Archives of Renal Diseases and Management
Survival and epidemiological, social, economic and clinical aspects of pediatric peritoneal dialysis: An integrative review
Mariana Rodrigues Ramos1, Maria Goretti Moreira Guimarães Penido1,2* and Sergio Veloso Brant Pinheiro2,3
2Pediatric Nephrology Unit, Department of Pediatrics, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
3Faculty of Medical Sciences of Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Cite this as
Ramos MR, Penido MGMG, Brant Pinheiro SV (2022) Survival and epidemiological, social, economic and clinical aspects of pediatric peritoneal dialysis: An integrative review. Arch Renal Dis Manag. 7(1): 016-022. DOI: 10.17352/2455-5495.000042Copyright
© 2022 Ramos MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: The kidneys are fundamental organs for survival and the progressive loss of their functions causes the loss of regulatory, excretory and endocrine functions, essentially affecting the entire balance of the organism. Chronic Kidney Disease (CKD) is considered a complex disease and a worldwide public health problem with the number of patients continuously increasing, even in the pediatric population. CKD often progresses to end-stage requiring Renal Replacement Therapy (RRT). Kidney transplantation is the treatment of choice to maximize the survival, growth, and development of pediatric patients, however, if dialysis is necessary, Peritoneal Dialysis (PD) is a high-quality and low-cost RRT modality preferred therapy for children and adolescents with End-Stage Kidney Disease (ESKD). In this scope, this study aimed to carry out an integrative review of the survival and the epidemiological, clinical, social and economic profile of children and adolescents with PD.
Methods: It is an integrative review whose data collection was carried out between January 2019 and January 2021 following the methodology suggested by the literature, using a validated data collection instrument. The following health science descriptors (DECs) from the VHL portal (virtual health library) were used: children, adolescents, chronic peritoneal dialysis, survival and epidemiology.
Results: Thirty-five studies were selected and evaluated using the Critical Appraisal Skills Program (CASP). The level of evidence of the articles was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) scale. Survival and epidemiological, social, economic and clinical aspects of pediatric PD in Brazil and worldwide have been described and reviewed.
Conclusions: The complexity and costs involved in the care of pediatric patients in RRT impact their survival. Mortality and morbidity are higher than in healthy children and life expectancy is considerably lower. ESKD is more severe in the pediatric population and interferes with general development, weight and height gain, regulation of mineral metabolism and causes definitive cardiovascular calcifications. Kidney transplantation is the treatment of choice to maximize the survival, growth and development of pediatric patients. However, if dialysis is necessary PD is the first-choice modality in this population. Unfortunately, data are scarce in the literature on its survival and its epidemiological, social, economic and clinical aspects.
Introduction
The kidneys are fundamental organs for maintaining the homeostasis of the human body. The progressive decrease in the glomerular filtration rate observed in Chronic Kidney Disease (CKD) causes the loss of regulatory, excretory and endocrine functions, essentially affecting the entire balance of the organism. CKD is considered a complex and serious disease, constituting a worldwide public health problem with the number of patients continuously increasing [1]. It affects 750 million people worldwide and its main risk factors for the adult population are diabetes mellitus, arterial hypertension, aging, obesity and family history [2]. In the pediatric population, the main diseases that cause CKD are congenital anomalies of the kidney and urinary tract (CAKUT), primary glomerular diseases, cystic diseases and hereditary or congenital diseases [3].
End-Stage Kidney Disease (ESKD) has a global incidence of between 5.5 and 9 persons per million inhabitants (ppm) [4], and a prevalence of between 23 and 65 ppm in children under 15 years of age [5,6]. In Brazil, the rate of prevalence of all patients on dialysis, regardless of age group, corresponds to 684 ppm. According to the Brazilian Census of Chronic Dialysis, published in 2021 by the Brazilian Society of Nephrology, it is estimated that the number of patients on dialysis in the country was 148.363 in the year 2021, and 1.1% were between one and 19 years old [7].
Studies with Brazilian pediatric patients on renal replacement therapy (RRT) are scarce. In 2015, Konstantyner, et al. showed that this number in Brazil reached 1,283, with a prevalence of 20 pmp cases and an incidence of 6.6 pmp. There was a huge difference between the regions of the country, both in relation to the profile of the population and the type and quality of treatment offered [8].
In the US (2018) the prevalence of ESKD in the US pediatric population was 5,410 pmp with an incidence of 841 pmp [9].
Peritoneal Dialysis (PD) is a high-quality and low-cost RRT modality. In the last decade, there has been an expansion of PD in China, Thailand and the USA. In contrast, this replacement therapy has declined in Europe and Oceania. It is known that the main barriers to increasing its use are costs, lack of trained professionals, lack of infrastructure and regional differences, especially in developing countries [2].
PD is the preferred therapy for children and adolescents and its clinical results and those related to the quality of life are comparable, if not better, than those obtained with hemodialysis (HD) [2]. In general, children have difficulty obtaining vascular access for HD. Considering that these young patients still have a way to go until kidney transplantation (Tx), it is extremely important to preserve these vascular accesses for HD and Tx.
More than 50% of pediatric patients with ESKD start RRT with PD [4]. The aim of this therapy is to prepare the patient for a timely kidney transplant [3,10]. There are advantages of PD for pediatric patients when compared to HD. The following can be mentioned: the preservation of residual renal function, fewer dietary restrictions, the possibility of regularly attending school, availability of the modality although it can be far from a pediatric dialysis center, no vascular access and social life [11].
In addition to the Glomerular Filtration Rate (GFR), other factors such as growth retardation, changes in mineral metabolism, and cardiovascular and psychosocial impairment, must be considered to define the initiation of RRT [12]. The absolute indications for dialysis are a GFR < 8 ml/min / 1.73 m2, symptomatic uremic syndrome, or refractory metabolic and/or hemodynamic changes. Relative indications are a GFR between 9-14 ml/min / 1.73 m2 associated with fatigue, malnutrition, impairment of height, weight growth and/or cognition, fluid and electrolyte imbalance, anemia, and mineral metabolism abnormalities, among others [13].
Available dialysis modalities (HD and PD) promote renal replacement by withdrawing solutes and water, restoring electrolyte and metabolic balance. Unlike HD, which is based on the passage of blood through an extracorporeal circuit through vascular access, PD makes the exchange of solutes and water between the blood in the peritoneal capillaries and the dialysis solution placed in the peritoneal cavity (dialysate) through a catheter, using the peritoneal membrane as the dialysis surface. The selection of dialysis modality depends on the type of patient and their family. However, the absolute indications for PD are weight < 5 kg, lack of vascular access and inability to receive anticoagulation. Contraindications are patients with omphalocele, gastroschisis, bladder exstrophy, dysfunctional peritoneal membrane and diaphragmatic hernia. The presence of abdominal ostomies is not an absolute contraindication [14].
This therapy requires permanent supervision and continuous adjustment, considering that the pediatric patient is constantly growing. So that these patients can be well managed and oriented, it is necessary to indicate the best RRT and to know their social and economic environment. The scarcity of data related to this specific population makes it difficult to develop actions and improvements for them. In this context, this study aimed to carry out an integrative review of the survival and the epidemiological, clinical, social and economic profile of children and adolescents with PD.
Methods
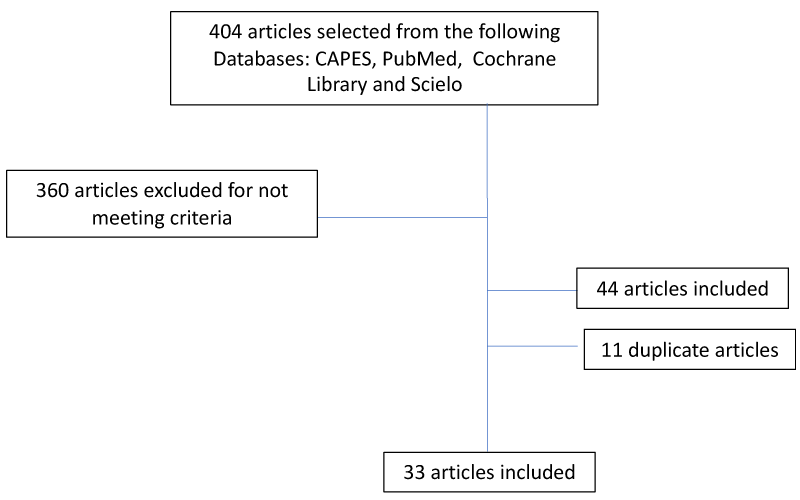
This is an integrative review whose data collection was carried out between January 2019 and January 2021 following the methodology suggested by the literature, using a validated data collection instrument. The following health science descriptors (DECs) from the VHL portal (virtual health library) were used: children, adolescents, chronic peritoneal dialysis, survival and epidemiology. Articles published in the last seven years in English, Portuguese and Spanish were accepted for this review. The research was carried out in the following CAPES, PubMed, Cochrane Library and Scielo databases. Article references were also included if they met the inclusion criteria. Initially, 404 articles were found, which were later selected, with 33 articles fulfilling the criteria for this review (Figure 1). These articles were evaluated using the Critical Appraisal Skills Program (CASP). The level of evidence of the articles was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) scale.
Results and discussion
Kidney transplantation is the treatment of choice for children who have lost renal function and over the preceding years, it has made great progress. This improvement in the survival of these patients is the result of greater surgical care, adequate immunological management, better infection control, cardiovascular care and optimization of nutritional reserves. Allied with all of this, pediatric kidney transplantation includes improved linear growth and the potential for remarkable advances in social and intellectual development. In this way, these patients should be viewed and treated differently from adult kidney transplantation, with a focus on the unique challenges specific to pediatrics.
Survival
The complexity and costs involved in caring for patients with CKD, the lack of financial and human resources, different health priorities and the lack of infrastructure have consequences for access to RRT and directly impact the survival of these patients, especially in low and middle-income countries. In the pediatric population, CKD is associated with a significant increase in morbidity and mortality compared to healthy children [5]. According to a study carried out by Kramer, et al. which analyzed the European ERA-EDTA database between 1985 and 2004, the average life expectancy of pediatric patients with CKD who started RRT under the age of 18 years is only 38 years for those receiving dialysis and 63 years for those with a kidney transplant [15]. The risk of mortality is greatest during the first year of dialysis. Late referral for follow-up with pediatric nephrology may be a risk factor for early mortality, especially for children in whom HD is chosen as the initial treatment [3].
For Chesnaye, et al. several factors are associated with mortality, such as age, gender, race, primary kidney disease, anthropometry, associated comorbidities, RRT modality, time on dialysis, presence/absence of residual renal function and GFR at baseline of RRT [16]. The same authors concluded that the main causes of death in this population are cardiovascular diseases (30%) and infections (20%). The risk of mortality in these children is multifactorial due to (1) the complexity and multiple causes of CKD, (2) the intrinsic characteristics of patients, (3) the country’s economic situation, and (4) the quality of treatment [16]. The mortality rate for all causes is up to 30 times higher for children on dialysis compared to the general pediatric population and is still considerably higher than that of the transplanted population. The overall five-year survival rate in European children starting dialysis between 2005 and 2010 was 89.5% [17]. A descriptive and retrospective Portuguese pediatric study, from January 1991 to August 2014, showed that the causes most frequently associated with mortality in children with ESKD in the first year of life were cardiac or infectious diseases. In that study, overall mortality was 30%, in agreement with the literature, and mortality in children who start PD before the age of two was four times higher than that found in older children [18].
According to Fraser, et al. the survival of children with ESKD has improved in recent decades. The authors also report that there has been a significant increase in scientific knowledge in the area, in part, due to PD working groups, such as the European Pediatric Peritoneal Dialysis Working Group and the International Pediatric Peritoneal Dialysis Registry [19]. Associated with this are also the improvement of catheters and the preventive focus on education and training on therapy with family members [20].
Epidemiological, social and economic aspects
In 2018, the GBD (Genitourinary Diseases Expert Group) estimated that the global number of individuals with CKD represents 752.7 million, with 417.0 million women and 335.7 million men [21]. In many countries, the rate of renal failure and the attention for this disease are defined by socioeconomic, cultural, and political factors that lead to disparities in the burden of disease, even in developed countries [22].
Kidney diseases have not yet been widely recognized in global politics, becoming neglected diseases. According to Crews, et al. [22], the World Health Organization (WHO) in the 2013 Global Action Plan for the Prevention and Control of Noncommunicable Diseases (NCDs) focused on cardiovascular diseases, cancer, chronic respiratory diseases and diabetes, but not kidney disease [23].
This fact is very worrying, as estimates from the Global Burden of Disease study in 2015 showed that about 1.2 million people were known to have or die from CKD, and more than 2 million people died in 2010 because they did not have access to dialysis [24]. It is possible, therefore, that kidney disease may contribute to more deaths than the four main CNCDs targeted by the current CNCD action plan [23]. According to Gouveia, et al. the Brazilian Society of Nephrology has been working with the Ministry of Health in order to insert the CKD theme in health programs. Its objectives are to support the adoption of effective surveillance, prevention, treatment and control measures for this disease [25].
The incidence of CKD and the need for RRT varies worldwide, due to genetic and environmental factors, including the availability and provision of adequate treatment. The average incidence of CKD in children under 19 years of age worldwide is 9 cases (range 4 years to 18 years) per million population (pmarp). In Europe, the incidence of RRT in children under 20 years of age was estimated at 8.9 pmarp, while in the US it was 14.2 pmarp [3]. Among children undergoing RRT, 80% of them are in North America, Europe, and Japan, where access to therapy is practically universal [3]. In 2018, Niang, et al. showed that PD is only available in 29% of low-income countries and in 68% of middle-income countries [26]. However, it is available in virtually 100% of high-income countries [26]. In Europe there was a 2% reduction in the incidence and prevalence of PD between the years 2005 and 2012. Several reasons were associated with this reduction in PD in relation to HD, including the organization of dialysis care, patient education on the modalities and lack of motivation and/or experience of nephrologists [2].
In Brazil, the overall incidence of children and adolescents with CKD on RRT was 6.6 pmarp cases in 2012 [8]. The southern region had the highest rate of new pediatric cases under this therapy: 11.0 pmarp cases, and the lowest rate was found in the region Northeast: 3.8. The proportion of male/female was 52.5% and female 47.5%, respectively, practically without a difference. The prevalence of adolescents (64.8%, 95% CI: 56.9-71.9) was significantly higher than the prevalence of children (35.2%, 95% CI: 28.1-43.1), and HD was proportionally more used than PD (74.9%, 95% CI: 66.9–81.6, vs 25.1%, 95% CI: 18.4–33.1, respectively) [8]. The 2021 Brazilian Dialysis Census showed that the unified health system (SUS) is the main source of payment for dialysis treatment in the country (82%). Almost half of the dialysis clinics are in the Southeast region and 94% were on HD and 5.8% on PD, with 30,439 (21%) on the transplant waiting list [6]. The census also showed that the predominance of automated PD remains, which corresponds to 5.0% of the total number of patients, followed by continuous ambulatory peritoneal dialysis (0.8%) [6].
A Brazilian multicenter cohort study with 75 centers that attended children on dialysis from all regions of Brazil, carried out from December 2004 to January 2011, showed that the mean age of the studied population was 10.2 ± 4.9 years. Forty-one percent were female, 52.7% had pre-dialysis follow-up, and family income was less than two Brazilian minimum wages per person in 32.6% [27].
Recently, Rezende, et al. demonstrated in a cross-sectional and observational study with 82 children and adolescents aged 0 to 17 years and 11 months, with a mean age of 9.25 years on RRT that the profile of the patients was a boy of short stature, in HD, over seven years old, not resident in the city where he was undergoing treatment, cared for by his mother, with a per capita family income of less than one minimum wage, regularly attending school, enrolled in the Basic Health Unit, and receiving RRT and transportation from the government for their treatment [28]. The authors also concluded that these findings are close to those in the literature, especially in those developing countries [28].
Clinical aspects
The pediatric population suffers more from CKD because, in addition to the disorders caused by primary kidney disease, they have their general development affected. Pediatric CKD interferes with general development, delays weight gain, favors deregulation of mineral and bone metabolism, affects height growth and causes permanent cardiovascular calcifications [26].
As aforementioned, the main causes of pediatric CKD are congenital anomalies of the kidney and urinary tract (CAKUT), primary glomerular diseases, cystic, hereditary, or congenital diseases, and secondary glomerular diseases [3]. When the diagnosis of CKD is on time, conservative treatment is indicated and carried out in specialized centers with a pediatric nephrologist, pediatric urologist, nutritionist, psychologists, nurses and social worker. This attitude can postpone the evolution to the complete loss of renal functions. However, when there is renal failure, the patient will need some therapy to replace the function of the kidneys: transplant or dialysis.
PD is the modality of the first choice for the pediatric population and especially for infants and young children. According to Woodrow, et al. and Brown, et al. many factors influence the low offer of PD in services, such as precarious infrastructure, lack of specialized staff, late referral to RRT and inadequate remuneration of professionals [29,30]. In many countries, the results of PD are superior to those of HD, in addition to the low cost and easy handling.26 The National Institute for Health and Care Excellence (NICE) Clinical Guideline 125 (2011) recommends PD as the initial dialysis treatment of choice for stage 5 CKD to children aged two years or older, people with residual renal function and adults without significantly associated comorbidities [31]. For the first time, this guideline includes recommendations related to PD in children, however, not for infants.
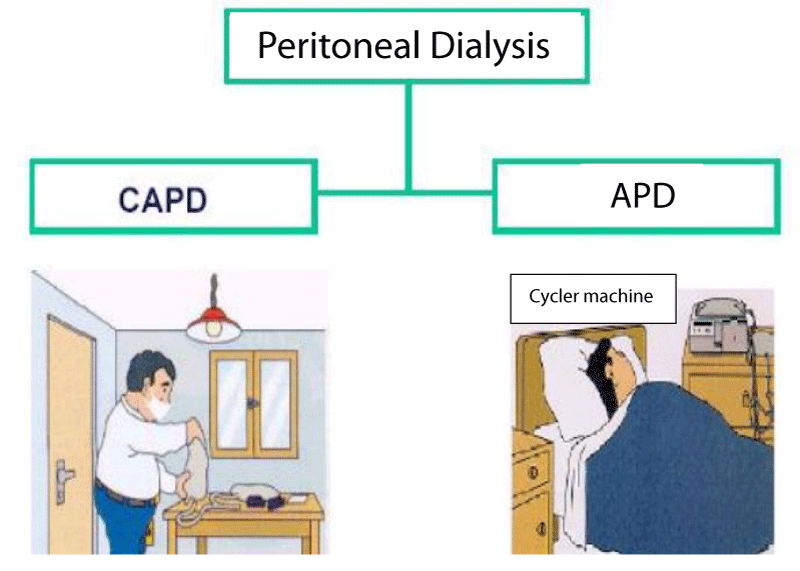
There are two types of PD: CAPD and APD. CAPD is the modality in which the abdominal cavity is always filled with the dialysis solution and the patient/relative needs to change this liquid in the cavity, usually 4 - 5 times a day or according to clinical needs. APD is the dialysis modality in which the patient performs the infusion and withdrawal of the dialysis solution through a cycler machine, normally only at night [32]. The patient and the family member/caregiver receive training given by a specialized team on the procedure, care, complications, and records. It is a modality in which the patient has some advantages: flexibility of schedule, the possibility of travel, a method performed daily continuously removing fluids and solutes and flexibility in the diet [32] (Figure 2).
PD can present complications, both infectious and non-infectious [25]. The main non-infectious complications are related to the catheter: (1) peri catheter leakage - related to the break-in (the period between catheter implantation and the onset of PD); (2) drainage failure: a) the catheter infuses and does not drain (may be related to constipation or catheter tip migration) or “omental sequestration”, b) the catheter does not infuse and does not drain (occurs due to kinks and intramural obstruction); (3) catheter translocation (resolved with the use of laxative, repositioning by guidewire or catheter exchange [32]. Other non-infectious and less common complications are hernias (especially inguinal), hydrothorax, hypervolemia, hyperglycemia, and hypertriglyceridemia. Hernias can result from increased intra-abdominal pressure and often require surgical intervention [32]. Hydrothorax is a rare complication that occurs due to the passage of dialysate into the pleural space via the lymphatics or through a congenital diaphragmatic defect. Hypervolemia is related to excessive salt and water intake, loss of residual renal function, non-adherence to dialysis prescription and excessive dialysate absorption. Hypertriglyceridemia and hyperglycemia can occur due to glucose absorption and a caloric overload with consequent weight gain. Treatment includes a hypocaloric diet, increased physical activity, and restriction of water intake, aiming to minimize the use of hypertonic bags [32]. Other catheter-related complications include flow dysfunction, catheter exit site, and tunnel infections, intra-abdominal injuries and encapsulated peritoneal sclerosis [20,32].
According to Swartz, et al. PD catheter-related infections involve the exit site, tunnel, or peritoneum itself (peritonitis) and can lead to dialysis complications, urgent hospitalizations, surgeries, unplanned or unwanted transitions to HD and even deaths [33].
Sclerosing encapsulating peritonitis is a rare complication of PD, with high morbidity and mortality. It usually results from intestinal obstruction or malnutrition and is characterized by a fibro collagenous membrane surrounding the small intestine, resulting in symptoms of intestinal obstruction [34]. The symptoms are anorexia, nausea, vomiting, weight loss, anemia, and hypoalbuminemia [32]. It may also present as hemoperitoneum and recurrent sterile peritonitis. Laparotomy is the only way to make the definitive diagnosis, but it is usually not performed due to the high risk. PD should be stopped and nutritional support provided [32]. Corticosteroids, tamoxifen and immunosuppression have been described as alternative therapies, however, they are of inconclusive benefit [35].
As mentioned above, peritonitis is the most serious complication of PD and is still the main factor for technique failure. Patients with PD with abdominal pain should always have a diagnostic suspicion of peritonitis. Abdominal pain, cloudy dialysis fluid and peritoneum with reactive signs and symptoms raise suspicion of peritonitis, which is confirmed by a cell count greater than 100 leukocytes/μL of dialysis fluid and at least 50% predominance of polymorphonuclear cells. The culture of the dialysis liquid establishes the etiological agent. Intraperitoneal treatment is preferable, however, it can be done with systemic antibiotics for 14 to 28 days, depending on the organism found and with doses corrected for renal function. Repeated infections can reduce the exchange area of the peritoneal membrane and consequently decrease the effectiveness of dialysis treatment [32].
Treatment for gram-positive and gram-negative bacteria should be started soon after collecting material for culture. The International Society of Peritoneal Dialysis (ISPD) recommends that the selection of antibiotics should be performed considering the site and the history of sensitivity to the agents [33]. A first-generation cephalosporin or vancomycin (gram-positive coverage) combined with a cephalosporin of third-generation or aminoglycoside (gram-negative coverage) can be chosen. The administration of intraperitoneal antibiotics can be done continuously (in all dialysis) or intermittently (once a day) and in this case, the contents of the peritoneal fluid bag containing the antibiotic, must remain in the cavity for at least six hours. After identifying the etiologic agent from the culture results, the antibiotic should be adjusted (Table 1) [30,36].
Treatment can be on an outpatient basis if the patient is in good general condition and without signs of systemic infection. There is an improvement in the symptoms, mainly, if there is a clear and transparent effluent. If there is no response after five days of adequate treatment, refractory peritonitis is diagnosed, which may require the removal of the catheter and transfer to HD [32,36].
If the PD is inadequate, there are signs and symptoms of uremia, cardiovascular impairment, hypervolemia, growth impediment, resistant arterial hypertension and persistent hydroelectrolytic alterations. Faced with these complications, the following should be evaluated: (1) whether the prescription is adequate for the type of transport of the peritoneal membrane, (2) whether the dialysis dose through Kt/v is adequate, (3) whether there has been a loss of residual renal function, (4) if there was the loss of the peritoneal surface, (5) the positioning of the catheter and (6) if there is adherence to the treatment [37].
A prospective Brazilian study evaluated the incidence, risk factors, microbiology, treatment and outcome of peritonitis in pediatric patients with PD. Patients < 18 years of age at their first episode of peritonitis were included. Of 491 pediatric patients, 237 had episodes of peritonitis. The overall rate of peritonitis was one episode per 27.9 patients/month or 0.43 episodes per patient/year. The authors concluded that peritonitis remains a common complication of PD in children and negative cultures and those with Pseudomonas had a negative impact on technique failure [27].
Finally, the complications of PD can be mitigated by planning in catheter implantation procedures, with care during catheter insertion and providing specialized support for the family.
Conclusions
Although PD is the preferred therapy for pediatric patients, data are scarce in the literature on its survival and its epidemiological, social, economic and clinical aspects.
PD is only available in 29% of low-income countries and 68% of middle-income countries. However, it is available in virtually 100% of high-income countries.
Kidney transplantation is the treatment of choice to maximize the survival, growth, and development of pediatric patients, however, if dialysis is necessary, the ideal therapy will be the one that guarantees better growth and biochemical balance, prevents cardiovascular deterioration, and allows achieving kidney transplantation under stable conditions [37].
The future of pediatric PD depends on research focusing on the peritoneal membrane, such as preventing damage, repairing its cells and transplanting stem cells [20].
- Schoolwerth AC, Engelgau MM, Hostetter TH. A public health action plan is needed for chronic kidney disease. Adv Chronic Kidney Dis. 2005 Oct;12(4):418-23. doi: 10.1053/j.ackd.2005.07.012. PMID: 16198282.
- Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ, Lameire N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017 Feb;13(2):90-103. doi: 10.1038/nrneph.2016.181. Epub 2016 Dec 28. PMID: 28029154.
- Rees L, Schaefer F, Schmitt CP, Shroff R, Warady BA. Chronic dialysis in children and adolescents: challenges and outcomes. Lancet Child Adolesc Health. 2017 Sep;1(1):68-77. doi: 10.1016/S2352-4642(17)30018-4. Epub 2017 Jul 20. PMID: 30169229.
- Harambat J, Bonthuis M, Groothoff JW, Schaefer F, Tizard EJ, Verrina E, van Stralen KJ, Jager KJ. Lessons learned from the ESPN/ERA-EDTA Registry. Pediatr Nephrol. 2016 Nov;31(11):2055-64. doi: 10.1007/s00467-015-3238-8. Epub 2015 Oct 24. PMID: 26498279.
- Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012 Mar;27(3):363-73. doi: 10.1007/s00467-011-1939-1. Epub 2011 Jun 29. Erratum in: Pediatr Nephrol. 2012 Mar;27(3):507. PMID: 21713524; PMCID: PMC3264851.
- Brazilian Dialysis Census of the Brazilian Society of Nephrology 2021. https://www.censo-sbn.org.br/censosAnteriores - Accessed on 25/11/2022
- Nerbass FB, Lima HN, Saldanha Thomé F, Vieira Neto OM, Lugon JR, Sesso R. Brazilian Dialysis Census 2020. Braz. J. Nephrol. Jul-Sep 2022; 44 (3) https://doi.org/10.1590/2175-8239-JBN-2021-0198 .
- Konstantyner T, Sesso R, de Camargo MF, de Santis Feltran L, Koch-Nogueira PC. Pediatric Chronic Dialysis in Brazil: Epidemiology and Regional Inequalities. PLoS One. 2015 Aug 18;10(8):e0135649. doi: 10.1371/journal.pone.0135649. PMID: 26285019; PMCID: PMC4540415.
- US Renal Data System. End stage renal disease: ESKD among children and adolescents. In: 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2020; 2:7.
- Rees L. Assessment of dialysis adequacy: beyond urea kinetic measurements. Pediatr Nephrol. 2019 Jan;34(1):61-69. doi: 10.1007/s00467-018-3914-6. Epub 2018 Mar 26. PMID: 29582148; PMCID: PMC6244854.
- Fischbach M, Warady BA. Peritoneal dialysis prescription in children: bedside principles for optimal practice. Pediatr Nephrol. 2009 Sep;24(9):1633-42; quiz 1640, 1642. doi: 10.1007/s00467-008-0979-7. Epub 2008 Sep 20. PMID: 18807074; PMCID: PMC2719743.
- Winnicki E, McCulloch CE, Mitsnefes MM, Furth SL, Warady BA, Ku E. Use of the Kidney Failure Risk Equation to Determine the Risk of Progression to End-stage Renal Disease in Children With Chronic Kidney Disease. JAMA Pediatr. 2018 Feb 1;172(2):174-180. doi: 10.1001/jamapediatrics.2017.4083. PMID: 29255845; PMCID: PMC5839269.
- Warady BA, Neu AM, Schaefer F. Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis. 2014 Jul;64(1):128-42. doi: 10.1053/j.ajkd.2014.01.430. Epub 2014 Apr 7. PMID: 24717681.
- Schaefer F, Warady BA. Peritoneal dialysis in children with end-stage renal disease. Nat Rev Nephrol. 2011 Sep 27;7(11):659-68. doi: 10.1038/nrneph.2011.135. PMID: 21947118.
- Kramer A, Stel VS, Tizard J, Verrina E, Rönnholm K, Pálsson R, Maxwell H, Jager KJ. Characteristics and survival of young adults who started renal replacement therapy during childhood. Nephrol Dial Transplant. 2009 Mar;24(3):926-33. doi: 10.1093/ndt/gfn542. Epub 2008 Oct 7. PMID: 18840894.
- Chesnaye NC, van Stralen KJ, Bonthuis M, Harambat J, Groothoff JW, Jager KJ. Survival in children requiring chronic renal replacement therapy. Pediatr Nephrol. 2018 Apr;33(4):585-594. doi: 10.1007/s00467-017-3681-9. Epub 2017 May 15. PMID: 28508132; PMCID: PMC5859702.
- Chesnaye NC, Schaefer F, Groothoff JW, Bonthuis M, Reusz G, Heaf JG, Lewis M, Maurer E, Paripović D, Zagozdzon I, van Stralen KJ, Jager KJ. Mortality risk in European children with end-stage renal disease on dialysis. Kidney Int. 2016 Jun;89(6):1355-62. doi: 10.1016/j.kint.2016.02.016. Epub 2016 Apr 13. PMID: 27165828.
- Deuchande S, Mano T, Novais C, Machado R, Stone R, Almeida M. Diálise Peritoneal nos Dois Primeiros Anos de Vida: Experiência de uma Unidade de Nefrologia e Transplantação Renal Pediátrica [Peritoneal Dialysis in the First Two Years of Life: Experience of a Nephrology and Renal Transplantation Pediatric Unit]. Acta Med Port. 2016 Sep;29(9):525-532. Portuguese. doi: 10.20344/amp.6913. Epub 2016 Sep 30. PMID: 28060690.
- Fraser N, Hussain FK, Connell R, Shenoy MU. Chronic peritoneal dialysis in children. Int J Nephrol Renovasc Dis. 2015 Oct 7;8:125-37. doi: 10.2147/IJNRD.S82419. PMID: 26504404; PMCID: PMC4603717.
- Weaver DJ Jr, Somers MJG, Martz K, Mitsnefes MM. Clinical outcomes and survival in pediatric patients initiating chronic dialysis: a report of the NAPRTCS registry. Pediatr Nephrol. 2017 Dec;32(12):2319-2330. doi: 10.1007/s00467-017-3759-4. Epub 2017 Jul 31. PMID: 28762101.
- Bikbov B, Perico N, Remuzzi G; on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron. 2018;139(4):313-318. doi: 10.1159/000489897. Epub 2018 May 23. PMID: 29791905.
- Crews DC, Bello AK, Saadi G. 2019 World Kidney Day Editorial - burden, access, and disparities in kidney disease. J Bras Nefrol. 2019 Jan-Mar;41(1):1-9. doi: 10.1590/2175-8239-JBN-2018-0224. Epub 2019 Feb 28. PMID: 31063178; PMCID: PMC6534018.
- World Health Organization - WHO. Global Action Plan for the prevention and control of non-communicable diseases. 2022; 20132020. https://www.who.int/publications/i/item/9789241506236
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016 Oct 8;388(10053):1459-1544. doi: 10.1016/S0140-6736(16)31012-1. Erratum in: Lancet. 2017 Jan 7;389(10064):e1. PMID: 27733281; PMCID: PMC5388903.
- Gouveia DSES, Bignelli AT, Hokazono SR, Danucalov I, Siemens TA, Meyer F, Santos LS, Martins ZCL, Mierzwa TC, Furquim R. Analysis of economic impact between the modality of renal replacement therapy. J Bras Nefrol. 2017 Apr-Jun;39(2):162-171. English, Portuguese. doi: 10.5935/0101-2800.20170019. Epub 2017 Apr 27. PMID: 28489179.
- Niang A, Iyengar A, Luyckx VA. Hemodialysis versus peritoneal dialysis in resource-limited settings. Curr Opin Nephrol Hypertens. 2018 Nov;27(6):463-471. doi: 10.1097/MNH.0000000000000455. PMID: 30148722.
- Ponce D, de Moraes TP, Pecoits-Filho R, Figueiredo AE, Barretti P. Peritonitis in Children on Chronic Peritoneal Dialysis: The Experience of a Large National Pediatric Cohort. Blood Purif. 2018;45(1-3):118-125. doi: 10.1159/000484344. Epub 2017 Dec 14. PMID: 29241184.
- Rezende CF, Penido MGMG, Alvarenga AS, Cherchiglia ML, Nery VL. Pediatric patients on renal replacement therapy: clinic, epidemiological, social and economic profile. Urol Nephrol Open Access J. 2021; 9(1):6‒10.
- Woodrow G, Fan SL, Reid C, Denning J, Pyrah AN. Renal Association Clinical Practice Guideline on peritoneal dialysis in adults and children. BMC Nephrol. 2017 Nov 16;18(1):333. doi: 10.1186/s12882-017-0687-2. PMID: 29145808; PMCID: PMC5691857.
- Brown EA, Bargman J, van Biesen W, Chang MY, Finkelstein FO, Hurst H, Johnson DW, Kawanishi H, Lambie M, de Moraes TP, Morelle J, Woodrow G. Length of Time on Peritoneal Dialysis and Encapsulating Peritoneal Sclerosis - Position Paper for ISPD: 2017 Update. Perit Dial Int. 2017 Jul-Aug;37(4):362-374. doi: 10.3747/pdi.2017.00018. PMID: 28676507.
- Chronic kidney disease (stage 5): peritoneal dialysis. NICE Clinical guideline [CG125]. 27 July 2011. https://www.nice.org.uk/guidance/cg125. Accessed on 2022 November 25th.
- Andreoli MCC, Totoli C. Peritoneal Dialysis. Rev Assoc Med Bras (1992). 2020 Jan 13;66Suppl 1(Suppl 1):s37-s44. doi: 10.1590/1806-9282.66.S1.37. PMID: 31939534.
- Swartz SJ, Neu A, Skversky Mason A, Richardson T, Rodean J, Lawlor J, Warady B, Somers MJG. Exit site and tunnel infections in children on chronic peritoneal dialysis: findings from the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative. Pediatr Nephrol. 2018 Jun;33(6):1029-1035. doi: 10.1007/s00467-018-3889-3. Epub 2018 Feb 26. PMID: 29480421.
- Danford CJ, Lin SC, Smith MP, Wolf JL. Encapsulating peritoneal sclerosis. World J Gastroenterol. 2018 Jul 28;24(28):3101-3111. doi: 10.3748/wjg.v24.i28.3101. PMID: 30065556; PMCID: PMC6064970.
- Cestari AT, Conti ML, Prats JA, Sato Junior H, Abensur H. Peritonite esclerosante encapsulante pós-diálise peritoneal [Sclerosing encapsulating peritonitis after peritoneal dialysis]. J Bras Nefrol. 2013 Jan-Mar;35(1):65-8. Portuguese. doi: 10.5935/01012800.20130010. PMID: 23598754.
- Li PK, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, Kanjanabuch T, Kim YL, Madero M, Malyszko J, Mehrotra R, Okpechi IG, Perl J, Piraino B, Runnegar N, Teitelbaum I, Wong JK, Yu X, Johnson DW. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022 Mar;42(2):110-153. doi: 10.1177/08968608221080586. PMID: 35264029.
- Gajardo M, Cano F. ABC de la diálisis peritoneal en pediatría [ABC of the peritoneal dialysis in pediatrics]. Rev Chil Pediatr. 2020 Apr;91(2):265-274. Spanish. doi: 10.32641/rchped.v91i2.1242. PMID: 32730548.
- Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW. ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit Dial Int. 2016 Sep 10;36(5):481-508. doi: 10.3747/pdi.2016.00078. Epub 2016 Jun 9. Erratum in: Perit Dial Int. 2018 Jul-Aug;38(4):313. PMID: 27282851; PMCID: PMC5033625.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
