (Figure 3). These compounds were obtained as two unresolved diastereoisomers [16-17].
In the present article, we describe the introduction of chirality near the oxygen atom of a phenolic moiety of a calix[4]arene. Such a new concept should generate a particular behavior never explored at this time in a catalytic system. For this, we will perform the first grafting of Evans oxazolidinone [18-19] on the lower rim of a calix[4]arene containing or not a glycosyl substituent.
Experimental
General
All manipulations were carried out under dry argon. Routine 1H and 13C{1H} spectra were recorded with Bruker FT instruments (AC 300 and 500) and were referenced to residual protonated solvents (δ = 7.16 ppm and 77.16 ppm, respectively). Chemical shifts and coupling constants are reported in ppm and Hz, respectively. Elemental analyses were carried out by the Service de Microanalyse, Institut de Chimie, Université de Strasbourg. Optical rotations ([α]D20) were recorded with a Polarimeter Model 341 (Perkin–Elmer) at a wavelength of 589 nm in a 10 cm quartz cuvette.
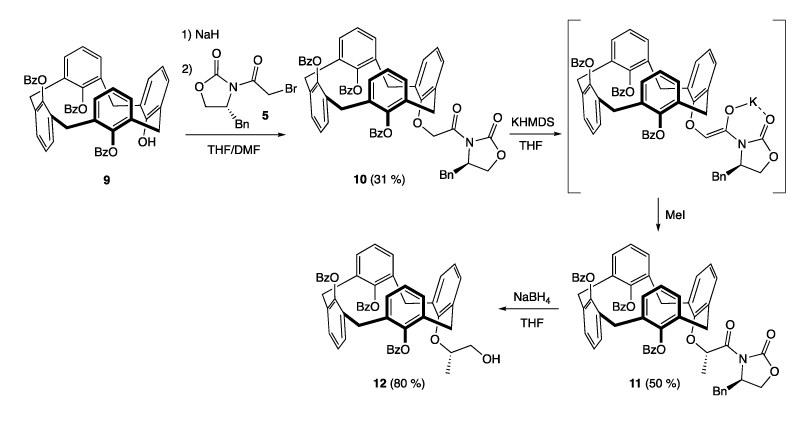
Synthesis of calixarenes 3 and 4
A mixture of calixarene 1 (489 mg, 0.75 mmol) and K2CO3 (120 mg, 1.1 equiv.) was heated in dry MeCN (10 mL) at 94°C for 5 h. Then a solution of 2 (1.118 g, 2.2 equiv.) in dry MeCN (10 mL) was added in one portion to the previous heterogeneous solution. The mixture was heated for 94 h at 88°C and the resulting homogeneous brown-orange solution was concentrated under reduced pressure to leave a brown solid which was dissolved in AcOEt (20 mL). Water (5 mL) and aqueous HCl 10% (0.5 mL) were added and the organic layer was washed with water (10 mL), brine (5 mL), dried over MgSO4, filtered, and concentrated to give a beige powder. The crude was purified by column chromatography on silica gel (AcOEt/cyclohexane 1:5 v/v as eluent) to afford the monosubstituted calixarene 3 (294 mg, 33 %) and the disubstituted calixarene 4 (287 mg, 22 %) as white solids.
Calixarene 3: [α]D20 = +40.8 (c = 1.12, CHCl3). 1H NMR (500 MHz, CDCl3): δ = 10.24 (s, 1H, ArOH), 9.47 (s, 1H, ArOH), 9.15 (s, 1H, ArOH), 7.91 (dd, 2H, Ar H of COPh, 3J = 8.0 Hz, 4J = 1.5 Hz), 7.69 (dd, 2H, Ar H of COPh, 3J = 8.0 Hz, 4J = 1.0 Hz), 7.65 and 6.95 (AB system, 4H, Ar H of CH3C6H4SO2, 2J = 7.5 Hz), 7.51-7.47 (m, 2H, Ar H of COPh), 7.35-7.29 (m, 4H, Ar H of COPh), 7.10 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.09 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.06 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.06 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.05 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.02 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.01 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.95 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.09 (t, 1H, CHOCOPh, 3J = 9.7 Hz), 5.44 (t, 1H, CHOCOPh, 3J = 9.7 Hz), 5.31 (d, 1H, CHOCH3, 3J = 3.5 Hz), 4.92-4.88 (m, 1H, ArOCH2CH), 4.72 (dd, 1H, CHOSO2, 3J = 10.0 Hz, 4J = 3.5 Hz), 4.36 and 3.19 (AB system, 2H, ArCH2Ar, 2J = 12.0 Hz), 4.30 and 3.51 (AB system, 2H, ArCH2Ar, 2J = 13.5 Hz), 4.30 and 3.48 (AB system, 2H, ArCH2Ar, 2J = 13.5 Hz), 4.21 and 3.51 (AB system, 2H, ArCH2Ar, 2J = 13.5 Hz), 4.20-4.10 (m, 2H, ArOCH2CH), 3.86 (s, 3H, OCH3), 2.21 (s, 3H, CH3C6H4SO2), 1.24 (s, 18H, C(CH3)3), 1.20 (s, 9H, C(CH3)3), 1.16 (s, 9H, C(CH3)3); 13C{1H} NMR (126 MHz, CDCl3): δ = 165.6 (s, OCOPh), 165.06 (s, OCOPh), 149.0-125.5 (Ar C), 98.4 (s, CHOCH3), 76.9 (s, CHOSO2), 75.6 (s, ArOCH2CH), 70.2 (s, CHOCOPh), 69.5 (s, CHOCOPh), 68.8 (s, ArOCH2CH), 57.3 (s, OCH3), 34.3 (s, C(CH3)3), 34.2 (s, C(CH3)3), 34.1 (s, C(CH3)3), 34.0 (s, C(CH3)3), 33.2 (s, ArCH2Ar), 33.1 (s, ArCH2Ar), 32.2 (s, ArCH2Ar), 31.6 (s, C(CH3)3), 31.6 (s, C(CH3)3), 31.3 (s, ArCH2Ar), 31.3 (s, C(CH3)3), 21.8 (s, CH3C6H4SO2) ppm. Elemental analysis (%) calcd for C72H82O13S (1187.48): C 72.82, H 6.96 found: C 72.57, H, 6.93.
Calixarene 4: [α]D20 = +62.4 (c = 1.28, CHCl3). 1H NMR (500 MHz, CDCl3): δ = 7.96 (dd, 4H, Ar H of COPh, 3J = 8.5 Hz, 4J = 1.5 Hz), 7.72 (dd, 4H, Ar H of COPh, 3J = 8.5 Hz, 4J = 1.0 Hz), 7.67 and 7.02 (AB system, 8H, Ar H of CH3C6H4SO2, 2J = 8.0 Hz), 7.51-7.48 (m, 4H, Ar H of COPh), 7.37-7.30 (m, 8H, Ar H of COPh), 7.03 (s, 4H, Ar H of calixarene), 6.68 (s, 2H, ArOH), 6.62 (s, 4H, Ar H of calixarene), 6.02 (t, 2H, CHOCOPh, 3J = 9.7 Hz), 5.26 (t, 2H, CHOCOPh, 3J = 9.7 Hz), 5.14 (d, 2H, CHOCH3, 3J = 3.5 Hz), 4.73 (dd, 2H, CHOSO2, 3J = 10.0 Hz, 4J = 4.0 Hz), 4.60-4.56 (m, 2H, ArOCH2CH), 4.32 and 3.28 (AB system, 4H, ArCH2Ar, 2J = 13.5 Hz), 4.15 and 3.07 (AB system, 4H, ArCH2Ar, 2J = 13.0 Hz), 4.04-3.96 (m, 4H, ArOCH2CH), 3.79 (s, 6H, OCH3), 2.24 (s, 6H, CH3C6H4SO2), 1.31 (s, 18H, C(CH3)3), 0.82 (s, 18H, C(CH3)3); 13C{1H} NMR (126 MHz, CDCl3): δ = 165.6 (s, OCOPh), 165.0 (s, OCOPh), 150.6-124.9 (Ar C), 98.0 (s, CHOCH3), 76.7 (s, CHOSO2), 75.3 (s, ArOCH2CH), 69.9 (s, CHOCOPh), 69.7 (s, CHOCOPh), 69.0 (s, ArOCH2CH), 56.9 (s, OCH3), 33.9 (s, C(CH3)3), 33.9 (s, C(CH3)3), 31.8 (s, C(CH3)3), 31.5 (s, ArCH2Ar), 31.0 (s, C(CH3)3), 30.7 (s, ArCH2Ar), 21.7 (s, CH3C6H4SO2) ppm. Elemental analysis (%) calcd for C100H108O22S2 (1726.04): C 69.59, H 6.31 found: C 65.48, H, 6.09.
Synthesis of calixarenes 6 and 7
The calixarene 1 (610 mg, 0.94 mmol) was dissolved with K2CO3 (144 mg, 1.1 equiv.) in dry MeCN (9 mL) and the mixture was stirred at room temperature for 15 h. A solution of oxazolidinone 5 (678 mg, 2.4 equiv.) in dry MeCN (3.5 mL) was added and stirring was continued for 48 h at 86°C. The solvent was removed and the resulting solid was dissolved with AcOEt (20 mL). The organic layer was washed with an aqueous saturated NH4Cl solution (10 mL) water (10 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude brown solid was purified by column chromatography on silica gel (AcOEt/cyclohexane 1:5 to 1:1 v/v as eluent) to afford the monosubstituted calixarene 6 (89 mg, 11 %), and the disubstituted 7 calixarenes (599 mg, 59 %) as white solids.
Calixarene 6: [α]D20 = -30.1 (c = 1.0, CH2Cl2). 1H NMR (500 MHz, CDCl3): δ = 10.36 (s, 1H, ArOH), 9.46 (s, 1H, ArOH), 9.37 (s, 1H, ArOH), 7.39 (t, 2H, Ar H of CH2Ph, 3J = 7.7 Hz), 7.33-7.31 (m, 3H, Ar H of CH2Ph), 7.13 (s, 2H, Ar H of calixarene), 7.10 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.08 (s, 2H, Ar H of calixarene), 7.08 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.02 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.01 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 5.55 and 5.39 (AB system, 2H, ArOCH2CON, 2J = 17.5 Hz), 4.92-4.88 (m, 1H, NCHCH2Ph), 4.53 and 3.49 (AB system, 2H, ArCH2Ar, 2J = 13.0 Hz), 4.48 and 3.45 (AB system, 2H, ArCH2Ar, 2J = 13.0 Hz), 4.43-4.33 (m, 2H, OCH2CH(CH2Ph)), 4.40 and 3.46 (AB system, 2H, ArCH2Ar, 2J = 13.5 Hz), 4.36 and 3.47 (AB system, 2H, ArCH2Ar, 2J = 13.5 Hz), 3.47-3.45 (m, 1H, CH2Ph), 3.01 (a part of an ABX system, 1H, 2JAB = 13.2 Hz, 3JAX = 9.5 Hz, CH2Ph), 1.26 (s, 9H, C(CH3)3), 1.24 (s, 9H, C(CH3)3), 1.24 (s, 9H, C(CH3)3), 1.23 (s, 9H, C(CH3)3); 13C{1H} NMR (126 MHz, CDCl3): δ = 169.4 (s, NCOCH2), 153.4 (s, NCO2), 150.0-125.7 (Ar C), 74.4 (s, ArOCH2CON), 67.7 (s, OCH2CH(CH2Ph)), 55.0 (s, NCHCH2Ph), 37.8 (s, CH2Ph), 34.3 (s, C(CH3)3), 34.1, (s, C(CH3)3), 34.1 (s, C(CH3)3), 33.2 (s, ArCH2Ar), 32.7 (s, ArCH2Ar), 31.6 (s, C(CH3)3), 31.6 (s, C(CH3)3), 31.3 (s, C(CH3)3) ppm. Elemental analysis (%) calcd for C56H67NO7 (866.13): C 77.66, H 7.80, N 1.62 found: C 77.29, H, 7.69, N 1.43.
Calixarene 7: [α]D20 = -49.9 (c = 1.14, CH2Cl2). 1H NMR (500 MHz, CDCl3): δ = 7.33 (s br, 2H, ArOH), 7.32-7.27 (m, 6H, Ar H of CH2Ph), 7.20-7.19 (m, 4H, Ar H of CH2Ph), 7.07 (s, 4H, Ar H of calixarene), 6.82 (s, 4H, Ar H of calixarene), 5.36 and 5.25 (AB system, 4H, ArOCH2CON, 2J = 17.5 Hz), 4.81-4.76 (m, 2H, NCHCH2Ph), 4.49 and 3.38 (AB system, 4H, ArCH2Ar, 2J = 13.5 Hz), 4.49 and 3.34 (AB system, 4H, ArCH2Ar, 2J = 13.5 Hz), 4.33-4.30 (m, 1H, OCH2CH(CH2Ph)), 4.25-4.22 (m, 1H, OCH2CH(CH2Ph)), 3.39 (A part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 3.5 Hz, CH2Ph), 2.85 (B part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 9.5 Hz, CH2Ph), 1.30 (s, 18H, C(CH3)3), 1.00 (s, 18H, C(CH3)3; 13C{1H} NMR (126 MHz, CDCl3): δ = 168.8 (s, NCOCH2), 153.6 (s, NCO2), 150.6-125.2 (Ar C), 74.7 (s, ArOCH2CON), 67.5 (s, OCH2CH(CH2Ph)), 55.0 (s, NCHCH2Ph), 37.7 (s, CH2Ph), 34.0 (s, C(CH3)3), 34.0 (s, C(CH3)3), 32.0 (s, ArCH2Ar), 32.0 (s, ArCH2Ar), 31.8 (s, C(CH3)3), 31.1 (s, C(CH3)3) ppm. Elemental analysis (%) calcd for C68H78N2O10 (1083.35): C 75.39, H 7.26, N 2.59 found: C 75.14, H, 7.20, N 5.51.
Synthesis of calixarene 8
The calixarene 3 (125 mg, 0.10 mmol) was dissolved with K2CO3 (16 mg, 1.1 equiv.) in dry MeCN (4.5 mL) and the mixture was stirred at room temperature for 4 h. A solution of oxazolidinone 5 (47 mg, 1.5 equiv.) in dry MeCN (2 mL) was added and stirring was continued for 21 h at 86°C. The solvent was removed and the resulting solid was dissolved in AcOET (15 mL). The organic layer was washed with an aqueous saturated NH4Cl solution (5 mL) water (2 x 6 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude solid was purified by column chromatography on silica gel (AcOEt/cyclohexane 1:10 to 1:5 v/v as eluent) to afford 8 as a white-yellow solid (78 mg, 53 %). [α]D20 = +6.41 (c = 1.06, CH2Cl2). 1H NMR (500 MHz, CDCl3): δ = 7.87 (dd, 2H, Ar H of COPh, 3J = 8.5 Hz, 4J = 1.0 Hz), 7.67 (dd, 2H, Ar H of COPh, 3J = 8.0 Hz, 4J = 1.0 Hz), 7.60 and 6.94 (AB system, 4H, Ar H of CH3C6H4SO2, 2J = 8.5 Hz), 7.48-7.44 (m, 2H, Ar H of COPh), 7.38 (t, 2H, Ar H of CH2Ph, 3J = 7.5 Hz), 7.32-7.27 (m, 7H, Ar H of COPh and CH2Ph), 7.19 (s, 1H, ArOH), 7.11 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.08 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 7.05 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.93 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.76 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.70 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.69 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.62 (d, 1H, Ar H of calixarene, 4J = 2.5 Hz), 6.65 (s, 1H, ArOH), 6.03 (t, 1H, CHOCOPh, 3J = 9.7 Hz), 5.28 (t, 1H, CHOCOPh, 3J = 9.7 Hz), 5.20 and 5.12 (AB system, 2H, ArOCH2CON, 2J = 18.0 Hz), 5.15 (d, 1H, CHOCH3, 3J = 3.5 Hz), 4.87-4.82 (m, 1H, NCHCH2Ph), 4.81-4.77 (m, 1H, ArOCH2CH), 4.67 (dd, 1H, CHOSO2, 3J = 10.0 Hz, 4J = 3.5 Hz), 4.44 and 3.33 (AB system, 2H, ArCH2Ar, 2J = 13.0 Hz), 4.41 and 3.33 (AB system, 2H, ArCH2Ar, 2J = 13.0 Hz), 4.38-4.28 (m, 2H, OCH2CH(CH2Ph)), 4.32 and 3.31 (AB system, 2H, ArCH2Ar, 2J = 13.0 Hz), 4.10 and 3.11 (AB system, 2H, ArCH2Ar, 2J = 13.5 Hz), 4.06-4.03 (m, 2H, ArOCH2CH), 3.78 (s, 3H, OCH3), 3.41 (A part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 3.2 Hz, CH2Ph), 2.95 (B part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 9.5 Hz, CH2Ph), 2.21 (s, 3H, CH3C6H4SO2), 1.33 (s, 9H, C(CH3)3), 1.29 (s, 9H, C(CH3)3), 0.91 (s, 9H, C(CH3)3), 0.87 (s, 9H, C(CH3)3); 13C{1H} NMR (126 MHz, CDCl3): δ = 168.4 (s, NCOCH2), 165.7 (s, OCOPh), 165.1 (s, OCOPh), 153.7 (s, NCO2), 150.7-125.1 (Ar C), 97.5 (s, CHOCH3), 77.1 (s, CHOSO2), 75.0 (s, ArOCH2CH), 74.7 (s, ArOCH2CON), 70.3 (s, CHOCOPh), 69.8 (s, CHOCOPh), 68.7 (s, ArOCH2CH), 67.6 (s, OCH2CH(CH2Ph)), 56.6 (s, OCH3), 54.9 (s, NCHCH2Ph), 37.9 (s, CH2Ph), 34.0 (s, C(CH3)3), 34.0 (s, C(CH3)3), 33.9 (s, C(CH3)3), 32.1 (s, ArCH2Ar), 31.9 (s, C(CH3)3), 31.8 (s, C(CH3)3), 31.5 (s, ArCH2Ar), 31.4 (s, ArCH2Ar), 31.3 (s, ArCH2Ar), 31.1 (s, C(CH3)3), 31.0 (s, C(CH3)3), 21.7 (s, CH3C6H4SO2) ppm. Elemental analysis (%) calcd for C84H93NO16S (1404.70): C 71.82, H 6.90, N 1.00 found: C 71.74, H, 6.90, N 0.94.
Synthesis of calixarene 10
Oil-free NaH (55 mg, 1.4 equiv.) was added to a white slurry solution of calixarene 9 (1.216 g, 1.64 mmol) in dry DMF (14 mL) at room temperature. After 35 min at the same temperature, a solution of oxazolidinone 5 (501 mg, 1.0 equiv.) in dry DMF (5 mL) was added to the previous green solution. Stirring was continued for 20 h at ambient temperature and then the mixture was diluted with AcOEt (40 mL) and water (10 mL). The organic layer was washed with water (8 x 10 mL), and brine (5 mL), dried over Na2SO4, filtered, and concentrated under a vacuum. The resulting solid was purified by chromatography on silica gel (AcOEt/cyclohexane 1:9 to 1:5 v/v as eluent) to afford starting material 9 (371 mg) and a white solid containing the desired product with some impurities detected by NMR. Recrystallization (CH2Cl2/cyclohexane 1:3 v/v) was necessary to obtain the pure product 10 as a white solid (493 mg, 31 %). [α]D20 = -16.1 (c = 1.07, CH2Cl2). 1H NMR (500 MHz, CDCl3): δ = 7.87-7.85 (m, 4H, Ar H of COPh), 7.72 (t, 2H, Ar H of COPh, 3J = 7.5 Hz), 7.65-7.62 (m, 1H, Ar H of COPh), 7.60-7.49 (m, 8H, Ar H of COPh), 7.41 (t, 2H, Ar H of CH2Ph, 3J = 7.2 Hz), 7.36-7.31 (m, 3H, Ar H of CH2Ph), 7.27-7.23 (m, 2H, Ar H of calixarene), 6.71-6.56 (m, 9H, Ar H of calixarene), 6.49 (t, 1H, Ar H of calixarene, 3J = 7.5 Hz), 5.18 and 5.11 (AB system, 2H, ArOCH2CON, 2J = 17.5 Hz), 4.88-4.83 (m, 1H, NCHCH2Ph), 4.39 (dd, 1H, OCH2CH(CH2Ph), 3J = 9.0 Hz, 4J = 8.0 Hz), 4.32 (dd, 1H, OCH2CH(CH2Ph), 3J = 9.0 Hz, 4J = 3.0 Hz), 4.05 and 3.60 (AB system, 2H, ArCH2Ar, 2J = 15.0 Hz), 4.04 and 3.59 (AB system, 2H, ArCH2Ar, 2J = 15.0 Hz), 3.63 and 3.61 (AB system, 4H, ArCH2Ar, 2J = 15.0 Hz), 3.50 (A part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 3.0 Hz, CH2Ph), 2.94 (B part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 9.5 Hz, CH2Ph); 13C{1H} NMR (126 MHz, CDCl3): δ = 167.9 (s, NCOCH2), 164.6 (s, OCOPh), 164.6 (s, OCOPh), 164.3 (s, OCOPh), 153.8 (s, NCO2), 156.3-122.8 (Ar C), 70.6 (s, ArOCH2CON), 67.6 (s, OCH2CH(CH2Ph)), 55.1 (s, NCHCH2Ph), 38.2 (s, CH2Ph), 37.3 (s, ArCH2Ar), 37.3 (s, ArCH2Ar), 37.2 (s, ArCH2Ar) ppm. Elemental analysis (%) calcd for C61H47NO10 (954.03): C 76.80, H 4.97, N 1.47 found: C 76.67, H, 4.93, N 1.60.
Synthesis of calixarene 11
A solution of KHMDS (0.5M in toluene, 0.65 mL, 2.2 equiv.) was added dropwise to a solution of calixarene 10 (146 mg, 0.15 mmol) in dry THF (2.5 mL) at -78°C. The mixture was stirred for 30 min at the same temperature before the addition of neat MeI (105 μL, 11.2 equiv.) Stirring was continued for 1 h allowing the temperature to rise to -15°C. The reaction was quenched with an aqueous saturated NH4Cl solution (0.5 mL) followed by an aqueous HCl 10% (1 mL) until pH 2. The solution was diluted with water (5 mL) and AcOEt (10 mL) and the organic layer was washed with water (2 x 4 mL), brine (4 mL), dried over Na2SO4, filtered, and concentrated under vacuum to give a white solid. The crude was purified by chromatography on silica gel (AcOEt/cyclohexane 1:2 v/v as eluent) to afford compound 11 (73 mg, 50 %) as a white solid. [α]D20 = -46.3 (c = 1.0, CHCl3). 1H NMR (500 MHz, CDCl3): δ = 7.91 (dd, 2H, Ar H of COPh, 3J = 8.5 Hz, 4J = 1.0 Hz), 7.78-7.74 (m, 4H, Ar H of COPh), 7.66 (t, 1H, Ar H of COPh, 3J = 7.2 Hz), 7.60-7.53 (m, 4H, Ar H of COPh), 7.53-7.47 (m, 3H, Ar H of COPh and calixarene), 7.40-7.34 (m, 5H, Ar H of COPh, CH2Ph and calixarene), 7.30 (t, 1H, Ar H of CH2Ph, 3J = 7.2 Hz), 7.22 (d, 2H, Ar H of CH2Ph, 3J = 7.0 Hz), 6.74-6.70 (m, 2H, Ar H of calixarene), 6.67-6.64 (m, 3H, Ar H of calixarene), 6.61-6.56 (m, 4H, Ar H of calixarene), 6.44 (t, 1H, Ar H of calixarene, 3J = 7.5 Hz), 6.35 (q, 1H, ArOCH(CH3)CON, 3J = 6.5 Hz), 4.57-4.53 (m, 1H, NCHCH2Ph), 4.56 and 3.46 (AB system, 2H, ArCH2Ar, 2J = 14.5 Hz), 4.12 (dd, 1H, OCH2CH(CH2Ph), 3J = 9.5 Hz, 4J = 2.5 Hz), 4.05-4.01 (m, 1H, OCH2CH(CH2Ph)), 4.04 and 3.55 (AB system, 2H, ArCH2Ar, 2J = 14.5 Hz), 3.63 and 3.60 (AB system, 4H, ArCH2Ar, 2J = 15.0 Hz), 3.26 (A part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 3.5 Hz, CH2Ph), 2.82 (B part of an ABX system, 1H, 2JAB = 13.5 Hz, 3JAX = 9.5 Hz, CH2Ph), 1.62 (d, 3H, ArOCH(CH3)CON, 3J = 6.5 Hz); 13C{1H} NMR (126 MHz, CDCl3): δ = 172.6 (s, NCOCH2), 164.7 (s, OCOPh), 164.4 (s, OCOPh), 164.1 (s, OCOPh), 153.3 (s, NCO2), 154.3-122.4 (Ar C), 71.5 (s, ArOCH(CH3)CON), 66.8 (s, OCH2CH(CH2Ph)), 55.2 (s, NCHCH2Ph), 38.1 (s, ArCH2Ar), 37.7 (s, CH2Ph), 37.3 (s, ArCH2Ar), 37.3 (s, ArCH2Ar), 18.8 (s, ArOCH(CH3)CON) ppm. Elemental analysis (%) calcd for C62H49NO10 (968.05): C 76.92, H 5.10, N 1.45 found: C 76.46, H, 5.05, N 1.46.
Synthesis of calixarene 12
A solution of NaBH4 (10 mg, 4.2 equiv.) in water (0.1 mL) was slowly added to a solution of calixarene 11 (63 mg, 0.07 mmol) at 0°C. The white heterogeneous mixture was stirred for 5.5 h allowing the temperature to rise to ambient temperature. The reaction was quenched with water (2 mL), an aqueous HCl 10% solution (0.2 mL) until pH 1, and then diluted with CH2Cl2 (5 mL). The organic layer was washed with water (3 mL), and brine (2 mL), dried over Na2SO4, filtered, and concentrated under a vacuum to give a white solid. Purification of the crude by chromatography on silica gel (AcOEt/cyclohexane 1:2 v/v as eluent) afforded the alcohol 12 (41 mg, 80 %) as a white solid. [α]D20 = +3.1 (c = 1.02, CH2Cl2). 1H NMR (500 MHz, CDCl3): δ = 7.88 (dd, 2H, Ar H of COPh, 3J = 9.0 Hz, 4J = 1.0 Hz), 7.86 (dd, 2H, Ar H of COPh, 3J = 9.0 Hz, 4J = 1.0 Hz), 7.80-7.77 (m, 2H, Ar H of COPh), 7.68-7.64 (m, 1H, Ar H of COPh), 7.61-7.58 (m, 4H, Ar H of COPh), 7.47-7.41 (m, 4H, Ar H of COPh), 7.33 (dd, 1H, Ar H of calixarene, 3J = 8.5 Hz, 4J = 2.5 Hz), 7.26 (dd, 1H, Ar H of calixarene, 3J = 8.5 Hz, 4J = 2.5 Hz), 6.71-6.57 (m, 9H, Ar H of calixarene), 6.45 (t, 1H, Ar H of calixarene, 3J = 7.5 Hz), 4.70-4.64 (m, 1H, ArOCH(CH3)), 3.92-3.82 (m, 2H, CH2CH(CH3)), 3.88 and 3.62 (AB system, 2H, ArCH2Ar, 2J = 14.0 Hz), 3.86 and 3.54 (AB system, 2H, ArCH2Ar, 2J = 14.0 Hz), 3.61 and 3.58 (AB system, 4H, ArCH2Ar, 2J = 14.5 Hz), 1.10 (d, 3H, ArOCH(CH3), 3J = 7.0 Hz); 13C{1H} NMR (126 MHz, CDCl3): δ = 164.6 (s, OCOPh), 164.5 (s, OCOPh), 164.1 (s, OCOPh), 153.5-122.4 (Ar C), 67.5 (s, CH2CH(CH3)), 38.2 (s, ArCH2Ar), 37.8 (s, CH2Ph), 37.3 (s, ArCH2Ar), 37.3 (s, ArCH2Ar), 15.7 (s, ArOCH(CH3)) ppm. Elemental analysis (%) calcd for C52H42O8 (794.88): C 78.57, H 5.33 found: C 78.46, H, 5.36.
Results and discussions
The Evans oxazolidinone was grafted on the lower rim of calixarene 3 bearing a glycosyl substituent. The latter compound was obtained in 33 % yield by reaction of tetra-hydroxycalix[4]arene 1 with iodopyranose 2 in MeCN. Note that the mono glycosylation was not exclusive and the formation of disubtituted calixarene 4 was isolated in 22 %. It can be mentioned that the two glycosylated calixarenes 3 and 4 substituted at the 6-C position of the pyranose fragment were not reported. We found only related calixarene compounds described in the literature by Dondoni et al. in which the pyranose units were anchored at the anomeric position [20-22]. As expected, calixarene 8 has lost its plane of symmetry as shown on its 1H spectrum by the presence of eight doublets in the range 7.11-6.62 ppm (4J = 2.5 Hz) attributed to the eight aromatic protons of the calixarene skeleton. Optionally, the calixarene 8 was obtained by grafting the Evans oxazolidinone on calixarene 1, leading to the formation of the intermediate 6, followed by substitution of the pyranoside derivative (Scheme 1).
It is well established that the Evans chiral auxiliary allows the enantioselective introduction of an electrophile such as a methyl group, specifically at the methylene position [23] as illustrated for calix[4]arene 10, which was isolated as a unique diastereoisomer. Indeed, due to the coordination of the potassium cation to the enolate intermediate, the electrophilic addition is specifically directed on the less hindered side namely at the opposite side of the benzyl substituent [24]. Finally, the Evans oxazolidinone calixarene 11 was treated with NaBH4 to obtain the corresponding alcohol derivative 12 in 80 % yield with the release of the oxazolidinone moiety [25] (Scheme 2).
Conclusion
We have reported new optically pure calixarenyl derivatives in which their lower rims were substituted with Evans oxazolidinone and/or pyranose moieties. The introduction of the latter substituents reduced the symmetry of the macrocycle which, in the case of the oxazolidinone/pyranose-disubstituted macrocycle, results in the presence of eight doublet corresponding to the eight aromatic protons. Stereocontrolled alkylation of the Evans auxiliary allowed us to introduce a methyl substituent near a phenolic position of the macrocycle. The cleavage of the chiral auxiliary has generated alcohol, which would be exploited in future work to develop chiral organic or organometallic catalysts.
We gratefully acknowledge the CNRS for a CRCT to C. B.
Author contributions
Conceptualization, C. B. and D. S.; methodology, C. B; validation, C. B. and D. S.; formal analysis, C. B.; investigation, C. B.; writing-original draft preparation, C. B. and D. S.; writing-review and editing, D. S; All authors have read and agreed to the published version of the manuscript.
- Wieser C, Dieleman CB, Matt D. Calixarene and resorcinarene ligands in transition metal chemistry. Coord. Chem. Rev. 1997; 165:93-161.
- Antipin IS, Kazakova EK, Habicher WD, Konovalov AI. Phosphorus-containing calixarenes. Russ. Chem. Rev. 1998; 67:905-922.
- Harvey PD. Wide-rim and outer-face functionalizations of calix[4] arene. Coord. Chem. Rev. 2002; 233-234:289-309.
- Homden DM, Redshaw C. The use of calixarenes in metal-based catalysis. Chem Rev. 2008 Dec;108(12):5086-130. doi: 10.1021/cr8002196. PMID: 18956902.
- Acharya A, Samanta K, Rao CP. Conjugates of calixarenes emerging as molecular entities of nanoscience. Coord. Chem. Rev. 2012; 256:2096-2125.
- Chinta JP, Ramanujam B, Rao CP. Structural aspects of the metal ion complexes of the conjugates of calix[4]arene: crystal structures and computational models. Coord. Chem. Rev. 2012; 256:2762-2794.
- Sémeril D, Matt D. Synthesis and catalytic relevance of P(III) and P(V)-functionalised calixarenes and resorcinarenes. Coord. Chem. Rev. 2014; 279:58-95.
- Rodik R, Cherenok S, Kalchenko O, Yesypenko O, Lipkowski J, Kalchenko V. Functional calixarenes for material and life science. Curr. Org. Chem. 2018; 22:2196-2218.
- Santoro O, Redshaw C. Metallocalix[n]arenes in catalysis: a 13-year update. Coord. Chem. Rev. 2021; 448:214173.
- McIldowie MJ, Mocerino M, Ogden MI. A brief review of Cn-symmetric calixarenes and resorcinarenes. Supramol. Chem. 2010; 22:13-39.
- Szumna A. Inherently chiral concave molecules--from synthesis to applications. Chem Soc Rev. 2010 Nov;39(11):4274-85. doi: 10.1039/b919527k. Epub 2010 Sep 30. PMID: 20882239.
- Arnott GE. Inherently Chiral Calixarenes: Synthesis and Applications. Chemistry. 2018 Feb 6;24(8):1744-1754. doi: 10.1002/chem.201703367. Epub 2017 Dec 11. PMID: 28809457.
- Durmaz M, Halay E, Bozkurt S. Recent applications of chiral calixarenes in asymmetric catalysis. Beilstein J Org Chem. 2018 Jun 8;14:1389-1412. doi: 10.3762/bjoc.14.117. PMID: 29977403; PMCID: PMC6009176.
- Jeunesse C, Dieleman C, Steyer S, Matt D. Calix[4]arene-derived diphosphines, diphosphinites and diphosphites as chelating ligands for transition metal ions. Encapsulation of silver(I) in a calix-crown diphosphite. J. Chem. Soc. Dalton Trans. 2001; 881-892.
- Karpus A, Yesypenko O, Boiko V, Daran JC, Voitenko Z, Kalchenko V, Manoury E. Synthesis of an enantiomerically pure inherently chiral calix[4]arene phosphonic acid and its evaluation as an organocatalyst. J. Org. Chem. 2018; 83:1146-1153.
- Elaieb F, Sémeril D, Matt D, Pfeffer M, Bouit PA, Hissler M, Gourlaouen C, Harrowfield J. Calix[4]arene-fused phospholes. Dalton Trans. 2017; 46:9833-9845.
- Elaieb F, Sémeril D, Bauder C, Matt D, Bailly C, Karmazin L. Synthesis and structure of two crowded trans-[PdCl2L2] complexes based on a chiral, calix[4]arene-fused phosphole. Polyhedron. 2018; 139:172-177.
- Evans DA, Takacs JM, McGee LR, Ennis MD, Mathre DJ, Bartroli J. Chiral enolate design. Pure Appl. Chem. 1981; 53:1109-1127.
- Evans DA, Ennis MD, Mathre DJ. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of a-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 1982; 104:1739-1740.
- Marra A, Scherrmann MC, Dondoni A, Casnati A, Minari P, Ungaro R. Sugar calixarenes: preparation of calix[4]arenes substituted at the lower and upper rims with O-glycosyl groups. Angew. Chem. Int. Ed. Engl. 1994; 33: 2479-2481.
- Dondoni A, Marra A, Scherrmann MC, Casnati A, Sansone F, Ungaro R. Synthesis and properties of O-glycosyl calix[4]arenes (calixsugars). Chem. Eur. J. 1997; 3:1774-1782.
- Dondoni A, Marra A. Calixarene and calixresorcarene glycosides: their synthesis and biological applications. Chem Rev. 2010 Sep 8;110(9):4949-77. doi: 10.1021/cr100027b. PMID: 20496911.
- Evans DA, Ennis MD, Mathre DJ. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of a-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 1982; 104:1737-1739.
- Smith TE, Richardson DP, Truran GA, Belecki K, Onishi M. Acylation, diastereoselective alkylation, and cleavage of an oxazolidinone chiral auxiliary. A multistep asymmetric synthesis experiment for advanced undergraduates. J. Chem. Ed. 2008; 85:695-697.
- Prashad M, Har D, Kim HY, Repic O. A new, economical, practical and racemization-free method for the reductive removal of 2-oxazolidinones from N-acyloxazolidinones with sodium borohydride. Tetrahedron Lett. 1998; 39:7067-7070.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
