Journal of Biology and Medicine
Results of a preliminary study on the effects of a compound on telomeres length, biological and physiological parameters
Christophe de Jaeger*, Carla Lamberti, Virginie Van Leeuwen, Elena Voronska and Saskia Kruiskamp
Cite this as
de Jaeger C, Lamberti C, Van Leeuwen V, Voronska E, Kruiskamp S (2021) Results of a preliminary study on the effects of a compound on telomeres length, biological and physiological parameters. J Biol Med 5(1): 008-015. DOI: 10.17352/jbm.000025Telomeres are ribonucleoprotein structures forming a protective buffer at the ends of chromosomes. They preserve the integrity of the genetic material during the cell cycle. Replicative telomere erosion can be compensated by a telomerase. The average size of telomeres is a marker of the biological age of individuals and their exposure to various physiological and pathological conditions. We are reporting the findings of an open prospective pre-study in healthy volunteers of a new association of molecules on telomere elongation and its cardiovascular action. Ten healthy volunteers (6 men, 4 women), average age 59.4 ± 8.19 years old, with no specific history. This pre-study did reveal significant results concerning the size of the average and median telomeres. The speed of the propagation of the pulse wave on the carotid- femoral and arterial segment shows a reduction (8.74 ± 1.72 m / s at T0 for the 10 subjects, versus 8.18 ± 2.18 at M6). The study shows a significant improvement of the average PAO of 10 subjects, from 1.51 ± 0.13 mmol / l to T0, versus 1.68 ± 0.16 mmol / l at M1 (p <0.0006) and 1.58 ± 0.18 mmol / l at M6 (p <0.05).
Introduction
Telomeres are ribonucleoprotein structures forming a protective buffer at the ends of chromosomes [1] Photo 1. They preserve the integrity of the genetic material during the cell cycle and correspond to tandem repetitions of nucleotide sequences (TTAGGG) associated with several dozens of regulatory proteins including telomerase, which is the only enzyme capable of replicating telomeres. In the absence of telomerase activity, which depends on the catalytic subunit Telomerase Reverse Transcriptase (TERT), the end of the DNA molecule is truncated by about 50 to 200 nucleotides at each S phase of the cell cycle. The TERT gene is suppressed in almost all postnatal somatic cells, with the exception of stem cells and lymphoid cells. The telomere length is therefore truncated in older organisms [2].
This shortening of aging telomeres is directly related to cell division. Indeed, DNA polymerases cannot replicate the ends of linear chromosomes. Thus, each cycle of DNA replication has a loss of genetic material [2]. Since the telomere does not contain coding sequences, there is no loss of genomic information. In addition, telomeres prevent chromosome ends from being recognized as DNA damage. Telomeres are therefore involved in the process of preserving the integrity of the genome and are essential for proper cell function.
In those cases where no mechanism comes into play to regenerate the telomeres, if telomere shortening occurs at each cell replication cycle, this indicates that the cell cannot live indefinitely.
The division of cells in the absence of an effective telomere maintenance mechanism, will in fact lead to the formation of short telomeres which will in turn lose their protective function and be reported to the cell as damaged DNA. An activation of the cellular senescence pathways called p53 and pRb / p16 [3] can then be observed, which induces an interruption of cellular proliferation, an entry into senescence or death by apoptosis, according to cellular type.
The Hayflick limit is the maximum number of cell divisions that a cell can undergo [4]. It provides a link between the length of the telomere and the lifespan of the cell.
Replicative telomere erosion can be compensated by the action of an enzyme, telomerase, a specialized reverse transcriptase that uses a specific template RNA to extend the 3 ‘strand of the chromosome ends. There are also telomerase-independent telomerase elongation mechanisms based on homologous recombination events called ALT (alternative lengthening of telomeres) [5].
In addition to cellular proliferation, chronic oxidative stress has been shown to be a major causal factor in telomere shortening and cellular senescence [6].
Measured by various techniques in peripheral blood leukocytes, the average size of telomeres is a marker of the “biological age” of individuals and their exposure to chronic stress under various physiological and pathological conditions [7]. In humans, the telomere size varies on average from 10 to 4 kb from birth to 80 years of age and undergoes erosion of a few dozen bases per year. The length of the telomeric sequence, shorter in men than in women, is linear and inversely correlated with age. It is a genetically determined individual characteristic (heritability of 70-80%), which is characterized by a large variability. At a given age, the average size of leukocyte telomeres is therefore the result of three variables: inherited length, the proliferation rate of immune cells, and the level of exposure to chronic oxidative stress.
Numerous studies have determined that telomere shortening in peripheral blood leukocytes constitutes a risk factor for cardiovascular (atherosclerosis, early infarction, hypertension, vascular dementia), metabolic (diabetes, obesity, insulin resistance) and cancerous [7- 10] pathologies. Hematopoietic cells and endothelial cells share the same embryogenic origins [11], which could be one of the essential elements of the link between telomere size, atherosclerosis and cardiovascular pathologies. Thus, regardless of age, telomeres could be involved in the initiation and /or progression of cardiovascular disease.
Overall, for the same chronological age, the mortality rate from infections or cardiovascular disease in subjects over 60 years of age with the shortest telomeres is three to eight times higher than those with the longest telomeres [12]. This data accounts for the critical role of telomeres at the interface of the molecular systems involved in aging, cell proliferation, tissue renewal, oxidative stress, inflammation, and carcinogenesis [13].
Several molecules have appeared on the market in recent years, due to their supposed action on telomeres. Astragalus, a plant used in traditional Chinese medicine, could act as an activator of telomerases. Astragalus and one of its derivatives (Astragalosides) appear to be metabolized to Cycloastragenol (CA), a telomerase activator [14]. Some products from Astragalus have shown their value in vitro and in animal experiments [15-17] and have been evaluated in humans with encouraging first results. In a randomized, double-blind versus placebo-controlled study of 117 subjects aged 53 to 87 years, Salvador et al. showed that Astragalus derivatives significantly increased telomerase size over a one-year follow-up period compared to the placebo [18].
We are reporting the findings below of an open prospective pre-study in healthy volunteers, the interest of a new ASFC molecule on telomere elongation and its cardiovascular action.
Methodology
Population
Ten healthy volunteers (6 men, 4 women), average age 59.4 ± 8.19 years old (average age females: 55.2 ± 9.14 years old, average age males: 63.6 ± 4.87 years), with no specific history included between November 2017 and February 2018. All subjects signed informed consent before inclusion in the protocol. The volunteer subjects came all from our medical and paramedical team. No subject had active cardiovascular disease at the time of inclusion. All changes in physical activity or drug intake during the study period, as well as the occurrence of any negative side effects, were reported.
ASTCOQ02
The telomerase activator tested ASTCOQ02, combining Astragalus extracts (including astragaloside IV and cycloastragenol, olive fruit (including hydroxytyrosol), zinc oxide and seed extract). The product was in the form of an oral capsule, used by this clinical pre-study in healthy volunteers, with a dosage of 2 capsules (one in the morning and one in the evening) per day. The daily dose (2 capsules per day) included in total:
➣ Astragalus extract: 250 mg
- Including astragaloside IV: 40 mg
- Including cycloastragenol: 25 mg
➣ Zinc oxide: 14.46 mg (144.6 % VNR*)
➣ Grape seed extract:160 mg
➣ Olive fruit extract : 140 mg
- That is 28 mg of hydroxytyrosol and its derivatives
It is a product marketed as a food supplement with no recognized toxicity.
Evaluation of the size of telomeres
Blood samples for measurement of telomere size were collected at TO, 1 month and 6 months after inclusion in the study. Results were provided in KB (kilo base). Telomeres were measured by the Q-FISH fluorescence in situ hybridization technique (LIFELENGTH - Calle Faraday 7, Madrid, 28049, Spain) as published [19].
In situ hybridization using Q-FISH methodology allows the characterization of restriction fragments bearing TTAGGG repetitions. It involves digesting the telomeric DNA with one or more restriction enzymes and then the restriction fragments are separated according to their size by gel electrophoresis. The fragments are then transferred to a nitrocellulose or nylon membrane which will bear a replica of the position of the fragments in the gel. The DNA is then denatured, arranged, and hybridized with a fluorescent probe. The position of the fragments is then revealed by radiography showing one or more black bands. The size of the terminal fragments is estimated through the comparison between the distance they have traveled in the gel and that covered by fragments of known length. This technique has the advantage of determining the length of any telomere, regardless of its size, but has the disadvantage of being a long technique.
This HT Q-FISH technique measures median and average telomere length, short telomere rate and measurement, shortest telomere percentile; and the global distribution of telomeres.
Cardiovascular evaluation and other measures
The medical and socio-economic characteristics of the subjects were collected by a standardized questionnaire, including the medical-surgical antecedents and cardiovascular co-morbidities. All subjects benefited at TO, M1 (1 month) and M6 (6 months):
- a health questionnaire with standardized complete clinical examination,
- a measurement of the length of the telomeres by HT Q-FISH (length of the median, average, and short telomeres, and percentile of the shortest telomeres),
- a biological exam with CRPus
- a study of plasma antioxidant potency (total PAO in mmol / l - VR between 1.35 and 1.65) (Dr C. Garrel, Laboratory of Biochemistry, CHU Grenoble),
- a measure of arterial stiffness, by measuring the rate of propagation of the pulse wave on the carotid-femoral and carotid-radial arterial segments (Complior Analysis, ALAM Medical, Vincennes, France).
- evoked cognitive potentials measured by electroencephalography, with evaluation of P300 (Galileo NT, EBNeuro, Florence, Italy).
- and the length of the PQ interval at the ECG.
The evoked potentials P300 constitute late cognitive evoked potentials demonstrating the quality of the cortical activities. The P300 is very clearly correlated with cerebral aging and provides a distinction between neurodegenerative diseases such as Alzheimer’s disease and thymic disorders [20-22].
Statistical analysis
Mean values and standard deviations of the overall population of 10 healthy volunteers and 2 independent groups: men and women were gathered according to clinical and biological parameters, telomere size, arterial compliance results, P300. The average of the different groups were evaluated by a parametric statistical test: student’s t-test, with a significance level p set at 5%.
Results
1 / Compliance and tolerance
Ten volunteer subjects of mean age 59.4 ± 8.19 years were included. There were 4 women (mean age: 55.2 ± 9.14 years) and 6 men (mean age: 63.6 ± 4.87 years), ambulatory, with no particular antecedent. The 10 subjects completed the study at 6 months with no adverse side effects reported. No changes in weight, blood pressure or heart rate were found. Biological assessments have not revealed any biological alteration. The medium-sensitive CRP was not changed during the study. The analysis of the T0, M1 and M6 electrocardiogram showed no abnormality in independent analysis by a cardiologist in a single blind study concerning the PQ space.
2 / On the length of telomeres
The “long-life” telomere activator requires a few weeks’ time for incorporation and action. This is observed in our pre-study between T0 to M1, where one simply observes the physiological shortening of telomeres before the “long-life” action.
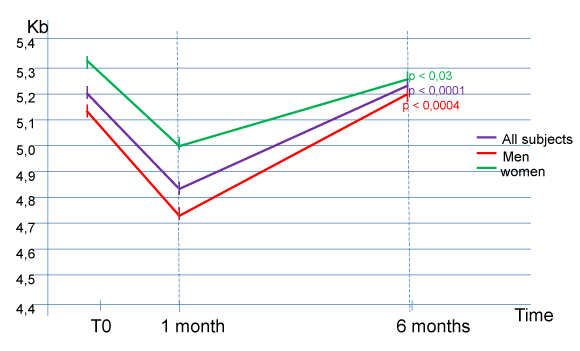
Results show, after the lag phase and the incorporation of the product between T0 and M1, a very significant increase in the short telomere length between M1 and M6 for all healthy volunteers, (average size for the 10 volunteers: 4.83 ± 0.49 Kb to M1, versus 5,22 ± 0,58 Kb to M6, p < 0,0001) (Figure 1). The results are particularly clear in humans (average size for the 6 male volunteers: 4.72 ± 0.48 Kb at M1, versus 5.20 ± 0.55 Kb at M6, p <0.0004). There is also a statistically significant increase in the size of short telomeres in women (average size for the 4 female volunteers: 5.0 ± 0.54 Kb at M1, versus 5.25 ± 0.70 Kb at M6, p < 0.03).
This clinical pre-study did not reveal any significant results concerning the size of the average and median telomeres (Table 1). Indeed, there is a regular decrease in the size of the median (average median telomere size for the 10 volunteers: 9.88 ± 0.77 Kb at M1, versus 9.61 ± 0.86 Kb at M6) and average telomeres (the average size of these telomeres for the 10 subjects: 11.48 ± 0.86 Kb at M1, versus 11.18 ± 0.89 Kb at M6) as well as in the 2 male and female populations of the study. However, given the natural evolution towards the shortening in size of the telomeres over time, and the absence of a control population, it is impossible to eliminate a significant effect of the “long-life” activator from this simple pre-study on these telomeres through slower natural speed of shortening.
3 / On arterial rigidity
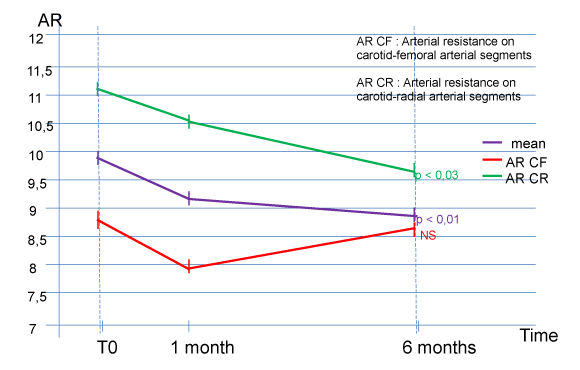
The evaluation of arterial stiffness shows a significant improvement at 6 months of total arterial compliance (9.90 ± 2.20 m / s at T0 for the 10 subjects, versus 8.93 ± 2.17 at M6, p <0.01) and carotid-radial arterial compliance (11.05 ± 2.07 m / s at T0 for the 10 subjects, versus 9.67 ± 2.00 at M6, p <0.03) (Figure 2). The speed of the propagation of the pulse wave on the carotid- femoral and arterial segment also shows a reduction (8.74 ± 1.72 m / s at T0 for the 10 subjects, versus 8.18 ± 2.18 at M6) but does not reach the threshold of significance (Figure 2).
4 / On the p300
The measurement of the P300 cognitive potentials evoked also shows an improvement, but insignificant, at 6 months of P300 for all 10 healthy volunteers (298.10 ± 19.51 msec at T0 for the 10 subjects, versus 282.40 ± 29.94 msec to M6).
5 / Study of plasmatic anti-oxidizing power
Plasma antioxidant analysis was performed at the reference center of Biochemistry Laboratory, Grenoble University Hospital (Dr. C. Garrel) (total PAO in mmol / l - VR between 1.35 and 1.65). The study shows a significant improvement of the average PAO of 10 subjects, from 1.51 ± 0.13 mmol / l to T0, versus 1.68 ± 0.16 mmol / l at M1 (p <0.0006) and 1.58 ± 0.18 mmol / l at M6 (p <0.05).
Discussion
This pre-study evaluated the compliance and the good tolerance of the “long-life” product at 6 months.
All healthy volunteers continued the study to term and no side effects were reported during the entire six-month follow-up period. Biological assessments have not revealed any biological alteration. Analysis of the T0, M1 and M6 electrocardiogram showed no abnormality in independent analysis by a cardiologist through a single-blind study. The “long-life” telomere activator requires a few weeks’ time for incorporation and effects. This is observed in our pre-study between T0 to M1, in which one can simply observe the physiological shortening of telomeres before “long-life” effects.
Our study revealed, after the lag phase of the product from T0 to M1, a very significant increase in short telomere length between M1 and M6 for all healthy volunteers, (average size for the 10 volunteers): 4.83 ± 0.49 Kb at M1, versus 5.22 ± 0.58 Kb at M6, p <0.0001); and particularly in humans (average size for the 6 volunteers: 4.72 ± 0.48 Kb at M1, versus 5.20 ± 0.55 Kb at M6, p <0.0004). The results are also statistically significant in women (average size for the 4 female volunteers: 5.0 ± 0.54 Kb at M1, versus 5.25 ± 0.70 Kb at M6, p <0.03).
This clinical pre-study did not reveal any significant results concerning the size of the average and median telomeres.
At this stage of development of the “long-life” molecule, the results of this pre-study can only be interpreted with caution, concerning the median and average telomeres. The limited duration of follow-up (6 months), the limited number of subjects included, and above all the absence of a control population; which could have allowed us to see a significant difference because of the natural shortening with time and the size of the telomeres, the lack of effectiveness cannot be concluded on this type of telomere.
In fact, the telomere point measurement has no value as such. It is the measure of the variation in the size of our telomeres, in one way or another, which constitutes a major piece of information on the future quality of longevity.
It is also possible that the “long-life” telomerase activator has a more targeted and rapid effects on short telomeres and is the most likely to bring the cell into senescence and apoptosis.
New studies on the action mechanism of the “long-life” molecule, at different dosages, during a longer study period, a larger number of subjects included, and with the presence of a control group, are in progress.
Our pre-study has also shown interesting cardiovascular results. The increase in arterial stiffness has been validated as a strong independent marker of cardiovascular mortality in both hypertensive and normotensive patients [23].
The long-life telomerase activator resulted in a significant improvement at 6 months in total arterial and carotid-radial arterial compliance in the subjects included, as well as a non-significant improvement in the carotid and femoral arterial segment.
Similarly, we observed an improvement, but insignificant at 6 months of the P300 for all 10 healthy volunteers.
The “long-life” telomerase activator seems to possess an activity on the vascular wall through its anti-oxidant action (PAO) demonstrated by our study, and not on the areas of inflammation, evidenced by the absence in the modification of CRP ultra-sensitive subjects included, throughout the follow-up period of our study.
Several studies have demonstrated a formal link between telomere length and senescence, including endothelial cells [24], but also with numerous cardiovascular atherosclerotic pathologies [25-27]. The respective roles of chronic inflammation, oxidative stress, and telomere shortening on the occurrence of atherosclerosis remain debatable [28,29].
Thus, the size of telomeres is inversely correlated with obesity [29,30], smoking [31,32], stress and depression [33], type 2 diabetes [34] and high blood pressure [35]. On the contrary, physical activity have a positive impact in telomere strength and aging [36].
In many cases, coronary diseases appear within the context of atherothrombosis and cellular senescence, which have been the focus of attention for many years [37-39]. It has been suggested that the length of telomeres within vascular cells plays a key role in the development of coronary pathologies through the establishment of a particular senescence phenotype of smooth muscle and endothelial cells [40,41]. Brouilette et al, in a case-control study of 104 subjects (45 with a family history of coronary pathology and 59 control subjects), showed an association between family history of coronary disease and shortening of telomeres [42]. These results suggest that the presence of short telomeres would be one of the primary abnormalities of atherosclerotic coronary disease. More recently, Goglin, et al. have shown a close relationship between cardiovascular mortality and telomere size, independent of common cardiovascular risk factors [43].
In 2011, Depinho et al reported an improvement in tissue degeneration in elderly mice by activation of telomerases [44]. This work has been confirmed on many animal models.
However, there is currently little data on the possible therapeutic strategies that can be used to protect telomere length or elongation, particularly in the context of cardiovascular disease. Physical exercise appears to positively influence telomere length [45].
The WOSCOPS study showed that statins could have a beneficial effect on telomere length, thus contributing, in addition to their lipid-lowering effect, to preserving the integrity of the vessel wall of patients at risk, by decreasing the senescence of endothelial cells [46,47]. Oral antidiabetic drugs such as Pioglitazone, belonging to another pharmacological class, appear to have some beneficial properties on telomere biology by increasing telomerase activity [48,49]. However, no clinical studies have been reported to date.
Currently no other therapeutic class (fibrates, NSAIDs, aspirin, corticosteroids, betablockers, insulin, ACE inhibitors, angiotensin II receptor antagonists etc ...) seems to have any effect on telomere biology.
Conclusion
Thus, these different molecules have opened up a therapeutic approach in the early primary prevention of cardiovascular diseases by having a direct effect on one of the contributors of vascular cell aging. Due to the first interesting results of this pre-study, new protocols evaluating the effectiveness of the “long-life” telomerase activator on telomere size and cardiovascular impact, are under way in randomized studies, versus placebo.
- Blasco AB (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6: 611-622. Link: https://bit.ly/3apuO8R
- Chatterjee S (2017) Telomeres in health and disease. J Oral Maxillofac Pathol 21: 87-91. Link: https://bit.ly/3pkDKAJ
- D’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, et al. (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198. Link: https://bit.ly/2Nticoj
- Blasco M (2007) Telomere length, stem cells and aging. Nat Chem Biol 3: 640-649. Link: https://bit.ly/3s2PN7v
- Nittis T, Guittat L, Stewart SA (2008) Alternative lengthening of telomeres (ALT) and chromatin: is there a connection ? Biochimie 90: 5–12. Link: https://bit.ly/3anPBcK
- Wolkowitz OM, Epel ES, Mellon S (2008) When blue turns to grey: do stress and depression accelerate cell aging? World J Biol Psychiatry 9: 2-5. Link: https://bit.ly/2Zykqpf
- Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88: 557-79. Link: https://bit.ly/3amRGFW
- Fuster JJ, Adres V (2006) Telomere biology and cardiovascular disease. Circ Res 99: 1167-1180. Link: https://bit.ly/3amRKpa
- Toupance S, Labat C, Temmar M, Rossignol P, Kimura M, et al. (2017) Short Telomeres, but Not Telomere Attrition Rates, are Associated With Carotid Atherosclerosis. Hypertension 70: 420-425. Link: https://bit.ly/3rXed1Z
- McNally EJ, Luncsford PJ, Armanios M (2019) Long telomeres and cancer risk: the price of cellular immortality. J Clin Invest 129: 3474–3481. Link: https://bit.ly/3duvkob
- Aviv A (2012) Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res 730: 68–74. Link: https://bit.ly/3qoQDuF
- Cawthon RM, Smith K, O’brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361: 393-395. Link: https://bit.ly/3rXewd9
- Turner KJ, Vasu V, Griffin DK (2019) Telomere Biology and Human Phenotype. Cells 8: 73. Link: https://bit.ly/2ODy341
- Yu Y, Zhou L, Yang Y, Liu Y (2018) Cycloastragenol: An exciting novel candidate for age-associated diseases. Exp Ther Med 16: 2175-2182. Link: https://bit.ly/2Zn7FNT
- Harley CB, Liu W, Flom PL, Raffaele JM (2013) A natural product telomerase activator as part of a health maintenance program: metabolic and cardiovascular response. Rejuvenation Res 16: 386-395. Link: https://bit.ly/2N1e4fp
- de Jesus BB, Schneeberger K, Vera E, Tejera A, Harley CB, et al. (2011) The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10: 604-621. Link: https://bit.ly/3u63CUC
- Le Saux CJ, Davy P, Brampton C, Ahuja SS, Fauce S, et al. (2013) A novel telomerase activator suppresses lung damage in a murine model of idiopathic pulmonary fibrosis. PLoS One 8: e58423. Link: https://bit.ly/37AnsOb
- Salvador L, Singaravelu G, Harley CB, Flom P, Suram A, et al. (2016) A Natural Product Telomerase Activator Lengthens Telomeres in Humans: A Randomized, Double Blind, and Placebo Controlled Study. Rejuvenation Res 19: 478-484. Link: https://bit.ly/2LV5KgD
- de Pedro N, Díez M, García I, García J, et al. (2020) Analytical Validation of Telomere Analysis Technology® for the High-Throughput Analysis of Multiple Telomere-Associated Variables. Biological Procedures Online 22: 2. Link: https://bit.ly/3qucuRy
- Hedges D, Janis R, Mickelson S, Keith C, Bennett D, et al. (2016) P300 amplitude in Alzheimer Disease: a meta-analysis and meta-regression. Clin EEG Neurosci 47: 48-55. Link: https://bit.ly/3qriWZo
- Tsolaki AC, Kosmidou V, KomparsiarisI Y, Hadjileontiadis L, Adam A, et al. (2017) Brain source localization of MMN and P300 ERPs in mild cognitive impairment and Alzheimer disease : a high-density EEG approach. Neurobiol Aging 55: 190-201. Link: https://bit.ly/37jjfOz
- Cintra MTG, Avila RT, Soares TO, Cunha CLM, Silveira KD, et al. (2018) Increased N200 and P300 latencies in cognitively impaired elderly carrying ApoE ƹ-4 allele. Int J Geriatr Psychiatry 33: e221-e227. Link: https://bit.ly/3povxLN
- Darné B, Girerd X, Safar M, Cambien F, Guize L, et al. (1989) Pulsatile versus steady component of blood pressure. A cross-sectional and prospective analysis on cardiovascular mortality. Hypertension 13: 392-400. Link: https://bit.ly/3dpD0aS
- Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, et al. (2010) Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension 55: 1389-1397. Link: https://bit.ly/3alyCIf
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, et al. (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349: g4227. Link: https://bit.ly/3qvUG8Q
- D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, et al. (2015) Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet 8: 82-90. Link: https://bit.ly/2LX5hdO
- Xu X, Hu H, Lin Y, Huang F, Ji Y, et al. (2019) Differences in Leukocyte Telomere Length between Coronary Heart Disease and Normal Population: A Multipopulation Meta-Analysis. Biomed Res Int 2019: 5046867. Link: https://bit.ly/2OCNzgt
- Aviv A, Levy D (2012) Telomeres, atherosclerosis, and the hemothelium: the longer view. Annu Rev Med 63: 293-301. Link: https://bit.ly/3anQEJI
- Gielen M, Hageman GJ, Antoniou EE, Nordfjal Kl, Mangino M, et al. (2018) Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am J Clin Nutr 108: 453-475. Link: https://bit.ly/2OHOTyR
- Nordfjall K, Eliasson M, Stegmayr B, Melander O, Nilsson P, et al. (2008) Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 16: 2682-2689. Link: https://bit.ly/3qq9yFA
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, et al. (2005) Obesity, cigarette smoking, and telomere length in women. Lancet 366: 662-664. Link: https://bit.ly/3jQnpmd
- Morla M, Busquets X, Pons J Sauleda J, MacNee W, et al. (2006) Telomere shortening in smokers with and without COPD. Eur Respir J 27: 525-528. Link: https://bit.ly/2ZlQdJO
- Lung FW, Chen NC, Shu BC (2007) Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet 17: 195-199. Link: https://bit.ly/3pt4uim
- Tentolouris N, Nzietchueng R, Cattan V, Poitevin G, Lacolley P, et al. (2007) White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care 30: 2909-2915. Link: https://bit.ly/2OICdYw
- Lung FW, Ku CS, Kao WT (2008) Telomere length may be associated with hypertension. J Hum Hypertens 22: 230-232. Link: https://bit.ly/37fkvSM
- Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L (2017) Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget 8: 45008-45019. Link: https://bit.ly/2LT0lqb
- Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, et al. (2006) Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res 99: 156-164. Link: https://bit.ly/3po8ZuF
- Davies MJ,Woolf N, Rowels PM, Pepper J (1988) Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Br Heart J 60: 459-464. Link: https://bit.ly/3rYS7MA
- Burrig KF (1991) The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb 11: 1678-1689. Link: https://bit.ly/2NbHSFV
- Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, et al. (2004) Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24: 546-550. Link: https://bit.ly/2LSV6qw
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, et al. (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105: 1541-1544. Link: https://bit.ly/2N2IRZi
- Brouilette SW, Whittaker A, Stevens SE, van der Harst P, Goodall AH, et al. (2008) Telomere length is shorter in healthy offspring of subjects with coronary artery disease: support for the telomere hypothesis. Heart 94: 422-425. Link: https://bit.ly/3dhu2wD
- Goglin SE, Farzaneh-Far R, Epel ES, Lin J, Blackburn EH, et al. (2016) Change in Leukocyte Telomere Length Predicts Mortality in Patients with Stable Coronary Heart Disease from the Heart and Soul Study. Plos One 11: e0160748. Link: https://bit.ly/3rYSs1T
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, et al. (2011) Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469: 102-106. Link: https://go.nature.com/3u5IxcA
- Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, et al. (2010) The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One 5: e10837. Link: https://bit.ly/2OztzeI
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Shepherd J, et al. (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369: 107-114. Link: https://bit.ly/2NdfEuD
- Satoh M, Minami Y, Takahashi Y, Tabuchi T, Itoh T, et al. (2009) Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clin Sci (Lond) 116: 827-835. Link: https://bit.ly/2ZkUM78
- Werner C, Gensch C, Pöss J, Haendeler J, Böhm M, et al. (2011) Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis 216: 23-34. Link: https://bit.ly/3u2ibID
- Srinivas N, Rachakonda S, Kumar R (2020) Telomeres and Telomere Length: A General Overview. Cancers (Basel) 12: 558. Link: https://bit.ly/3b2XvHC
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
