International Journal of Aquaculture and Fishery Sciences
Secondary metabolites production combined with lead bioremediation by Halamphora sp. marine diatom microalgae and their physiological response
Ines Dahmen-Ben Moussa1,2*, Saoussan Boukhriss2, Khaled Athmouni2 and Habib Ayadi2
2Department of life sciences, Laboratory of Biodiversity and Aquatic Ecosystems, Ecology and planktonology, University of Sfax Tunisia, Faculty of Sciences, Unit UR 11 ES 72/Street of Soukra Km 3.5, B.P. 1171, CP 3000 Sfax, Tunisia
Cite this as
Dahmen-Ben Moussa I, Boukhriss S, Athmouni K, Ayadi H (2022) Secondary metabolites production combined with lead bioremediation by Halamphora sp. marine diatom microalgae and their physiological response. Int J Aquac Fish Sci 8(2): 025-036. DOI: 10.17352/2455-8400.000075Copyright License
© 2022 Dahmen-Ben Moussa I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.This study was designed to investigate the physiological and biochemical response of the diatom microalgae Halamphora sp. (SB1 MK575516.1) to the toxicity of lead (Pb) as well as its ability as phytoremediation. Four different concentrations of Pb (50, 100, 150, and 200 mg L-1) were applied for 10 days. Fatty acid profile, mineral composition, secondary metabolite contents, and physiological responses have been determined in Halamphora biomass. We found that this metal was mainly removed by bio adsorption on cell surfaces and that Halamphora sp. could acclimatize upon long-term exposure to Pb stress. A decrease in the cell’s number and size, polyunsaturated fatty acids as well as mineral content in Halamphora sp were observed under Pb stress. However, an increase in polyphenol, flavonoid, and carotenoid contents has been recorded at 100 mg Pb L-1, with stimulation of the antioxidant capacity as measured by DPPH and ABTS radical scavenging activities. An increase in MDA, proline, and H2O2 levels were also observed. On the other hand, the deleterious effect of Pb resulting from the cellular oxidative state can be alleviated by the enzymatic system such as Superoxide dismutase (SOD), Glutathione peroxidase (GPx), and catalase (CAT). The present study indicates the ability of Halamphora sp. to remove heavy metals from the aquatic environment and produce antioxidant biomolecules.
Abbreviations
ABTS: 2,2’-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid); Car: Carotenoids; CAT: Catalase; CE: Catechin Equivalent: Chl: Chlorophyll; DPPH; Diphenylpicrylhydrazyl: DW: Dry Weight; GAE: Acid Equivalent; GPx: Glutathione Peroxidase; MDA: Malondialdehyd; MUFA: Monounsaturated Fatty Acids; Pb: Lead; PUFA: Polyunsaturated Fatty Acids; ROS: Reactive Oxygen Species; SFA: Saturated Fatty Acids; SOD: Superoxide Dismutase
Introduction
Among the most common heavy metals, lead (Pb) is involved in acute and/or chronic effects on organisms [1]. The concentration of this toxic metal occurs at ultra-trace concentrations in environments through natural processes such as weathering. Nevertheless, lead pollution has risen dangerously due to human activities [2]. Lead from anthropogenic sources can lead to concentrations above 10,000 ppm and may induce severe harm to aquatic life even at a low concentration of 10ppm [3]. This heavy metal can cause damage to the nervous system, kidneys, and disturbance of vitamin D metabolism, especially in children when the blood lead levels exceed 25µg/dL [4].
Microorganisms play an important role in the removal of various chemicals and physical pollutants from the environment. Therefore, bioremediation techniques are considered safe and sustainable methods to remove toxic substances from contaminated water. The bioremediation process uses different microbes such as bacteria, algae, fungi, and yeast to treat oil spills, contaminated soil, and contaminated water. Previous studies have well been reported on the approach of microalgae for the removal of various contaminants. In algae-based bioremediation, algae absorb nutrients like carbon, phosphate, and heavy metals from wastewater and produce new biomass, which is useful in the generation of bioenergy [5-8].
The algae have many characteristics that make them ideal candidates for the elimination and concentration of heavy metals, which include high tolerance to heavy metals, autotrophic and heterotrophic growth capacity, and genetic manipulation as well as the development of biofuels [9,10]. Several studies have shown that microalgae species have been used as biomarkers and bioindicators of water pollution and in determining the impact of toxic metals [11]. Recently, Al-Homaidan, Al-Ghanayem [12] demonstrated that the green algae Enteromorpha and/or Cladophora were used to estimate heavy metal concentrations in many parts of the world. In this regard, some species have already been used for the removal of heavy metals from contaminated water [13]. In recent years, the use of microalgae in the detoxification of wastewater has aroused great attention because of their key role in the fixation of carbon dioxide. In addition, Amphora biomass produced has great potential as feedstock for biofuel production [14-17]. These bioremediation capabilities of microalgae are useful for environmental sustainability [11]. In addition, recent investigations regarding microalgae were carried out to remediate domestic and industrial wastewater [7,8,18]. To our knowledge, literature pertaining to the remediation of toxic heavy metals by the diatom microalgae Halamphora sp. is relatively scarce. Moreover, the present survey for the first time has been conducted to remediate hypersaline waters.
The aim of this present study was to assess the phycoremediation potential of this wild microalga, Halamphora sp., by determining the bioaccumulation capacity and toxicity of lead to this microalga. The effect of lead on the biochemical and physiological responses of this alga was investigated.
Materials and methods
Strain and cultural conditions
Halamphora sp. SB1 MK575516.1 was isolated via micromanipulation and serial dilutions from the Sfax Solar Saltern pond with an average salinity of 107p.s.u. Isolated and purified microalgae were inoculated in 2L Erlenmeyer flasks containing 1000mL autoclaved F/2 culture medium, which was enriched with sodium silicate (Na2SiO3). The cultures were kept at 25°C under a 16h light/8h dark cycle provided by cool white fluorescence lights at 6000Lux irradiance and were fed by a CO2 bubbling rate of 0.1vvm. The culture was then transferred to a 15L glass air bubble photobioreactor with a working volume of 10L.
Heavy metal exposure
Exponentially grown cells used for different treatments were harvested and cultured in 2000 mL flasks with 1000mL of F/2 medium with final Pb concentrations of 0, 50, 100, 150 and 200mgL-1. Pb solutions were prepared by dissolving analytical-grade PbCl2 in sterilized F/2 medium at the desired concentrations just before the experiment. During the whole experiment, all cultures were kept under the same previous conditions.
Biomass was separated from the culture medium by sedimentation and lyophilized at -70°C.
Determination of fixed lead
Fixed lead was evaluated using a modified method of Stauber and Florence [19] described by Folgar and Torres [20].
Total lead removed: total lead removed in cells was measured by filtration of 25mL aliquots from each culture through two superposed 1.2-µm Millipore filters. The lead concentrations were determined in both filters and the lower filter was used as blank.
Lead removed intracellularly: Intracellular lead was determined in the following way.
The aliquots of 25mL of each culture were centrifuged at 4500rpm for 15min and the pellet was resuspended for 20min in 25mL of 0.02M EDTA dissolved in an F/2 medium. EDTA’s function is to eliminate the lead adsorbed onto the cell surface, thus allowing only the intracellular lead fraction to be measured. Then, the culture was centrifuged for 15min at 4500rpm and the pellet was washed twice with seawater and centrifuged again at 4500rpm for 15min.
Bioadsorbed lead: The quantification of adsorbed lead onto the cell surface was calculated by subtracting the intracellular lead concentration from the total lead removed (adsorbed lead = total lead - intracellular lead).
Measurement of lead level: Each one of the filters from the determination of total lead removed and the pellets from the determination of lead removed intracellularly were separately digested in a mixture of 5mL of 1M HNO3 and 0.2mL of HCl (37 %). Digested samples were brought to a final volume of 25mL with distilled water. Pb presenting the samples was measured by atomic absorbance spectroscopy (205 AAS; USA).
Growth
The cell density in the cultures was measured using a hemocytometer. Ten µL of Lugol’s solution was added to 50µL Halamphora culture to immobilize and stain the cells. Growth inhibition was calculated according to the following equation:
Where % Ir is the percent inhibition in average specific growth rate, µc is the average specific growth rate (µ) in the control group, and µr is the average specific growth rate for the treatment replicate.
Mineral composition analysis
Analyses of sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), Copper (Cu2+), and Zinc (Zn2+) minerals content in Halamphora sp. were performed using the inductively coupled plasma optical emission spectrophotometer (ICP-OES) (Model4300 DV, Perkin Elmer, Shelton, CT, USA).
Determination of pigments contents
Carotenoid content in the algal cultures was determined spectrophotometrically according to the method described by Lichtenthaler and Buschmann [21].
Extraction of polyphenols was executed as reported by Gouvinhas, Santos [22], with minor modifications. Briefly, samples (0.1g) were mixed with 1.5mL of methanol/distilled water (80:20 v/v), vortexed, and incubated in the dark for 20min under agitation at 40°C. Subsequently, the mixture was centrifuged for 2min at 6000rpm. The supernatants were stored at 4°C.
Total phenol content in Halamphora extracts of different groups was estimated by the Folin–Ciocalteu method [23]. The absorbance of the mixture was recorded at 760nm after incubation for 30min in the dark. Gallic acid was used as a reference standard, and the content of total phenolics was expressed as milligram gallic acid equivalent (mg GAE g-1 DW).
The total flavonoids content of microalga extracts was determined according to the modified method of [24]. Absorbance was measured at 510nm against the blank. Total flavonoid content was expressed as milligram catechin equivalent (mg CE g-1 DW).
Antioxidant activity
Diphenylpicrylhydrazyl (DPPH) free radical scavenging assay: The measurement of the DPPH radical scavenging activity was carried out as described by Blois [25], with a slight modification. The diluted phenolic extracts (0.06–1mg mL−1) were reacted with 1mL of DPPH radical solution 0.1mM in ethanol, and 450μL of 50mM Tris–HCl buffer (pH 7.4) was added. The solution was incubated for 30min at room temperature, and the reduction of DPPH free radicals was measured by reading the absorbance at 517nm. The control solution was prepared by mixing ethanol and DPPH radical solution. The scavenging activity percentage was determined according to the following equation:
Where Acontrol is the absorbance of ethanolic DPPH solution and Atest is the absorbance of samples. The antioxidant activity of each extract was expressed as IC50 values, defined as the concentration of the extract required to inhibit 50% of radical.
ABTS radical scavenging activity
This assay, based on the ability of substances to scavenge 2, 2’- azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS·+) radical cation, was performed according to Katalinic, Modun [26] with minor modifications. Briefly, the ABTS radical cation (ABTS·+) was prepared by mixing 7mM ABTS stock solution with 2.45mM potassium persulfate and incubating the mixture for 12–16h before use. The ABTS·+ solution was diluted with phosphate-buffered saline (PBS; pH 7.4) until absorbance of 0.70 ± 0.02 at 734nm. The photometric assay was conducted on 2 mL of diluted ABTS·+ solution and 100μL of tested extract, or Trolox standard. The reaction mixture was incubated for 30min at 30°C. The decrease in absorbance was recorded at 734nm. All measurements were performed in triplicate. ABTS radical scavenging activity was expressed as µmol Trolox equivalent (µM TEq g-1 DW).
Fatty acids profiling
Fatty Acid Methyl Esters (FAME) were prepared from the lipid extract by transesterification using a direct transmethylation method [15]. Then, the FAMEs were extracted with hexane and analyzed quantitatively by GC-MS (HP 5975B inert MSD) equipped with FID and HP-5MS capillary column (30m length; 0.25mm i.d.; 0.25mm film thickness). The carrier gas (He) was used at a 1mLmin-1 flow rate. The oven temperature program was 1 min at 100°C ramped from 100 to 250°C at 4°Cmin-1 and 10min at 260°C. The split ratio was 1:100. Components identification was assigned by matching their mass spectra with Wiley and NIST library data.
Antioxidant enzymes biomarkers
The algal pellets were suspended in phosphate buffer solution (PBS, pH 7.4). Cells were physically disrupted by three cycles of freezing (-80°C). Broken cells were centrifuged at 10,000rpm for 5 min at 4°C, and the supernatants were used for the quantification of antioxidant status.
Catalase (CAT) activity was determined spectrophotometrically according to Radi, and Torrens [27]. The absorbance was measured at 240 nm and the results were expressed as µmol H2O2 min-1 mg-1 of protein.
Glutathione Peroxidase (GPx) activity was estimated according to Vega-López, and Ayala-López [28]. The absorbance was measured at 340 nm and the results were expressed as µmol GSH min-1mg-1 of protein.
Superoxide Dismutase (SOD) activity was determined according to the method of Cano-Europa, López-Galindo [29] by measuring its ability to inhibit the photoreduction of nitrobluetetrazolium (NBT). SOD activity was expressed as units (U) mg-1 protein.
Determination of oxidative stress biomarkers
Proline was extracted using 3% sulphosalicylic acid and estimated using L-proline as a standard as described by Bates, and Waldren [30].
For the determination of H2O2 content in microalga, cells were centrifuged at 10,000rpm for 10 min and the pellet was homogenized in 0.1% w/v TCA solution. The homogenate was centrifuged at 10,000rpm for 10min. 0.5ml supernatant was mixed with 0.5mL of 10mM phosphate buffer (pH 7.0) and 1mL of 1MKI. Absorbance was recorded at 390nm. H2O2 content was expressed as µmoL H2O2 g-1 fresh weight [31].
For the determination of lipid peroxidation, microalgal cells were harvested by centrifugation at 10,000rpm for 10min. The cell pellet was homogenized in 2mL of 80:20 (v/v) ethanol: water. The homogenate was centrifuged at 10,000rpm for 10min. 1mL supernatant was mixed with 1 mL of 0.65% TBA prepared in 20% TCA solution containing 0.01% BHT and heated at 95°C for 25min. After cooling at room temperature, the mixture was centrifuged at 10,000 rpm for 10min. Absorbances of supernatants were read at 450nm, 532nm, and 600nm. MDA content was calculated using the following equation [32]:
MDA (µmol gStatistical analysis fresh weight) = [6.45 × (OD532 – OD600)] – [0.56 × OD450]/ fresh weight (g) (5).
Statistical analysis
Statistics were performed on SPSS software (version 20). Data were expressed as the mean ± standard deviation and analyzed by one-way ANOVA. The significance level was determined (p<0.05) and a significant difference was separated using Duncan’s Multiple Range Test (DMRT).
Results
Phycoremediation potential
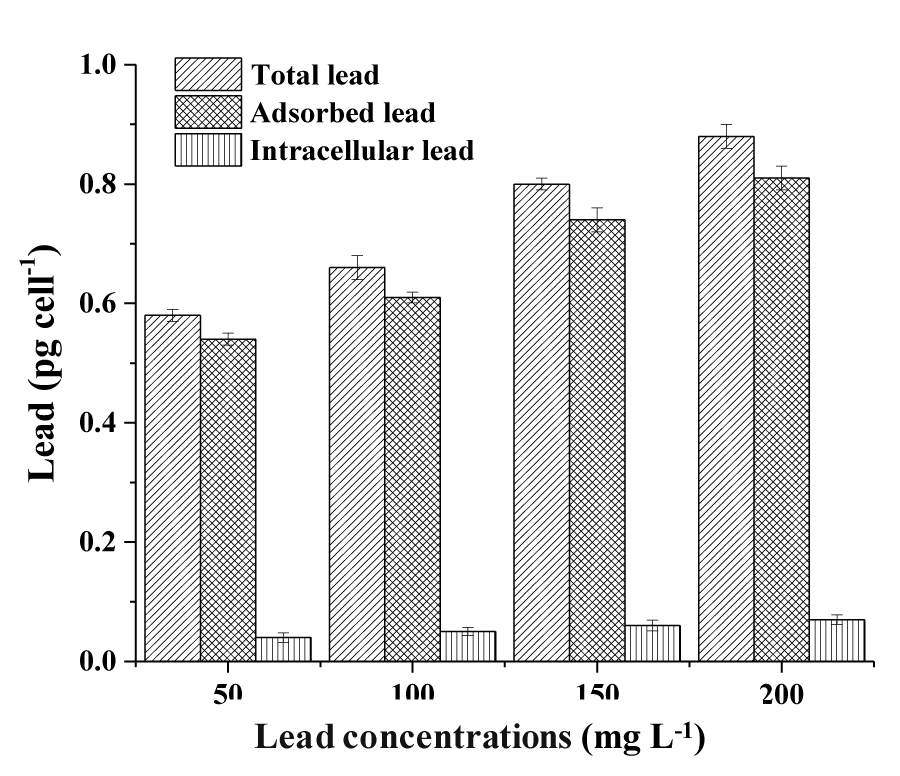
The content of total lead, adsorbed lead, and intracellular lead removed by the Halamphora sp. cells were observed in the least different lead concentrations (50, 100, 150 and 200mg L-1). The total removed lead by the cells increased proportionally as much as the concentration of Pb in the culture media increased (Figure 1). The maximum removal of Pb occurred for a concentration of 100mg L-1 recording 27.64%. However, the Pb removal efficiency decreased by increasing the initial concentration of Pb to 150 and 200mg L-1 and reached 24.65 and 20.65%, respectively.
The determination of the Pb bio adsorbed to the cells surface of Halamphora sp. is given in Figure 1. For the lowest Pb concentration used (50mgL-1), bio adsorbed Pb represents 99.21% of the total Pb removed. Also, for the highest Pb concentration (200mgL-1), the bio adsorbed fraction represents 97.36% of the total Pb removed.
Figure 1 also represents the quantity of Lead removed intracellularly by the cells of Halamphora sp. Pb ion removal by Halamphora sp. biomass depends largely on the initial concentration of the metal ions in the solution phase. For the lower concentration of 50mg PbL-1, Halamphora sp. was capable to remove 0.04±0.01pg celL-1 which corresponds to 6.89% of the total removed Pb. The metal accumulation into Halamphora sp. cell increased at the higher concentration (200mgL-1) and reached 0.07±0.02 pg celL-1, which represents 7.95% of the total removed Lead.
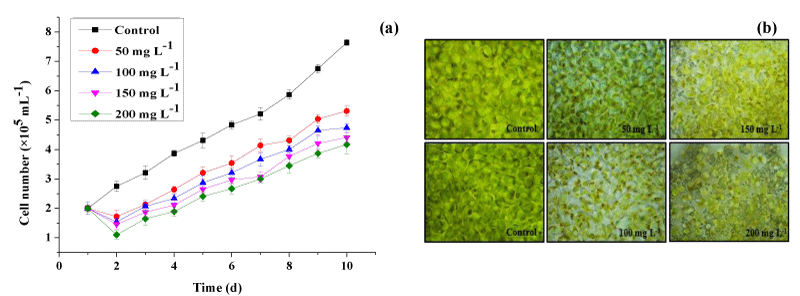
Effects of lead on Halamphora growth
The impact of Pb concentrations on Halamphora sp. growth was studied during 10 days of culture. The result revealed that the cell growth was significantly influenced by lead after 2 days of metal exposure (Figure 2a). In fact, Figure 2a shows that the number of cells of Halamphora sp. decreased proportionally with increasing the concentration of Pb in the microalgae medium. Cultures treated with the highest concentration of Pb (200mg L-1) showed the most marked phytotoxic effect of heavy metals on the growth of Halamphora sp. until the last day of culture. In this condition, the number of cells decreased by 49% as compared to the control group. This might be due to the degradation of indole acetic acid, the hormone which stimulates growth and multiplication, induced by the presence of Pb in the culture medium. At a Pb concentration of 50mgL-1, only a slight inhibition of algal growth occurred.
The toxicity symptoms were also observed in heavy metal-treated cultures of Halamphora sp. (Figure 2b). The morphological structure included partial disorganization of the cell wall, increasing in crystalline inclusions, and a reduction in cellular size at different lead concentrations.
Effects of lead on the mineral composition of Halamphora sp.
The changes in the concentration of mineral composition in the control and experimental group are presented in Table 1. Results of the ANOVA test showed that the mineral element contents of different groups treated with lead were significantly lower (p< 0.05) than that of the control group. Lower mineral contents were found in cells grown under different concentrations of Pb which could be a consequence of the growth inhibition during the response to metal stress.
Effects of lead exposure on Halamphora sp. fatty acid profiles
Fatty acids composition of Halamphora sp., exposed to Pb stress, was analyzed by extraction and transesterification of the lipids and analysis of the fatty acid methyl ester by GC-MS. Halamphora sp. accumulated fatty acids in the range of C14 to C20. Under control conditions, the major fatty acid components were C14:0, C16:0, C16:2, and C16:3 (Table 2). In addition, the levels of saturated fatty acids (SFA) were significantly lower, while the levels of polyunsaturated fatty acids (PUFA), including C16:2, C16:3, C20:4, and C20:5 were the highest compared to stressful conditions.
In this study, by the report to control culture, Halamphora sp. fatty acids profile showed an increase of SFA from 36.89% to 60.64% at 100mgL-1Pb and then decreased to reach 16.64% at 200mg L−1Pb (Table 2). As shown in table 2, Pb addition induced saturation of fatty acids by decreasing PUFA percentage and increasing SFA and monounsaturated fatty acids (MUFA) in Halamphora sp. cultures. At 150mgPbL-1, the proportions of C16:0 and C16:1 were highest, while those of C20:4 and C20:5 were lower.
Effects of lead stress on secondary metabolites of Halamphora sp.
Table 3 represents the effects of lead exposure on Halamphora sp. secondary metabolites and pigments. Results showed a pronounced increase in polyphenols content in Halamphora sp. at low Pb concentrations of 50 and 100 mg L-1 (1.58 ± 0.04 and 2.06 ± 0.07mg GAE g-1 DW respectively) compared to untreated cells (1.38 ± 0.03mg GAE g-1 DW) (Table 3). This means that moderate metal concentrations resulted in higher phenolic contents in Halamphora sp. culture. However, the amounts of phenolic contents per cell decreased at high concentrations of 150 mg L-1 and 200mg L-1.
In our study, the flavonoid content varied similarly to the phenolic content increasing from 0.29mg Catechin Equivalent (CE) g−1 DW in untreated cells to 0.43mg CE g−1 DW in treated cells at 100mgPb L−1 and then decreased to 0.25mgCE−1 DW at 200mgPb L−1 (Table 3).
Carotenoid and chlorophyll contents were also affected by Pb treatment (Table 3). A significant increase in carotenoid content was observed at 50 and 100mg Pb L-1 compared to control microalgae. An increase in carotenoid content in treated cells appears to be in response to the metal detoxification mechanism. On the other hand, the higher concentration of Pb (150 and 200mg Pb L-1) decreased carotenoid content when compared to control microalgae (Table 3). In contrast, Halamphora sp. showed significant reductions in chlorophyll a and b with the increased metal concentrations (p<0.05; Table 3).
Effects of lead stress on the antioxidant potential of Halamphora sp.
The antioxidant capacity of the phenolic extract of Halamphora sp. was estimated through the DPPH and ABTS scavenging tests (Tables 3,4). For the DPPH, the lowest IC50 (0.11mgmL-1) was obtained for the highest concentration of 200mgPbL-1, while the highest IC50 was 0.41mgmL-1 which was recorded for the mild Pb concentration (100mgL-1). The ABTS scavenging capacity of Halamphora sp. extracts increased with the Pb concentration, ranging from 0.54 µM Trolox equivalent (TEq) g-1 DW for the control to 0.75µM TEqg-1 DW at 100mgPbL-1. Then, this parameter decreased significantly and reached 0.20mMTEq g−1 DW at 200mgPbL-1.
Results showed the relative antioxidant efficiency of the diatom extracts against the DPPH radical. Antioxidants suppressed the absorbance at 515 nm on a time scale dependent on the antioxidant activity of the extracts.
The extracts derived from the diatom exposed to lead showed the highest radical scavenging activities IC50 of 0.41mgmL-1 at 100mgL-1 of lead exposure compared to the extract derived from the control (0.32mgmL-1). The different measurements showed that Halamphora sp. has an antioxidant capacity that rises with the increase in lead in the medium.
Effects of lead exposition on antioxidant enzymes and oxidative stress markers in Halamphora sp. cells
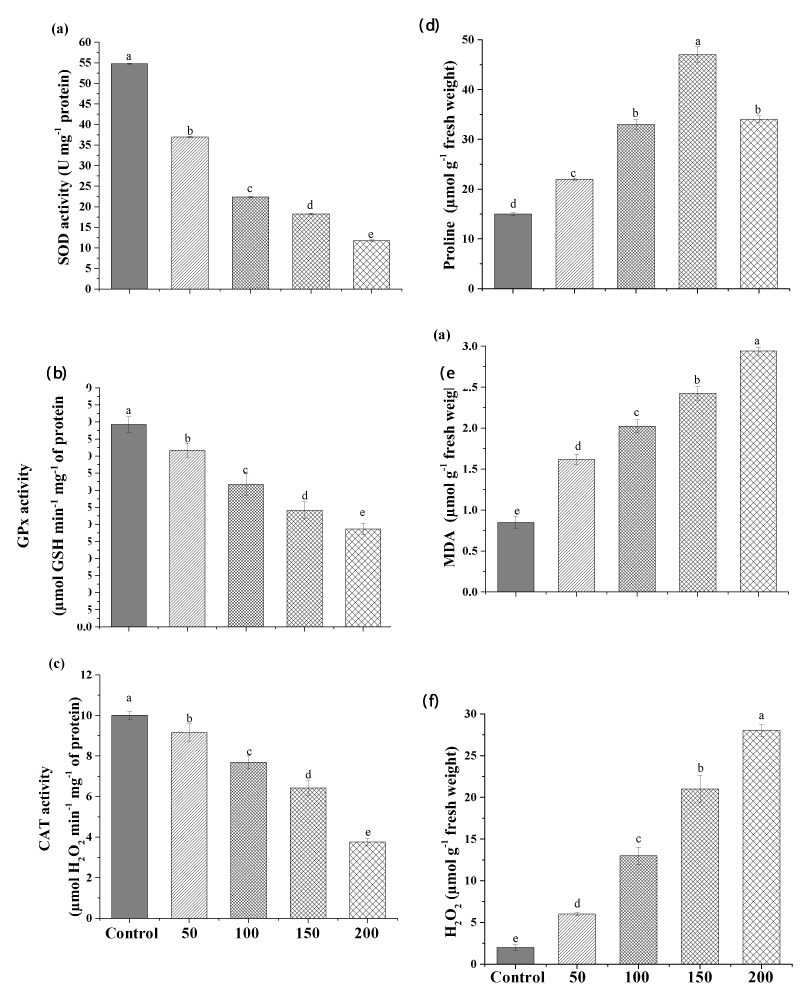
This is the first study to investigate the toxic effect of lead on the diatom alga Halamphora sp. The impact of different concentrations of Pb on the activities of the antioxidant enzymes SOD, GPx, and CAT of Halamphora are shown in Figure3. The result revealed that the activities of the antioxidant enzymes involved in antioxidant defense were negatively correlated with the concentrations of Pb (Figure 3).
The highest Pb concentration (200mgL-1) significantly decreased SOD, GPx, and CAT activities in Halamphora sp. by 78.51%, 51.68%, and 63% (p<0.01), respectively, compared to the control (Figure 3a, b, c).
Our result indicated an increase in the oxidative damage markers induced by Pb ions in Halamphora sp. where, a considerable increase in lipid peroxidation (MDA), proline, and H2O2 levels was recorded. This increase was dose-dependent. After 10 days of exposure, the MDA content was significantly enhanced (p<0.05) by about 3.4-folds by the high dose of Pb (200mgL-1) in Halamphora sp. Similar to the present study, Dahmen-BenMoussa, Athmouni [13] found an increase in lipid peroxidation level with the increase of nickel in the growth medium from 0 to 500mgL-1.
Similarly, H2O2 content was also extremely increased with increasing Pb concentration (Figure 3f), indicating that metal concentration was dependent on free radical production in the culture medium. The low concentration (50mgL-1) increased the H2O2 level from 2.42µmolg-1 FW to 6.34µmolg-1 FW (p<0.05) and the high concentration (200mgL-1) continued to increase it to 26.78µmolg-1FW. Similarly, Piotrowska-Niczyporuk, and Bajguz [2] reported increases in H2O2 amount in Acutodesmus obliquus exposed to different concentrations of lead (0-500µM).
Similarly, at the high concentration proline content was elevated from 15µmolg-1 FW to 34µmolg-1 FW (p<0.05). On the other hand, the maximum proline content was observed at the mild concentration of Pb, i.e., 47.45µmolg-1 FW (Figure 3e).
Generally, the production of proline increases under stress produced by some metals, since these molecules contribute to the antioxidant response against reactive oxygen species (ROS).
Discussion
During the exposition period, algae treated with (200mg PbL-1) contained the highest concentration of lead (0.88pg celL-1). This is often in agreement with the earlier study on Acutodesmus obliquus [2]. The tolerant diatom Halamphora sp. showed high efficiency of heavy metal biosorption. This may be due to their smaller size and larger cell wall surface area [10]. Therefore, even small differences in the organization and composition of the cell wall can greatly influence the tolerance of a particular metal to the different species of microalgae, as well as the level of tolerance toward metals. Microalgae-based technology is highly recommended for being applied for the biotreatment of heavy metals [33].
The mechanism of microalgal bioremediation of lead may occur in two ways i.e. biosorption process (a part of the lead removed by the cells is adsorbed on the cellular surface) and bioaccumulation process (another part is accumulated intracellularly). The results of this study agree well with the previous observation of Singh, et al. [34], who find that no more considerable increase in metal sorption is observed by a tandem increase of metal ions concentration. Uptake of Pb ions in algae occurs via the Ca2+ pathway. Since Pb can imitate Ca, a reasonable explanation for the increased absorption of Pb2+ could be that Pb2+ could block the Ca2+-dependent Pb2+ efflux system [35]. This second phase is known as the absorption of intracellular ions. It consists of the active sorption of heavy metal ions within the cytoplasm of algae cells and depends on cell metabolism (Shivaji and V L Dronamaraju 2019). It’s also been confirmed that the absorption of intracellular ions has a great contribution to the biosorption and detoxification of heavy metal ions [36].
A higher lead concentration was found to be present as loosely bound to the frustules instead of accumulating intracellularly, which suggests an efficient mechanism of metal-exclusion that binds the metal in the extracellular environment and prevents the entrance of lead into the cell. In our assay, the diatom Halamphora sp. leads to high adsorption removal capacity due to the rigidity, the high area surface, and therefore the strong binding affinity to heavy metals. There is some evidence to prove that sulfhydryl and amide groups of enzymes involve in binding Pb, altering their configuration and diminishing their activities [1]. Low molecular proteins such as phytochelatins, may also participate in binding toxic metals [2]. Algae uptake metals both passively and actively; some metals such as strontium (Sr) and Pb may be passively adsorbed by charged polysaccharides in the cell wall, and intracellular matrix [11], while others (eg Zn, Cd) are actively absorbed against a large intracellular concentration of gradients [37].
Our data indicate that the Halamphora sp. strain examined can tolerate the toxicity of Pb even at higher concentrations. Other algae such as Scenedesmus quadricauda, Phormidium ambiguum, and Chlorella sp. (3,38) are also able to tolerate Pb concentration up to 100mg L-1. It was determined that the toxic effect of Pb on growth and some key metabolic processes in Halamphora sp. cells is caused by its intracellular accumulation. Once in the cell, the lead induces the synthesis of class II phytochelatins and metallothionein which can enhance the detoxification effect [3]. Growth inhibition in microalgae is linked to the number of heavy metal ions bound to the algal cell surface, in some cases, and also to the quantity of intracellular heavy metal ions depending on their chemical nature Piotrowska-Niczyporuk, Bajguz [2,39]. The suppression of growth by lead was oberved in Chlorella Vulgaris as reported by Piotrowska-Niczyporuk, Bajguz [2], Teoh and Wong [3], Bajguz [40], Piotrowska-Niczyporuk, Bajguz [41].
The changes in morphological structure confirm the effect of this metal on the growth of Halamphora sp. A study with the diatom Amphora coffeaeformis reported that heavy metals such as copper and cadmium, at all concentrations, cause fewer branched filaments with the presence of many small vacuoles in the cells [42]. Similarly, Piotrowska-Niczyporuk, Bajguz [2,43] also observed a decrease in the cell size of the green microalga Acutodesmus obliquus, which may be associated with the toxic effect of Pb on the cell wall structure and the leakage of ions or cell components through the cytoplasmic membrane thus generating necrosis and irreversible cell damage. Recently, it has been demonstrated that Pb intoxication induces cellular death and oxidative stress, DNA damage, activation of caspase-3, and externalization of phosphatidylserine [1].
Lower mineral contents were found in cells cultured in the presence of Pb, which could be a consequence of reduced growth during the metallic stress response. Yedjou, Tchounwou [1] reported that one of the major mechanisms by which lead exerts its toxic effect is through biochemical processes that include lead’s ability to inhibit the action of calcium and to interact with proteins [44]. Within the cell, Pb may also compete with metallic cations for binding sites or alter the transport of essential metallic cations such as calcium [36].
The fatty acids profile of Halamphora sp., exposed to Pb stress showed a higher SFA and MUFA contents and a lower PUFA (C20:4 and C20:5) content in comparison with the control culture. Eicosapentaenoic acid (EPA) in control cells can be used as a nutraceutical or pharmacological agent for the prevention of cardiovascular and inflammatory diseases [45]. Moreover, EPA is the most valuable FA found in microalgae with a high content [46], which makes them suitable for aquaculture feeding [17,47]. Metal stress can be applied to alter the composition of fatty acids in microalgal cells to produce biodiesel [10].
A previous study reported that exposure of Amphora subtropica to nickel stress at 300mg L-1 led to an increase in C16:0, C18:0, and C18:1 lipid globules and a decrease in C18:2, C20:4, and C20:5 thylakoid membrane lipids (Dahmen-Ben Moussa, 2017). These changes in fatty acid composition resulted in a reduced degree of unsaturation of the total fatty acid pool. High levels of SFA and MUFA combined with low levels of PUFA are essential for biodiesel [48]. MUFAs are better than PUFAs for the cetane number and iodine value while SFA is favorable for combustion and oxidative stability–related biodiesel properties [48]. Finally, the coupling of heavy metal treatment and the production of biodiesel by microalgae makes it possible to combat eutrophication and industrial pollution linked to the production of renewable energies [18,35,36,49]. Nanda and Jaiswal [10] have also compared and reported similar findings for the use of microalgae in Pb bioremediation of contaminated wastewater in conjunction with biofuel production.
Polyphenols represent a group of chemical compounds arising from a common intermediate, phenylalanine, or a close precursor, shikimic acid [50]. Polyphenols can protect cells from oxidative stress by different mechanisms; they can chelate transition metal ions, directly scavenge molecular species of active oxygen can inhibit lipid peroxidation by trapping the lipid alkoxyl radicals [50].
The increase in the flavonoid content is explained by the fact that these molecules contribute to protecting the cells against the environmental pollution caused by the presence of lead in the medium at moderate concentrations. However, flavonoid content decreased, when higher concentrations of Pb were applied, as a result of the inhibitory effect of Pb on phenol synthesis and the cell damage affected by Pb at high concentrations [1].
The inhibition of pigment content observed in our experiment may be related to the sensitivity of the total carotenoid pigment towards the heavy metal ions at high concentrations. Some earlier studies have reported decreased carotenoid content, when higher concentrations of lead were used, as a consequence of the poisoning of the intracellular enzyme systems [51,52].
A wide range of valuable by-products (such as bioethanol and biodiesel), valuable nutrients, and bioactive compounds can be extracted from the produced biomass [35]. In this work, appropriate Pb concentrations (≤100mg Pb L-1) have enhanced phenolic, flavonoid, and carotenoid production. On the cost side, integrated algal-based wastewater treatment and bioactive compounds production can not only efficiently remove potentially hazardous contaminations such as toxic metal pollutants but also reduce the inputs and costs of algal biomass production [53].
In this research, the phenolic extract of Halamphora sp. showed a moderate antioxidant capacity. These results are consistent with the findings of many research groups that have reported a positive correlation between total phenolic content and antioxidant activity [54]. We suggest that the increase of non-enzymatic antioxidants, represented by phenolic compounds (including flavonoids) and the carotenoids, is responsible for the increase in the antioxidant capacity in Halamphora sp. Phenolic compounds from plants can act as antioxidants by chelating metal ions [55]. Consequently, plants exposed to heavy metals accumulate high amounts of phenolic compounds allowing their protection against cellular damage [56]. Similar to the present study, Belghith, Athmouni [57] reported that as Cd exposure increased up to 25mgL-1, phenolic contents of green alga Dunaliella salina increased. An increase in phenolic compound content has been reported also, in Phaeodactylum tricornutum exposed to high doses of copper (Cu(II)) [58]. These compounds help to minimize stress on the cell, which reduces the metabolic effort needed to absorb the metal [58]. Previous research has shown that flavonoids, a class of phenolic metabolites, have significant antioxidant and chelating properties [50].
As for chlorophyll content, showed decreasing values of both chlorophyll a and b for all Pb concentrations exposure. The increased Pb concentrations exposure may increase the inhibitory stress towards the photosynthesis of cells due to the destruction of chlorophyll pigments essential for photosynthesis. Hee, Shing [59] have supported the detrimental effects of Pb on the growth and chlorophyll content of Chlorella Vulgaris. They had reported a decrease in chlorophyll pigments per cell after an exposure of 32mgL-1 of Pb. When microalgae are exposed to Pb, the oxidative potential of metal ions causes the inhibition of key enzymes that play a crucial role in the CO2 fixation cycle. Such a decrease in the activity of these enzymes reduces the synthesis of ATP in the fixation of CO2 and the production of ATP and NADPH which therefore affects the rate of photosynthesis [3].
Several studies indicated that the heavy metal exposition affected the antioxidant enzymes activities implicated in the scavenging of ROS [13]. Batsalova, Teneva [60] found that microalgae are sensitive to exposure to lead due to its toxicological effect. The results of the present study are consistent with the observations of Piotrowska-Niczyporuk, Bajguz [2] who reported that lead produced ROS and the elevated concentrations of these species suppressed the antioxidant response of SOD, CAT, and GPx in Acutodesmus obliquus culture treated with 500μM Pb. Many researchers found that Pb exposure significantly decreases the antioxidant status in the green alga Cladophora [61]. For instance, Cao, Shi [62] reported a decrease in SOD activity in the green alga Cladophora, with the increase of Pb concentrations in the growth medium from 0 to 200mg L-1. Additionally, Huang, Lai [61] revealed that lead exposure induced oxidative stress in Phanerochaete chrysosporium by decreasing the catalase activity. Decreased expression of antioxidative enzymes may be due to a corresponding increase in oxidative damage. In our present study, the possible reason for suppressed activities of SOD, CAT, and GPx enzymes might be due to the down-regulation of gene expression of these enzymes. Another possible reason for decreased activity of antioxidant defense system might be due to brassinosteroids signaling (BRs). It has been reported that BRs enhanced the level of the antioxidant system (SOD, CAT and GPx) under heavy metals stress conditions. However, Bajguz [40] found that Pb exposure decreased BRs level in the green algae Chlorella Vulgaris.
Microalgae can annul any effects associated with the overproduction of ROS by enzymatic and non-enzymatic antioxidants. Proline is an important non-enzymatic biomolecule produced by microalgae to prevent stress [63]. Accumulation of proline by cells under metal stress is an adaptive strategy to reverse oxidative stress injury via scavenging free radicals, maintaining osmotic balance, regulating cellular redox potential, and protecting membranes, enzymes, and ribosomes [64]. Overall, high lipid peroxidation level and damage to the membrane structure suggest that the induced antioxidant enzymes may not have been able to maintain ROS below the toxic level. Previous studies assumed that the protective response of algae cells against heavy metal ions strongly depends on their resistance to oxidative damage [65,66]. In addition, the presence of excessive toxicity of heavy metal ions could lead to denaturation of the protein structure, replacing essential elements, or damaging the oxidative balance of living algae. The intensity of stress on algae cells depends on the protein and oxidized lipid content of these cells [67,68]. This stress tolerance was attributed to the high antioxidant defense system of Halamphora sp. and its biodegradation capacity.
Conclusion
In a conclusion, the data obtained in the current study demonstrate that the strain of Halamphora sp. studied was capable of tolerating high lead concentrations of at least 200mg L-1. Lead inhibited the Halamphora sp. cells’ growth, decreased the antioxidant status, and enhanced oxidative stress at high concentrations. Pb treatment affected the content of polyphenols, flavonoids, and pigments in Halamphora sp. as well as its fatty acids profile. Given the potential of Halamphora sp. in bioremediation, these metabolites most probably contribute to the use of Halamphora sp. to remove heavy metals and may help it to survive in Pb-contaminated environments.
Credit authorship contribution statement
IDB and SB designed and performed the experiments, analyzed the data, and wrote the paper. KA participated in the design and execution of the experiments. DB and HA critically reviewed the manuscript. All authors read and approved the final manuscript.
The authors acknowledge the “Ministry of Higher Education, Scientific Research and, Technology, Tunisia”, for the support of this study.
- Yedjou CG, Tchounwou HM, Tchounwou PB (2016) DNA Damage, Cell Cycle Arrest, and Apoptosis Induction Caused by Lead in Human Leukemia Cells. Int J Environ Res Public Health 13: 56. Link: https://bit.ly/3kepJEu
- Piotrowska-Niczyporuk A, Bajguz A, Talarek M, Bralska M, Zambrzycka E (2015) The effect of lead on the growth, content of primary metabolites, and antioxidant response of green alga Acutodesmus obliquus (Chlorophyceae). Environ Sci Pollut Res Int 22: 19112-19123. Link: https://bit.ly/3vgdiOH
- Teoh ML, Wong SW (2018) Influence Of Lead On Growth And Physiological Characteristics Of A Freshwater Green Alga Chlorella sp. Malaysian Journal of Science 37: 82-93. Link: https://bit.ly/3kex9rq
- Charkiewicz AE, Backstrand JR (2020) Lead toxicity and pollution in Poland. Int J Environ Res Public Health 17: 4385. Link: https://bit.ly/3OAhEb7
- Singhal M, Jadhav S, Sonone SS, Sankhla MS, Kumar R (2021) Microalgae Based Sustainable Bioremediation of Water Contaminated by Pesticides 12: 149-169. Link: https://bit.ly/38kS9Ki
- Jaiswal KK, Kumar V, Verma R, Verma M, Kumar A, et al. (2021) Graphitic bio-char and bio-oil synthesis via hydrothermal carbonization-co-liquefaction of microalgae biomass (oiled/de-oiled) and multiple heavy metals remediations. Journal of Hazardous Materials 409: 124987.
- Jaiswal KK, Kumar V, Vlaskin M, Sharma N, Rautela I, et al. (2020) Microalgae fuel cell for wastewater treatment: Recent advances and challenges. Journal of Water Process Engineering 38: 101549.` Link: https://bit.ly/3rRkmzr
- Arora N, Jaiswal KK, Kumar V, Vlaskin M, Nanda M, et al. (2020) Small-scale phyco-mitigation of raw urban wastewater integrated with biodiesel production and its utilization for aquaculture. Bioresour Technol 297: 122489. Link: https://bit.ly/3vf9LQI
- Singh A, Prasad S (2015) Remediation of heavy metal contaminated ecosystem: an overview on technology advancement. International Journal of Environmental Science and Technology 12: 353-366. Link: https://bit.ly/3kfLyU2
- Nanda M, Jaiswal KK, Kumar V, Vlaskin MS, Gautam P, et al. (2021) Micro-pollutant Pb (II) mitigation and lipid induction in oleaginous microalgae Chlorella sorokiniana UUIND6. Environmental Technology & Innovation 23: 101613. Link: https://bit.ly/38o02yK
- Pacheco D, Rocha AC, Pereira L, Verdelhos T (2020) Microalgae Water Bioremediation: Trends and Hot Topics. Applied Sciences 10:1886. Link: https://bit.ly/3vgF6CM
- Al-Homaidan AA, Al-Ghanayem AA, Alkhalifa AH (2011) Green algae as bioindicators of heavy metal pollution in Wadi Hanifah Stream, Riyadh, Saudi Arabia. International Journal of Water Resources and Arid Environments 1: 10-15. Link: https://bit.ly/38iWde1
- Dahmen-BenMoussa I, Athmouni K, Chtourou H, Ayadi H, Sayadi S, et al. (2017) Phycoremediation potential, physiological, and biochemical response of Amphora subtropica and Dunaliella sp. to nickel pollution. J Appl Phycol 1-11. Link: https://bit.ly/36LMA78
- Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Applied energy 87: 38-46. Link: https://bit.ly/3xTcKA0
- Dahmen-BenMoussa I, Chtourou H, Rezgui F, Sayadi S, Dhouib A (2016) Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresource Technology 218: 816-825. Link: https://bit.ly/3OEOEyO
- Chtourou H, Dahmen I, Jebali A, Karray F, Hassairi I, et al. (2015) Characterization of Amphora sp., a newly isolated diatom wild strain, potentially usable for biodiesel production. Bioprocess Biosyst Eng 38: 1381-1392. Link: https://bit.ly/3KaVcC0
- Hegel PE, Martín LA, Popovich CA, Damiani C, Leonardi PI (2019) Biodiesel production from Halamphora coffeaeformis microalga oil by supercritical ethanol transesterification. Chemical Engineering and Processing-Process Intensification 145: 107670. Link: https://bit.ly/3kbuQVM
- Nanda M, Jaiswal KK, Kumar V, Verma M, Vlaskin MS, et al. (2021) Bio-remediation capacity for Cd (II) and Pb (II) from the aqueous medium by two novel strains of microalgae and their effect on lipidomics and metabolomics. Journal of Water Process Engineering 44: 102404. Link: https://bit.ly/3rU3R5E
- Stauber J, Florence T (1990) Mechanism of toxicity of zinc to the marine diatom Nitzschia closterium. Marine Biology 105: 519-524. Link: https://bit.ly/3kdzwLa
- Folgar S, Torres E, Pérez-Rama M, Cid A, Herrero C, et al. (2009) Dunaliella salina as marine microalga highly tolerant to but a poor remover of cadmium. J Hazard Mater 165: 486-493. Link: https://bit.ly/3KlF4h4
- Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV‐VIS spectroscopy. Current protocols in food analytical chemistry 1: F4. 3.1-F4. 3.8. Link: https://bit.ly/37KMVaO
- Gouvinhas I, Santos R, Queiroz M, Leal C, Saavedra M, et al. (2018) Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Industrial Crops and Products 126: 83-91. Link: https://bit.ly/3xZ79bH
- Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal Food and Drug Analysis 10: 178-182. Link: https://bit.ly/3vfFBg6
- Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry 64: 555-559. Link: https://bit.ly/38iVLfP
- Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181: 1199-200. Link: https://go.nature.com/36NEg6V
- Katalinic V, Modun D, Music I, Boban M (2005) Gender differences in antioxidant capacity of rat tissues determined by 2, 2′-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp Biochem Physiol C Toxicol Pharmacol 140: 47-52. Link: https://bit.ly/3LgHWwY
- Radi R, Turrens JF, Chang LY, Bush KM, Crapo JD, et al. (1991) Detection of catalase in rat heart mitochondria. J Biol Chem 266: 22028-22034. Link: https://bit.ly/3vN93Jo
- Vega-López A, Ayala-López G, Posadas-Espadas BP, Olivares-Rubio HF, Dzul-Caamal R (2013) Relations of oxidative stress in freshwater phytoplankton with heavy metals and polycyclic aromatic hydrocarbons. Comp Biochem Physiol A Mol Integr Physiol 165: 498-507. Link: https://bit.ly/3OBfJ6e
- Cano-Europa E, López-Galindo GE, Hernández-García A, Blas-Valdivia V, Gallardo-Casas CA, et al. (2008) Lidocaine affects the redox environment and the antioxidant enzymatic system causing oxidative stress in the hippocampus and amygdala of adult rats. Life Sci 83: 681-685. Link: https://bit.ly/3KgbZDU
- Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205-207. Link: https://bit.ly/3OwcVr3
- Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science 151: 59-66. Link: https://bit.ly/3ENMloM
- Chokshi K, Pancha I, Trivedi K, George B, Maurya R, et al. (2015) Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour Technol 180: 162-171. Link: https://bit.ly/3kdvr9z
- Kumar V, Jaiswal KK, Verma M, Vlaskin MS, Nanda M, et al. (2021) Algae-based sustainable approach for simultaneous removal of micropollutants, and bacteria from urban wastewater and its real-time reuse for aquaculture. Science of The Total Environment 774: 145556. Link: https://bit.ly/3LhXAZb
- Singh R, Chadetrik R, Kumar R, Bishnoi K, Bhatia D, et al. (2010) Biosorption optimization of lead (II), cadmium (II) and copper (II) using response surface methodology and applicability in isotherms and thermodynamics modeling. Journal of Hazardous Materials 174: 623-634. Link: https://bit.ly/3OCJmUQ
- Pham TL, Dao S, Pham N, Bui H, Ngo T, et al. (2019) Lipid production combined with removal and bioaccumulation of Lead (Pb) by the green alga Scenedesmus sp. Polish Journal of Environmental Studies 28: 1-8.
- Chabukdhara M, Gupta S, Gogoi M (2017) Phycoremediation of Heavy Metals Coupled with Generation of Bioenergy. 163-188. Link: https://bit.ly/3ELPSnF
- Fathi AA (2002) Toxicological response of the green alga Scenedesmus bijuga to mercury and lead. Folia Microbiol 47: 667-671. Link: https://bit.ly/3OCJl3e
- Shanab S, Essa A, Shalaby E (2012) Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates). Plant Signal Behav 7: 392-399. Link: https://bit.ly/38u1NKQ
- Polonini HC, Brandao HM, Raposo NR, Brandao MAF, Mouton L, et al. (2015) Size-dependent ecotoxicity of barium titanate particles: the case of Chlorella Vulgaris green algae. Ecotoxicology 24: 938-948. Link: https://bit.ly/3rTI2D7
- Bajguz A (2011) Suppression of Chlorella Vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch Environ Contam Toxicol 60: 406-416. Link: https://bit.ly/38s2UdX
- Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Żyłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella Vulgaris (Chlorophyceae). Plant Physiol Biochem 52: 52-65. Link: https://bit.ly/36QAxWk
- Anantharaj K, Govindasamy C, Natanamurugaraj G, Jeyachandran S (2011) Effect of heavy metals on marine diatom Amphora coffeaeformis (Agardh. Kutz). Glob J Environ Res 5: 112-117. Link: https://bit.ly/3Mt0GcX
- Nam SH, An YJ (2015) Cell size and the blockage of electron transfer in photosynthesis: Proposed endpoints for algal assays and its application to soil alga Chlorococcum infusionum. Chemosphere 128: 85-95. Link: https://bit.ly/37I86KB
- Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, et al. (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int 23: 8244-8259. Link: https://bit.ly/37FIoXg
- Komprda T (2012) Eicosapentaenoic and docosahexaenoic acids as inflammation-modulating and lipid homeostasis influencing nutraceuticals: A review. Journal of Functional Foods 4: 25-38. Link: https://bit.ly/3Lkf07c
- Yen HW, Hu IC, Chen CY, Ho SH, Lee DJ, et al. (2013) Microalgae-based biorefinery–from biofuels to natural products. Bioresource Technology 135: 166-174. Link: https://bit.ly/3w3i8yf
- Gimpel JA, Henríquez V, Mayfield SP (2015) In metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity. Front Microbiol 6: 1376. Link: https://bit.ly/3kaVrT0
- Sajjadi B, Chen WY, Raman AAA, Ibrahim S (2018) Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renewable and Sustainable Energy Reviews 97: 200-232. Link: https://bit.ly/3Kg4UmH
- Salama ES, Roh HS, Dev S, Khan MA, Abou-Shanab RAI, et al. (2019) Algae as a green technology for heavy metals removal from various wastewater. World Journal of Microbiology and Biotechnology 35: 75. Link: https://bit.ly/3OAQcKh
- Rodrigo R, Libuy M (2014) Modulation of plant endogenous antioxidant systems by polyphenols. Polyphenols in Plants: Elsevier 65-85. Link: https://bit.ly/3veQONZ
- Chakilam S (2012) Metal effects on carotenoid content of cyanobacteria. International Journal of Botany 8: 192-197.
- Chakilam S (2012) Metal effects on carotenoid content of cyanobacteria. International Journal of Botany 8: 192.
- Solovchenko A, Chekanov K (2014) Production of Biomass and Bioactive Compounds Using Bioreactor Technology. 63-91. Link: https://bit.ly/3kaX9nq
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, et al. (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food chemistry 97: 654-660. Link: https://bit.ly/3xRX2Fg
- Kaurinovic B, Vastag D (2019) Flavonoids and Phenolic Acids as Potential Natural Antioxidants. Antioxidants. Link: https://bit.ly/3OOUpdJ
- Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish Journal Environmental Studies 15. Link: https://bit.ly/3xU9zrP
- Belghith T, Athmouni K, Bellassoued K, El Feki A, Ayadi H (2016) Physiological and biochemical response of Dunaliella salina to cadmium pollution. Journal of Applied Phycology 28: 991-999. Link: https://bit.ly/3vfwNqA
- Rico M, López A, Santana-Casiano JM, Gonzàlez AG, Gonzàlez-Dàvila M (2013) Variability of the phenolic profile in the diatom Phaeodactylum tricornutum growing under copper and iron stress. Limnology and oceanography 58: 144-152. Link: https://bit.ly/3EPQFUk
- Hee CW, Shing W, Chi CK (2021) Effect of Lead (Pb) exposure towards green microalgae (Chlorella Vulgaris) on the changes of physicochemical parameters in water. South African Journal of Chemical Engineering 37: 252-255. Link: https://bit.ly/36NgCHP
- Batsalova T, Teneva I, Belkinova D, Stoyanov P, Rusinova-Videva S, et al. (2017) Assessment of cadmium, nickel and lead toxicity by us-ing green algae Scenedesmus incras-satulus and human cell lines: Potential in vitro test-systems for monitoring of heavy metal pollution. Toxicol Forensic Med Open J 2: 63-73. Link: https://bit.ly/3Kd6DZT
- Huang C, Lai C, Xu P, Zeng G, Huang D, et al. (2017) Lead-induced oxidative stress and antioxidant response provide insight into the tolerance of Phanerochaete chrysosporium to lead exposure. Chemosphere 187: 70-77. Link: https://bit.ly/3MwESwZ
- Cao DJ, Shi XD, Li H, Xie PP, Zhang HM, et al. (2015) Effects of lead on tolerance, bioaccumulation, and antioxidative defense system of green algae, Cladophora. Ecotoxicol Environ Saf 112: 231-237. Link: https://bit.ly/3OC9Rtr
- Zhang C, Chen X, Wang J, Tan L (2017) Toxic effects of microplastic on marine microalgae Skeletonema costatum: interactions between microplastic and algae. Environ pollut 220: 1282-1288. Link: https://bit.ly/3rNPGPm
- Hayat K, Khan J, Khan A, Ullah S, Ali S, et al. (2021) Ameliorative effects of exogenous proline on photosynthetic attributes, nutrients uptake, and oxidative stresses under cadmium in Pigeon Pea (Cajanus cajan L.). Plants 10: 796. Link: https://bit.ly/3xQ0cJO
- Arunakumara KKIU, Zhang X (2008) Heavy metal bioaccumulation and toxicity with special reference to microalgae. Journal of Ocean University of China 7: 60-64. Link: https://bit.ly/3rRcyxy
- Shivaji S, Dronamaraju SVL (2019) Scenedesnus rotundus isolated from the petroleum effluent employs alternate mechanisms of tolerance to elevated levels of Cadmium and Zinc. Sci Rep 9: 8485. Link: https://bit.ly/3OBfqbA
- Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MP (2016) Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manage 181: 817-831. Link: https://bit.ly/3vf8JnO
- Hamed SM, Hassan SH, Selim S, Wadaan MAM, Mohany M, et al. (2020) Differential responses of two cyanobacterial species to R-metalaxyl toxicity: Growth, photosynthesis and antioxidant analyses. Environ Pollut 258: 113681. Link: https://bit.ly/37I7Knf

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
