International Journal of Veterinary Science and Research
Study on abattoir and clinical investigations on small ruminant reproductive disorders in Jigjiga, Ethiopia
Yikeber Walle1* and Mullusew Gashaw2
2Doctor of Veterinary Medicine, Addis Ababa University, Dawunt District Livestock Resource Development Office, North Wollo, Amhara, Ethiopia=
Cite this as
Walle Y, Gashaw M (2021) Study on abattoir and clinical investigations on small ruminant reproductive disorders in Jigjiga, Ethiopia. Int J Vet Sci Res 7(1): 005-0012 DOI: 10.17352/ijvsr.000074Across-sectional study was conducted from December, 2016 up to April, 2017 in and around Jigjiga town, Ethiopia to determine female small ruminant reproductive disorders on ewes 183 (56.8 %) and does 139 (43.2 %) at abattoir and clinic. Many factors contribute for low small ruminant productivity including; feed shortage, poor feed quality, ineffective husbandry, health constraints and poor services. Abattoir post-mortem examination revealed that 65 of the female small ruminants examined including 36.3% of ewes and 27.6% of doe’s were pregnant. A total of 26 (13%) and 12 (9.8%) female small ruminant reproductive disorders were observed in the abattoir/ postmortem and clinical investigations, respectively. Abortion/terminated pregnancy was observed in 19.4 % of the pregnant females including 2 clinical and 11 post-mortem abattoir cases. Prevalence of abortion/terminated pregnancy was relatively higher (p> 0.050) in doe’s, in younger (< 2 years) animals, and in middle gestation period. The reproductive disorders observed in non-pregnant females include clinical and post-mortem uterine infection 18 (7.1%); clinical retention of placenta 6 (2.4%) and post-mortem pyometra 1 (0.4%). Frequency of uterine infection and retained placental was similar in different species and age-groups. However, both conditions were higher in better body conditioned (p > 0.050) and recently parturient (p < 0.050) non-pregnant female small ruminants. A total of 40 specimens were taken in the clinical and abattoir investigations. This comprised of fetal fluid aspirates 16 (40 %), vaginal swabs 12 (30 %), endometrial swabs 10 (25 %) and aborted fetal skin swabs 2 (5 %). A total of 46 bacteria representing 8 different groups were isolated from the genital specimens. Short Gram Positive Bacilli (23.9%), Streptococcus species (19.6%) and S. aureus (17.4%) were the major genital bacteria isolates. Generally 20 (43.5%), 12 (26.1%) and 14 (30.4%) of the bacterial isolates were found from fetal fluid aspirate/skin swab, endometrial swabs and vaginal swabs, respectively. The majority of bacterial isolates (73.9%) came from healthy genital specimens whereas 17.4 % and 8.7 % were isolated from abortion and uterine infection cases. In conclusion, Abattoir investigation showed that ewes and does in the study area showed seasonal breeding tendency.
Introduction
Small ruminants play vital economic role as source of dietary proteins as well as skin and fur products for human use worldwide [1]. Ethiopia with its variable agro-ecological conditions is home to some 25.5 million sheep and 24.06 million goats [2]. Small ruminants account to 35 % and 14 % of the annual domestic meat and milk consumption [3] as well as to a considerable share of foreign exchange gained from export of live animals, hide and skin in Ethiopia [4]. However, the existing productivity and contribution of small ruminants is much lower than expected, given the size of national shoat populations [5].
Several factors contribute for low small ruminant productivity including; feed shortage, poor feed quality, ineffective husbandry, health constraints and poor services [6-8]. Shortage of grazing/browsing resources is a common challenge during the long dry season [9,10]. Recurrent droughts and short rains pose particularly sever feed shortages in most low-land goat producing areas of the country [11]. Various infectious and parasitic diseases have been associated with substantial small ruminant mortality (particularly young) and morbidity in Ethiopia [6]. Annual disease losses were estimated at 14-16 % in sheep and 11 -13 % in goats whereas helmenthic parasites alone imposed morbidity losses of up to 700 million birr. Prevalence of multiple trans-boundary diseases prevents Ethiopia from international markets [12].
Small ruminants are generally appreciated for higher fertility and faster reproductive rates compared to other farm animals. However, the reproductive performance of sheep and goat flocks in Ethiopia is low. In particular, pregnancy loss (abortion and still birth) associated to specific genital infections and post-natal offspring mortality represent major constraints for efficient small ruminant reproduction [13]. In Ethiopia, the magnitude of small ruminant pregnancy losses was estimated at 14% [14]. Reproductive output is further reduced by neonatal losses of up to 50% of all lambs and kids born/year [15]. Among major specific abortive genital pathogens, substantial sero-prevalence of Brucellosis [16], T. gonidi [17] and C. burnetii [18] has been reported from different parts of Ethiopia. Despite mounting evidence of potentially significant physiological and/or pathological implications in other mammalian species [19], the potential role of non-specific female genital tract microflora in small ruminant pregnancy and fertility complications is poorly understood.
Widespread practice of pregnant female slaughter has been reported as another serious shoat production constraint in poorer regions of the world [6,19]. This could be driven either by economic forces [21] or result from inefficient ante-mortem pregnancy screening systems [22,23]. The practice threatens sustainable supply of animal protein in developing countries [24-27]. Effective pregnancy detection system are lacking in most Ethiopian abattoirs [22], which opens room for substantial pregnancy wastage. In line with this, small ruminant abattoir pregnancy wastage of 72.2% has been reported in Asella leading to an estimated gross annual loss of 120,000 - 200,000 US $ [28].
The Ethiopian Somali Regional State (ESRS) is home to around 11.5 million shoats which play vital household nutrition and income generating functions particularly for vulnerable groups [29,30]. Informal Jigjiga University student and staff observations indicate common prevalence of abortion, neonatal mortality, placental retention and genital infections in Fafem zone small ruminant flocks. However, research evidence on prevalence, nature, causes and impacts of major reproductive disorders affecting ewes and doe’s in the region is scarce. Therefore, abattoir and clinic investigation was conducted in Jigjiga town of ESRS with an aim of helping to fill this gap. The specific objectives of this study were
• To estimate the prevalence of major gross reproductive disorders affecting ewes and does flocks in the study area.
• To roughly describe the aerobic bacteria associated with female genital tracts exhibiting different physiological states and/or gross pathological conditions.
Methodology
Study area
The study was conducted based in Jigjiga town of the Ethiopian Somali Regional State (ESRS). ESRS covers a land area of more than 350,000 km2 and is the largest of Ethiopia’s pastoralist regions. The population of ESRS is estimated to be around 4 million most of which represent rural, livestock dependent pastoral and agro-pastoral communities. Altitudes in the region range from 200 meters above sea level (masl) in the southern/central parts, to 1,800 masl in Jijiga Zone. The climate is mostly arid/semi-arid in lowland areas, cooler/wetter in the higher areas. Annual rainfall is 150 - 1,000mm per year. Temperatures range from 19°C (Jijiga Zone) to 40°C in the southern zones (SCUK/DPPB, 2004) [31]. Pastoralism and agro-pastoralism are the prominent livelihood systems in ESRS. The Region is estimated to have 23.6 million heads of livestock comprising of cattle (20%), sheep (33%), goat (36%), camel (10%) and equines (1%) (ESRS-MOFED, 2013/4).
Study design
Across-sectional investigation of female small ruminant reproductive disorder was conducted based in Jigjia municipality slaughter house and district veterinary clinic between December and April, 2016/17 G.C. The study considered dependent variables such as; type and frequency of reproductive problems and type and frequency of bacterial isolates. Independent variables comprised animal origin and type (species, breed, sex and age, body condition, health status, etc.), months, work setting, etc.
Sampling and sample size
Taking logistic convenience and absence of prior research in the study area, a systematic purposive, sampling procedure was used. Accordingly, busy abattoir (2) and clinical (2) working days of the week were selected for investigation. All ewes and doe’s encountered on corresponding days were included in the study. In this manner, a total of 322 animals including 183 ewes and 139 doe were sampled during abattoir (200) and clinical (122) investigations.
Study methods
Clinical and Abattoir (post-mortem) examinations were conducted to diagnose small ruminant reproductive abnormalities. Gross clinical and abattoir findings were recorded using formats prepared for the specific task. Abattoir (Post-mortem examination): Once the reproductive organs are removed at the slaughter line, presence/missing of each reproductive tract was checked. All the uteruses were collected and external appearance of both uterine horns (symmetric, one/both distend, one/both large/small) were observed and recorded. Uterine lumen was incised to detect presence/absence of pregnancy. For pregnant animals any pregnancy abnormalities observed (cloudy/bloody fetal fluid, defective fetus or other) were investigated. Fetal fluid deviations from normal (straw or yellowish) to cloudy, dark red/other were examined. Camera evidence was taken for any defect on maternal or fetal parts. Upon measurement of CRL by a ruler, fetal age was estimated using Richardson’s formula: developmental age (days) = 2.1(Y+17); Y = the length of Crown-Rump in “cm” [32]. In case of non-pregnant animals abnormalities like abnormal (substantial and off smelling) luminal fluid, color, smell, mucous membrane lesion, etc. were examined and recorded.
Clinical examination: History was taken by asking animal owners about those small ruminants with reproductive disorders .Any presence of disorder (pus/ blood, bad smelling, large volume) discharge around vulva, abortion/terminated pregnancy were observed and examined.
Sample collection: skin swab on aborted fetus, vaginal/uterine swab, and fetal fluid aspirates were aseptically collected for laboratory analysis. Samples were labeled (animal #, place, date and case) and transported to the laboratory under cold chain condition. Analyses of samples were conducted based in college of veterinary medicine – Jigjiga university microbiology laboratory.
Bacteriological study: Culture media used for isolation and purification of bacteria included: Nutrient broth, Blood agar, Nutrient agar, Mannitol salt agar and Eosin methylin blue. Media were prepared according to the manufacturer’s instructions. Inoculated media were incubated aerobically at 37 ºC for 24 hours. The combination of colonial morphology, growth conditions, bacterial morphology and reaction to gram stain were used to reach a presumptive identification. Gram and Giemsa stained smears were prepared and microscopically examined to identify abortion pathogens [13]. Bacteriological culture, Biochemical tests (catalase, oxidase, coagulase, IMVIC), isolations and identifications were conducted as per the microbiological protocols recommended for small ruminant infectious abortion pathogens [33].
Data analysis
Data collected from the surveys and laboratory investigations was entered on Microsoft (Ms) Excel spread sheet for coding, cleaning and validation. Data was analyzed using SPSS package version 20. Mean (SE) and ranges were used to summarize numerical variables and categorical were summarized by giving frequencies (n and %). Descriptive summary of study variables was presented using tables and bar graphs. Chi –square were used for analysis of variation and association. Statistical significance was determined at P < 0.05.
Results
Abattoir and clinical investigation of female small ruminant reproductive disorders was conducted in Jigjiga town between December 2016 and April 2017.
Description of study animals
Slightly more ewes (56.8 %) than doe (43.2 %) were included in this study. A higher proportion of study animals (62.1%) were found in abattoir investigation and 37.9 % represented clinical cases. Majority of study animals were aged 2 year or older 272 (84.5 %), and 50 (15.5 %) were younger than 2 years. The trend was similar in ewes and does. Proportion of younger (< 2 years) and more mature (> 2 years) animals observed in the abattoir (19 % and 81%) and clinical (9.8% and 90.2%) investigations showed significant variation (X2 = 4.85, p=0.038) (Table 1).
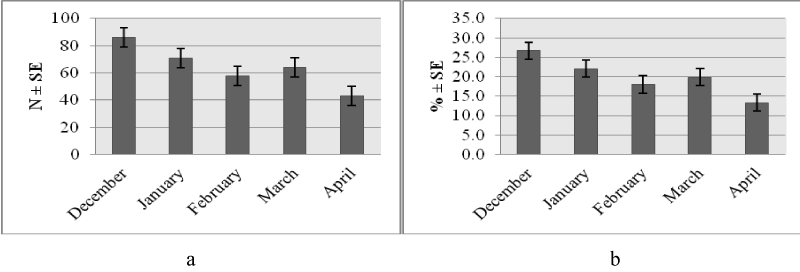
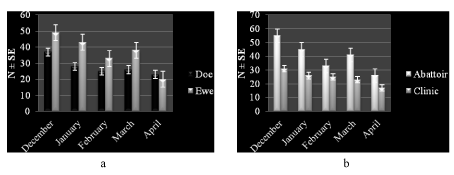
Higher number of study animals 139 (43.2 %) had a subjective body condition score of medium compared to poor 91 (28.1 %) or good 92 (28.6 %) scores. This trend was similar across species (Figure 1a) and places of work (Figure 1b). The monthly distribution (number and percentage) of study animals was variable with higher frequency observed in December and lower levels seen in April (Figure 1a,b). The trend was consistent relative to species (Figures 2a,3a) and work setting (Figures 2b,3b).
Body condition of study animals varied relative to working month (X2 = 15, p=0.059). A higher proportion of the animals investigated during April 2017 had poor body condition as compared to all other months (Table 2).
Body conditions coring system
Poor: When there is little evidence of fat deposition but some muscling in the hindquarters and the spinous processes feel sharp to the touch and are easily seen with space between them.
Moderate: When the spinous process can be felt with very firm pressure and they were round rather than sharp and there is evidence of moderate fat cover.
Good: When the Tail head had fat cover overwhole area and skin smooth but pelvis can be felt and end of horizontal process can only be felt with pressure; only slight depression in loin [34].
Physiological status
Majority of the study animals were non-pregnant 229 (71.1%) and the rest were either pregnant 67 (20.2%) or recently parturient 26 (8.1%). The proportion of non-pregnant, pregnant and recently parturient animals in ewes (70.5, 22.4 and 7.1 %) and doe (71.9, 18.7 and 9.4 %) was comparable (X2 = 1.04, p=0.589). Pregnant animals were mostly seen in abattoir study. However, both recently parturient and non-pregnant females were higher in clinic (X2 = 69, p=0.000) (Table 3).
Age group and physiological status of study animals did not show significant association (X2 = 1.46, p=0.425). Meanwhile, proportion of pregnant and parturient animals was relatively higher in young age-group (Figure 4a). Body condition of study animals showed significant association with physiological state (X2 = 11.3, p=0.013). A lower proportion of pregnant animals had poor body condition whereas medium body condition was higher in recently parturient animals (Figure 4b).
The frequency of pregnant and recently parturient female small ruminants showed significant variation in different months (X2 = 12.9, p=0.010). Accordingly, significantly higher proportions of both pregnant and recently parturient females were observed in December (Figure 5).
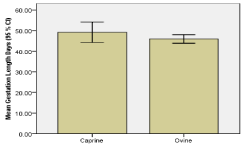
For pregnant ewes and does, gestation length/stage was estimated from fetal crown rump length (CRL) measurements. Mean CRL was 5.5 ± 0.5 cm’s and ranged from 1.5 to 25 cm’s. Accordingly, mean calculated gestation length was 47.2± 1.1 days and ranged from 38.85 to 88.2 days. Overall, majority 56 (83.6 %) of pregnant animals were in early (< 50 days) pregnancy and 11 (16.4 %) were in middle (50 – 100 days) pregnancy (Figure 5). The frequency of middle pregnancy was relatively higher (X2 = 1.4, p=0.315) in doe (23.1 %) compared to ewes (12.2 %). In line with this, mean gestation length was relatively higher in doe (Figures 5,6).
Gross reproductive disorders
A total of 26 (13%) and 12 (9.8%) female small ruminant reproductive disorders were observed in the abattoir/ postmortem and clinical investigations, respectively. Abortion/terminated pregnancy (dark bloody, off smelling fetal fluid, dead fetus and external fetal lesions) was observed in 19.4 % of the pregnant females including 2 clinical and 11 post-mortem abattoir cases. Among pregnant animals, frequency of abortion/terminated pregnancy was relatively higher (p> 0.050) in doe’s, younger (<2 years) animals, and animals in middle gestation but relatively lower (p> 0.050) in animals having good body condition (Table 4).
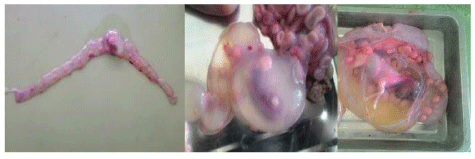
The reproductive disorders observed in non-pregnant females include uterine infection/inflammation (foul purulent exudates/discharge) on clinical and abattoir/post-mortem examination 18 (7.1%); retention of placenta 6 (2.4%) on clinical investigation; and pyometra 1 (0.4%) on abattoir/post-mortem examination. The case of pyometra was observed in young (< 2 years) ewe having good body condition (Figure 7). Frequency of uterine infection and retained placental was similar in different species and age-groups. However, both conditions were higher in better body conditioned (p > 0.050) and recently parturient (p < 0.050) non-pregnant female small ruminants (Table 5).
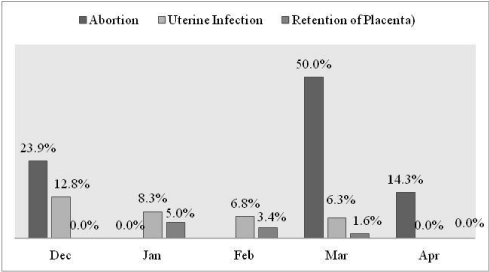
Detection frequency of abortion (X2=4.8, p=0.187) non-pregnant female reproductive disorders (X2=13.4, p=0.099) did not show significant variation across different study months (Figure 8).
Genital bacterial profile
A total of 40 specimens were taken in the clinical and abattoir investigations. This comprised of fetal fluid aspirates 16 (40 %), vaginal swabs 12 (30 %), endometrial swabs 10 (25 %) and aborted fetal skin swabs 2 (5 %). Fetal fluid aspirate was taken from 4 (25%) abortion and 12 (75%) normal pregnancy cases at post-mortem examination. Combined, swab samples were taken from abortion 4 (16.7%) and uterine infection 4 (16.7%) cases and 16 (66.7%) were from normal genital tracts.
A total of 46 bacteria representing 8 different groups were isolated from the genital specimens. Short Gram Positive Bacilli (SGPB), Streptococcus species (STRP) and S. aureus accounted for 60.9 % of the total genital bacteria isolates. Long Gram Positive Bacilli (LGPB), E. coli and other Gram Negative Bacilli (GNB) made up for 30 % of the isolates and these were followed by other Staphylococci and Micrococcus species (Figure 9,10).
Generally 20 (43.5%), 12 (26.1%) and 14 (30.4%) of the bacterial isolates were found from fetal fluid aspirate/skin swab, endometrial swabs and vaginal swabs, respectively. Relative isolation frequency of different bacteria groups varied between sample types. Other staphylococci and Micrococcus species were exclusive whereas E. coli was absent from fetal samples. GNB SGPB and STRP isolation frequency was higher in fetal samples. LGPB were more frequent in endometrial swabs the majority of which (80%) were collected from pregnant females. Meanwhile, E.coli and STRP were commonly isolated from vaginal swabs (Table 6).
The majority of bacterial isolates (73.9%) came from healthy genital specimens whereas 17.4 % and 8.7 % were isolated from abortion and uterine infection cases. S. aureus, E. coli and Micrococcus species were not isolated from abortion cases. Meanwhile, these three plus SGPB were the only bacteria isolated from uterine infection cases (Tables 6,7).
Discussion
According to results of the present study majority of the study animals (84.5 %) were aged 2 years or older. This is because as animals get older their reproduction performance and productivity decrease favoring more slaughter. Incidence of female reproductive disorders also increases in older age leading to more clinical presentation. In this study, 19 % of female small ruminants were slaughtered at immature age of less than 2 years. This has negative implications on flock expansion and financial return. Substantial proportion of the female small ruminants examined had a poor body condition. The trend was higher in April. This could reflect seasonal deficiency of local rainfall and plant growth trends which was exacerbated by the recent drought.
The monthly frequency of pregnant and parturient female small ruminants was higher in December. This could reflect seasonal tendency of breeding practices as suggested by Smith, et al. [35]. In Ethiopia, most sheep and goat conceptions occur during or after the period of short rains [15]. In fafem zone, this coincides with the period Karan (Gu) rains from beginning of August to end of September (SC-UK, 2004). Given average gestation length of around 5 months, a higher frequency of pregnancy and parturition would be expected between December and March.
Abattoir post-mortem examination revealed that 65 of the female small ruminants examined including 36.3% of ewes and 27.6% of doe’s were pregnant. This was lower than the 72.2% and 57.3% abattoir pregnancy wastage levels previously reported in Asella [28] and Jigjige [36], respectively. Likewise, higher levels of small ruminant pregnancy wastage has been reported from other parts of the world by Emady (1976) [37], 57.5% and Goossens, et al. [25] 60.0%. However, the current level was higher than the 10 % reported by Alosta, et al. [38]. The difference may be caused by variation of sample size and study periods. When applied on a nationwide basis, this rate of slaughter of the pregnant sheep and goat population represents a moderate loss in terms of production and income. One of the reasons could mostly be assumed is that owners have been sent animals for slaughtering because they were thought as barren. Gestation length estimated from CRL ranged from 38.85 to 88.2 days. Studies confirm that lamb/kid fetuses less than one month of age (< 0.5cm) were too small to measure and detect postmortem [21]. Less advanced stage of most pregnancies observed in this study could reflect effect difficulties in detecting pregnancy. Another reason for slaughtering of pregnant ewes and doe’s may be due to weak economic potential of farmers and nutritional factors such as availability of pasture and the effects of a dry season, the lengths of housing period and the stock of stored feed.
Abortion/terminated pregnancy (dark bloody, off smelling fetal fluid, dead fetus and external fetal lesions) was observed in 19.4 % of the pregnant females including 2 clinical and 11 post-mortem abattoir cases. This was comparable to previous small ruminant abortion prevalence of 14%reportwed from central Ethiopia [14]. Prevalence of abortion/terminated pregnancy was relatively higher (p> 0.050) in doe’s, in younger (< 2 years) animals, and in middle gestation period. Majority of specific small ruminant genital infections causing abortion are known to affect animals in after the first trimester. Meanwhile one of the major causes of abortion B. melitensis is primarily a caprine pathogen affecting animals in their first gestation [13].
Frequency of uterine infection and retained placental was similar in different species and age-groups. However, both conditions were higher in better body conditioned (p > 0.050) and recently parturient (p < 0.050) non-pregnant female small ruminants. Animals which give birth recently are expected to have somewhat better body condition owing to better care for late pregnant dry animals and/or better feed availability around season of birth.
Bacteria colonizing the vagina and uterus are likely to cause reproductive failure in ewes and doe other domestic ruminants. Vaginal bacteria get access into the uterus during the peripartum period leading to metritis and endometritis and subsequent reduction in the reproductive capacities of these animals [39]. It is therefore important to identify these bacteria with the view of providing remedial interventions that will restore fertility.
In the present study frequencies of bacterial isolation differ based on sample type and genital health status. Fetal fluid swab comprised relatively higher short gram positive bacilli, Streptococci, and gram negative bacilli. Uterine swabs had higher short gram positive bacilli and long gram positive bacilli while vaginal swab samples have higher streptococcus, E, coli and S. aurous. This is comparable with findings by Sokkar, et al. [40] who found E coli, Coryne bacterium pyogenes and Staphylococcus aurous as the most common uterine flora in some ewes, associated with endometritis and Mshelia, et al. [41,42] who observed Escherichia coli, Staphylococcus aureus and Klebsiella species were the most common genital bacterial isolates observed in ewes. On genital health basis, abortion cases contained higher short gram positive bacilli, streptococci and gram negative bacilli. Uterine infection comprised higher E. coli, S. aurous and gram negative bacilli while normal/apparently healthy animals had short gram positive bacilli, streptococci and S. aurous.
Conclusion and recommendations
Abattoir investigation showed that ewes and does in the study area showed seasonal breeding tendency. Pregnant slaughter and fetal wastage, particularly during middle gestation, were common findings at Jigjiga abattoir. Meanwhile, abortion in pregnant females and uterine infection and retention of placenta were observed in non-pregnant females particularly in animals that recently gave birth. Bacteria were isolated from both pregnant and non-pregnant genital tracts.
Therefore, based on these findings the following recommendations are forwarded
• Abattoir pregnancy screening system must be strengthened to avoid economic loss due to fetal wastage.
• Deeper investigation is required to identify and control the pathological agents associated with small ruminant genital infections and abortion.
• Improved flock management and vaccination systems need to be introduced to improve reproductive efficiency of small ruminant flocks.
First of all I would have a great pleasure to thank my advisor Befikadu Urga (PhD) for his voluntary guidance, follow up in working process and progress, devotion of time and for his suggestion to correct this paper.
Secondly my heartfelt goes to my family who assisted me in all aspects of my learning and achievements.
- Batavani RA, Mortaz E, Fallahian K, Dawoodi MA (2003) Study on frequency, etiology and some enzymatic activities of subclinical Ovine mastitis in Urmia, Iran. Small Ruminant Research 50: 45-50. Link: https://bit.ly/3p46sp5
- CSA (Central Statistical Agency) 2008 Federal Democratic Republic of Ethiopia, Agricultural Sample Enumeration Statistical abstract. Link: https://bit.ly/3p7cVzV
- Asfaw A, Ian BC, Bayou B (1998) The tanning industry, proceedings of control of sheep and goat skin disease to improve quality of hides and skin, 13-14 Feb. FAO, Addis Ababa, Ethiopia.
- Mahmud M (2000) Raw hides and skin improvement in Ethiopia, status and challenges. In: proceedings of the opportunities and Challenges of Goat Production in East Africa.
- Berhanu GD, Hoekstra G, Azege T (2006) Improving the Competitiveness of Agricultural Input Markets in Ethiopia: Experiences since 1991. Paper presented at the Symposium on Seed-fertilizer Technology, Cereal productivity and Pro-Poor Growth in Africa: time for New thinking 26th Triennial Conference of the International Association of Agricultural Economics (IAAE). Link: https://bit.ly/3rEsBMB
- Sanusi M, Abubakar M, Luka B (2006) Incidence of Foetal Wastages in Ruminant Animals Slaughtered at Bauchi and Jos Abattoirs. In; the Proceedings of the 2006 Annual Nigerian Society for Animal Production Conference 102-106.
- Tsedeke K (2007) Production and marketing of sheep and goats in Alaba, SNNPR. Msc thesis.Hawassa, Ethiopia. Link: https://bit.ly/3tLRR5c
- Getahun L (2008) Productive and Economic performance of Small Ruminant production in production system of the Highlands of Ethiopia. Ph.D.dissertation. University of Hohenheim, Stuttgart-Hoheinheim, Germany. Link: https://bit.ly/3tIxIwW
- Urgessa D, Duguma B, Demeke S, Tolamariam T (2012) Sheep and Goat Production Systems in Ilu Abba Bora Zone of Oromia Regional State, Ethiopia: Feeding and Management Strategies. Global Veterinaria 9: 421-429. Link: https://bit.ly/3tGSCg1
- Alemayehu M (1998) The Borana and the 1991-92 Drought: A Rangeland and Livestock Resource Study. Institute of Sustainable Development, Addis Ababa, Ethiopia 102. Link: https://bit.ly/3a4aOZa
- Shenkute B (2009) Production and marketing systems of small ruminants in Goma district of Jimma zone, western Ethiopia. Link: https://bit.ly/3rDJdUz
- Zewdie S, Lidetu D (2008) Sheep and goat flock health. In sheep and goat production for handbook Ethiopia, Alemu yami and RC Merkel (eds) Ethiopia sheep and goat productivity improving programme 213-272. Link: https://bit.ly/3pdhU24
- Parkinson TJ (2009) Specific infectious diseases causing infertility and subfertility in cattle. In: Noakes, D.E., Parkinson, T.J. and England G.C.W. Eds. Veterinary Reproduction and Obstetrics 9th Edn. Saunders Elsevier, UK
- Mugerwa M, Tekeleye B (1988) The Reproductive performance of Ethiopian highland sheep. Animal Reproduction Science 17: 95-102. Link: https://bit.ly/3jxx1SG
- Hirpa A, Abebe G (2008) Economic Importance of Sheep and Goats. In: Sheep and Goat Production Handbook for Ethiopia, Addis Ababa 1-4.
- Kasahun A, Regassa F, Robertson LJ, Martin AD, Skierve E (2012) Reproductive disorders in relation to Neospora caninum, Brucella spp and Bovine Viral Diarrhea virus serostatus in breeding and dairy farms of centeral and southern Ethiopia. Epidemiol Infect 1-9. Link: https://bit.ly/3tJEVgw
- Bekele T, Kasali OB (1989) Toxoplasmosis in sheep, goats and cattle in central Ethiopia. Vet Res Commun 13: 371-375. Link: https://bit.ly/3cXR5MP
- Balako G, Rebuma F, Lawrence Y, Teshale S, Tadele T, et al. (2013) Seroprevalence of Brucella and Q-fever in Southeast Ethiopia pastoral livestock. J Vet Sci Med Diagn 2: 10. Link: https://bit.ly/2Z2cI6h
- Hirh DC (1990) The genital tract of a microbial habitat. Review of Veterinary Microbiology 243-244.
- Tizhe MA, Kubkomawa HI, Waba YE, Addass PA (2010) Foetal Wastage In Ruminants And Sustainable Livestock Industry In Nigeria, Journal of Agriculture and Veterinary Sciences 2: 72-80.
- Kheradmand A, Batavani RA, Babaei H (2006) Study on the frequency of pregnant ewes slaughtered in Khorram Abad abattoir. Iranian Journal of Veterinary Research 7: 55-58. Link: https://bit.ly/3rCIDqf
- Abassa KP (1995) Prenatal wastage in Reproductive losses in small ruminants in Sub-Saharan Africa. A review on International Development Research Centre (IDRC), Ottawa, Canada, International Livestock Centre for Africa (ILCA), Addis Ababa, Ethiopia.
- Grandin T (2004) Restaurant Animal Welfare Audits of Stunning and Handling in federally inspected U.S. and Canadian beef, veal, pork, lamb, and poultry slaughter plants. Link: https://bit.ly/3jClgdq
- Singleton GH, Dobson H (1995) A survey of the reasons for culling pregnant cows. Vet Rec 136: 162-165. Link: https://bit.ly/3aOvpzP
- Goossens B, Osaer S, Kora S, Chandler KJ, Petrie L, et al. (1998) Abattoir survey of sheep and goats in the Gambia. Vet Rec 142: 277-281. Link: https://bit.ly/3a3BDgc
- Lawton DEB, Mead FM, Baldwin RR (2000) Farmer record of pregnancy status pre-slaughter compared with actual pregnancy status post-slaughter and prevalence of gross genital tract abnormalities in New Zealand dairy cows. N Z Vet J 48: 160-165. Link: https://bit.ly/3jzUPVW
- Ngbede EO, Raji MA, Kwanashie CN, Okolocha EC, Gugong VT, et al. (2012) Serological prevalence of Leptospirosis in cattle slaughtered in the Zango abattoir in Zaria, Kaduna State, Nigeria. Veterinaria Italiana 48: 179-184. Link: https://bit.ly/2Z0C2cK
- Chuko T, Wakayo BU, Mohammed O, Dawo TF (2015) Small Ruminant Fetal Wastage at Asella Abattoir in Central Ethiopia; Reflection on Potential Country Level Implications. International Journal of Livestock Research 5: 55-61. Link: https://bit.ly/3d5XFAC
- Save the Children - UK (1997) Stakeholder workshops on animal health services (Summarised English Version), 12th-15th August 1997. Save the Children (UK)-Regional Bureau of Agriculture Veterinary Services Support Project.
- Panthuliano S, Wekesa M (2008) Improving drought response in pastoral areas of Ethiopia. Somali and Afar Regions and Borena Zone of Oromiya region. Link: https://bit.ly/3a1YMQ6
- Somali Regional State, Ethiopia: Livelihood Zone (LZ) Map: Source: Field Surveys conducted by SCUK/DPPB. Food Security Monitoring and Early Warning Programme – Revised 2004
- Noakes DE, Parkinson TJ, England GCW (2001) Arthur’s Veterinary Reproduction and Obstetrics. 8th Edn. London, W. B. Saunders Co. 28: 557-570. Link: https://bit.ly/2OqsmGD
- Quinn PJ, Carter ME, Markey B, Carter GR (1999) Clinical Veterinary Microbiology. Mosby, New York USA.
- Edmonson A, Lean I, Weaver L, Farver T, Webster G (1989) A Body Condition Scoring Chart for Holstein Dairy Cows. J Dairy Sci 72: 68-78. Link: https://bit.ly/3aR0d2X
- Smith KC, Parkinson TJ, Long SE (1999) Abattoir survey of acquired reproductive abnormalities in the ewes. Vet Rec 144: 491-496. Link: https://bit.ly/3tCJI34
- Tariku T (2016) Study on lesion and bacteriological profile of small ruminants genital tracts, DVM thesis, Jigjiga University.
- Emady M (1976) Reproduction of the ewe and female goat in the province of Fars, Iran. Vet Rec 99: 208-209. Link: https://bit.ly/3cUnqUF
- Alosta RA, Vaughan L, Collins JD (1998) An abattoir survey of ovine reproductive tracts in Ireland. Theriogenology 50: 457-464. Link: https://bit.ly/3tHPX5K
- Levinson WE, Jawetz E (1994) Medical Microbiology and Immunology. Third edition. Prentice- Hall Int. Inc., Englewood Cliffs, New Jersey-USA 20-23.
- Sokkar SM, Kubba Al-Augaidy F (1980) Studies on natural and experimental endometritis. in ewes. Vet Pathol 17: 693-698. Link: https://bit.ly/3paDNPk
- Mshelia GD, Bilal VT, Maina VA, Okon K, Mamza SA, et al. (2014) Microbiological studies on genital infections in slaughtered ewes from tropical arid zone of Nigeria. Sokoto Journal of Veterinary Sciences 12: 18-22. Link: https://bit.ly/2N7sxpD
- Fayemi PO, Muchenjie V (2013) Maternal slaughter at abattoirs: history, causes, cases and the meat industry. Springer Plus 2: 125. Link: https://bit.ly/2NdqGQg
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
