Global Journal of Allergy
Relationship between gut microbiome and allergic asthma
Ryoji Hirota*
Cite this as
Hirota R (2022) Relationship between gut microbiome and allergic asthma. Glob J Allergy 8(1): 001-006. DOI: 10.17352/2455-8141.000025Copyright License
© 2022 Hirota R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Since the late 20th century, environmental exposure to endotoxins has been very low and type I allergic disease has increased. In addition, we have increased exposure to PM2.5 and other chemicals. Recently, there is concern that the daily use of hygiene products containing antimicrobial substances is associated with an increased prevalence of allergies. It has been noted that these antimicrobial substances may exacerbate allergies. In our study, we confirmed that intratracheal administration of aqueous mite solution as an inhaled antigen and antimicrobial substance as an aggravating chemical induced much stronger allergic bronchial asthma in mice than in mice that received intratracheal mite solution alone. Thus, allergies have been found to be exacerbated by simultaneous exposure to both environmental antigens and man-made chemicals (adjuvants). Next, we analyzed 16S rRNA of the gut microbiota of triclosan-treated mice that developed an allergy. The bacterial gene abundance of 16S rRNA of Deltaproteobacteria, Erysipelotrichi and Clostridia was increased in a dose-dependent manner in triclosan-treated mice, while Bacteroides were decreased in these mice. The composition of the gut microbiota was altered after triclosan treatment and correlated with the exacerbation of asthmatic disease in these mice.
In conclusion, because triclosan exacerbated the condition of allergic asthma in mice that inhaled mite antigens and were given triclosan to drink, and this condition was associated with an increase or decrease in certain bacteria in the gut, we suspect that if allergy sufferers continue to use triclosan, they will inhale dust mites and house dust on a daily basis, which may aggravate their allergy symptoms. Overall, the overuse of antimicrobials and preservatives in current daily life risks further increasing the number of allergic patients. The authors believe that it is time to rethink this lifestyle.
Introduction
The hygiene hypothesis and allergic diseases
Since the late 20th century, there has been an increase in type I allergic diseases involving IgE antibodies, such as atopic dermatitis, bronchial asthma, and hay fever. Since ancient times, humans have maintained a balance of health by living in harmony with various microorganisms, such as parasites and bacteria, that inhabit our living environment [1]. It has long been suggested that exposure to environmental microorganisms suppresses allergic diseases such as asthma, and this is known as the hygiene hypothesis. The hygiene hypothesis proposes that when we humans spend our neonatal and infant years in a hygienic environment, our immune systems are exposed to environmental antigens in an immature state without encountering environmental endotoxins, resulting in a predominance of the Th2-type immune system and an increased susceptibility to allergic diseases. Examples of this are the decrease in the prevalence of tuberculosis and the decrease in parasite carriers and the increase in the incidence of allergic diseases [1]. Epidemiological studies have also shown that the prevalence of hay fever (at ages 11 and 23) is lower with the number of older siblings at age 11 [2] and that the American Amish, who reject modern civilization, have about half (5%) the incidence of asthma as the same race of people living in urban Switzerland [3]. In addition, a significant decrease in allergic diseases has been observed in children born to pregnant mothers who work in the dairy industry, which is used as evidence to support the hygiene hypothesis [4].
Hay fever, also known as allergic rhinitis, is a common allergic condition characterized by symptoms such as sneezing, runny or stuffy nose, itchy eyes, nose, or throat, and fatigue. It is caused by an immune system response to allergens such as pollen, dust mites, and animal dander.
The prevalence of hay fever worldwide ranges from about 10% to 30% of the population [5]. Some Asian and European countries are known to have high prevalence rates of hay fever. In Japan, hay fever is a serious problem, with an estimated 29.8% to 49.2% of the population suffering from hay fever. During the season when hay fever symptoms worsen, many people take measures such as wearing masks [6].
Chemicals aggravate the allergic disease
Recently, there have been concerns about the aggravation of allergic reactions caused by the inhalation of Particulate Matter (PM2.5) from mainland China and Polycyclic Aromatic Hydrocarbons (PAHs) in diesel exhaust. PM2.5 is smaller than Suspended Particulate Matter (SPM: particles of 10 µm or less), for which environmental standards have been set and countermeasures taken [7]. It has also been found that inhaling PM2.5 and other chemicals attached to the particles can cause a narrowing of the airways and a decrease in heart rate as the parasympathetic nervous system, which regulates the respiratory and circulatory systems, becomes more dominant. There is also concern about the effects of diesel exhaust exposure on cardiac function [8].
In our study, we confirmed that intratracheal administration of aqueous mite solution as an inhaled antigen and diesel exhaust extracts as an aggravating chemical simultaneously induced much stronger allergic bronchial asthma in mice than in mice that received intratracheal mite solution alone [9]. Thus, allergies have been found to be exacerbated by simultaneous exposure to both environmental antigens and man-made chemicals (adjuvants).
Recent studies have suggested an association between changes in the gut microbiota and the exacerbation of chronic respiratory diseases [10]. In particular, the daily use of antimicrobial agents in hygiene products has been found to exacerbate the Th1/Th2 immune system [11]. Therefore, prolonged daily use may aggravate asthma. To avoid the deterioration of the Th1/Th2 balance, the intake of functional foods has been suggested as a fundamental way [12,13].
To prevent allergy
Based on these findings, the authors have been working on the prevention of allergic diseases through the intake of functional foods, believing that in order to prevent allergies, for which no basic treatment has been established, it is necessary to take preventive measures to avoid becoming allergic in the first place. For example, limonene, which is abundant in the rind of yuzu (Citrus junos ex. Tanaka), has been shown to be effective in reducing allergic bronchial asthma when inhaled daily in a mouse model of asthma [14]. In the limonene inhalation plus mite antigen sensitization group, IL-5, IL-13, eotaxin, MCP-1, and TGF-β1 levels in bronchoalveolar lavage fluid were reduced compared to the non-inhalation group (mite antigen sensitization only). Lung goblet cell hyperplasia, airway smooth muscle thickness, and airway fibrosis were also significantly reduced. In addition, airway resistance to acetylcholine (AHR) was significantly improved in limonene-treated mice. Other functional ingredients with anti-allergy activity include gallic acid in Goishi tea, a fermented tea produced in Kochi Prefecture [15] and daily consumption of beta-sitosterol in loquat seed tea [16], which has also been shown to reduce allergic bronchial asthma in a mouse model of asthma. It is expected that active consumption of such functional food ingredients will help people acquire a constitution that is less prone to allergies.
In addition, functional foods containing lactobacilli and bifidobacteria have recently been widely promoted in TV commercials and newspapers, claiming that they can reduce allergic diseases by regulating the intestinal environment [17-19]. It has also been found that Clostridium IV and Clostridium XIVa groups, which are representative intestinal bacteria, promote the production of TGF-β from colonic epithelial cells and induce the differentiation of helios-negative induced Treg (iTreg) and act in an allergy-suppressing manner [20]. These findings suggest that the gut microbiome plays an important role in allergy suppression.
The impact of antimicrobials and preservatives on the gut microbiome
We use a variety of hygiene products on a daily basis, including medicated soaps, gargles, dishwashing detergents, mouthwashes, toothpaste, hand sanitizers, and cosmetics containing antibacterial and antiseptic agents. These products have become indispensable for us to live hygienically in our modern society. However, we wonder if the antimicrobial agents and preservatives we use, when absorbed into the body through the skin or oral cavity, may disrupt the balance of the gut microbiome and kill beneficial bacteria, leading to an increase in allergic diseases.
With this concern in mind, the authors examine the association between the frequency of use of the antimicrobial triclosan and the antiseptic parabens and the prevalence of allergies. In particular, triclosan is known from animal studies to be an endocrine disruptor that can affect the thyroid, the female hormone estrogen, and the male hormone testosterone [21-23] and it has also been suggested that it may increase resistance to antibiotics. Furthermore, because medicated soaps do not have superior bactericidal efficacy compared to regular soaps, in September 2017, the US Food and Drug Administration (FDA) banned the sale of antibacterial soaps for general use that contain 19 different bactericides, including triclosan [24]. However, this action does not apply to commercial hygiene products.
Adjuvant effect of triclosan to exacerbate allergy
Triclosan is still used despite the fact that its harmful effects on the human body have been pointed out, but what kind of negative effects does it have when used by people prone to allergies? This has not been clear.
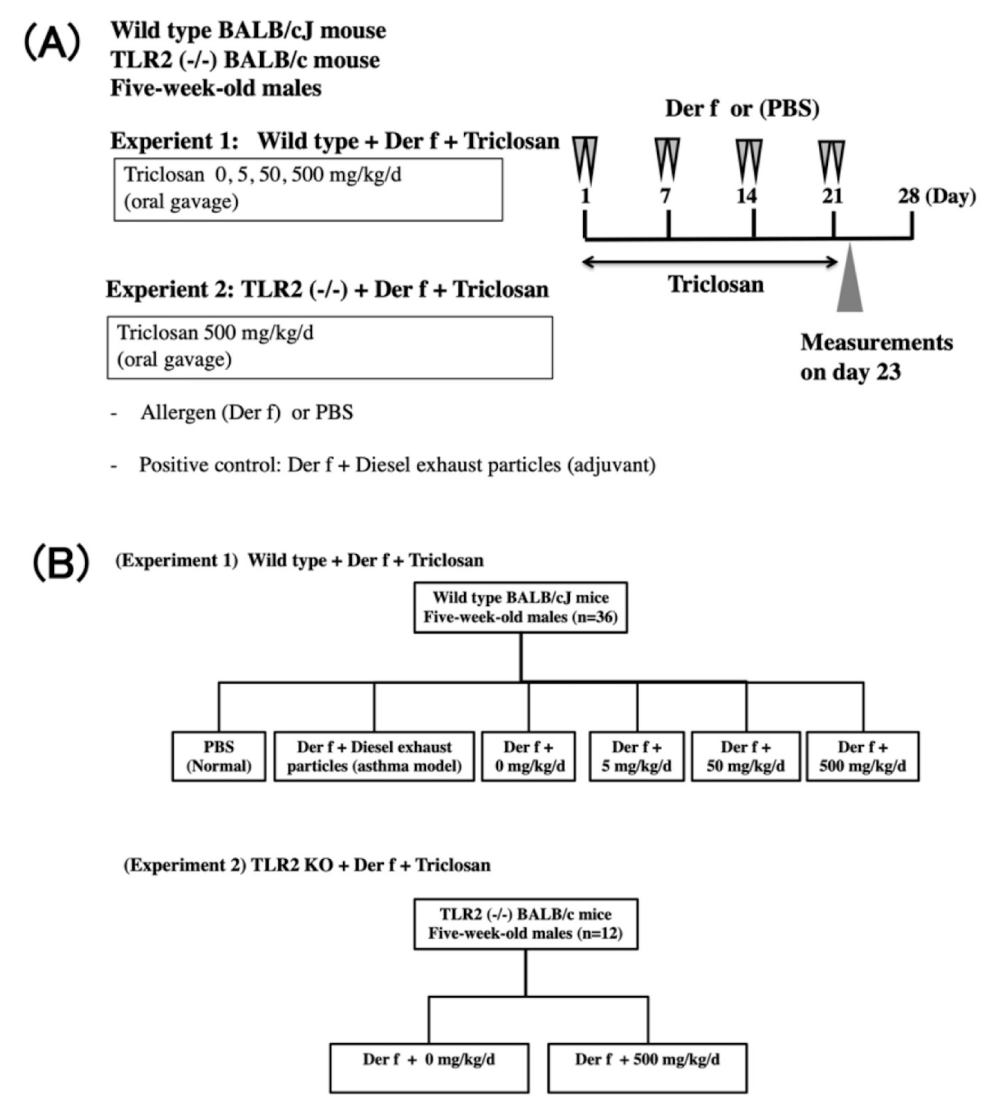
The authors’ group injected mite antigens into the trachea to induce allergic bronchial asthma in wild-type mice (presumed to be allergic) and gave them the antibacterial agent triclosan to compare with mice that were not given triclosan [25] (protocol: Figure 1A,1B) (result; Figure 2A). All of the experimental procedures were performed in accordance with the guidelines of the Laboratory Animal Research Kochi Medical School and were approved by the Institutional Animal Care and Use Committee of Kochi Medical School (J-0058, 28-0039). Triclosan-exposed mice received triclosan solution and were actively challenged with intratracheal instillation of Dermatophagoides Farinaeplus (Der f) on days 1-2-7-8-14-15-21-22, for a total of eight times. On day 23, we measured airway hyperresponsiveness (AHR) to acetylcholine under anesthesia and collected blood, Bronchoalveolar Lavage Fluid (BALF), lung tissue, and fecal samples. We measured serum allergen-specific IgE levels and BALF cytokine levels by ELISA, inflammatory cell counts in BALF, and histopathology of lung tissue. DNA from fecal pellets was amplified using 515 F and 806R primers targeting the V4 regions of bacterial 16S rRNA. The 16S rRNA gene was sequenced using Illumina MiSeq (500 cycles v2 kit), paired reads were assembled using Geneious software (Biomatters, Auckland, New Zealand) and taxonomic unit (OTU) selection and diversity analysis were performed using Quantitative Insights into Microbial Ecology (QIIME) software.
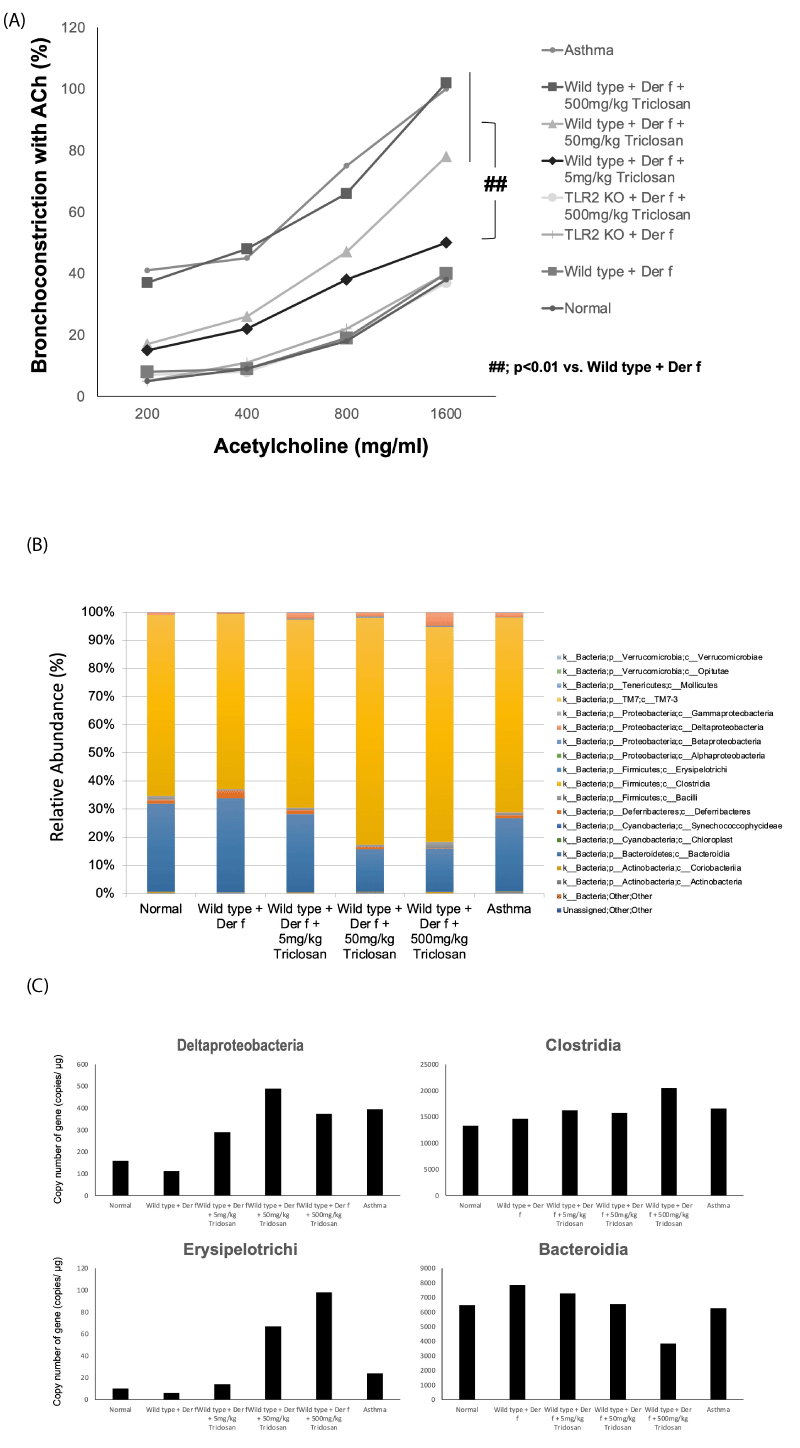
As a result, wild-type mice exposed to allergens and triclosan clearly exhibited the characteristics of airway asthma: exacerbated airway hyperresponsiveness, accumulation of eosinophils in the lungs, increased levels of Th2-type cytokines (IL-4 and IL-13) in lung lavage fluid, and increased serum allergen-specific IgE antibodies. In addition, increased collagen and eosinophils in lung tissue and increased goblet cell hyperplasia were observed. On the other hand, Toll-like receptor 2-deficient mice, which are genetically unresponsive to tick antigens (assuming they are not allergic), showed no asthmatic features at all when fed both tick antigens and triclosan.
Next, we analyzed bacterial genes in the feces of each experimental group of mice and found that in mice fed both tick antigen and triclosan, the copy numbers of bacterial 16S rRNA gene of c_Deltaproteobacteria, c_Clostridia and c_Erysipelotrichi in TCS-treated Der f-sensitized mice increased in a dose-dependent manner, whereas it markedly decreased in c_Bacteroidia (Figure 2B,2C).
A recent study concluded that fecal samples from triclosan-exposed mice showed changes in the composition of the gut microbiota characterized by an increase in harmful bacteria, including sulfate-reducing bacteria and Bacteroides, and a decrease in protective probiotics, butyrate-producing bacteria [26].
The role of regulatory T cells (Treg) in suppressing allergic responses is becoming better understood. Recent studies have reported that the Clostridium genus induces the accumulation of induced Treg cells in the intestinal tract of mice, and Clostridium butyricum infection activates TGF-β through the Smad pathway [27], suggesting that Clostridium suppresses allergic disease. In the present study, Clostridium was not classified in the samples, and there was no difference in the percentage of peripheral blood Treg cells between samples. Therefore, it was not possible to determine whether triclosan exposure reduced the number of Treg cells.
The authors’ data showed that the bacterial gene abundance of 16S RNA of Deltaproteobacteria, Erysipelotrichi, and Clostridia was increased in a dose-dependent manner in triclosan-treated mice, while Bacteroidia was decreased in these mice. The composition of the gut microbiota was altered after triclosan treatment and correlated with the exacerbation of asthmatic disease in these mice. These data suggest a potential relationship between triclosan-treated allergen-sensitized mice and the gut microbiota.
As noted above, certain species of Clostridium and Lactobacillus have been implicated in regulating the gut environment and suppressing allergies [17,18,28,29]. Whether the gut microbiota in this study is directly involved in allergy suppression needs to be clarified in future studies.
In conclusion, because triclosan exacerbated the condition of allergic asthma in mice that inhaled mite antigens and were given triclosan to drink, and this condition was associated with an increase or decrease in certain bacteria in the gut, the author suspects that if allergy sufferers continue to use triclosan, they will inhale dust mites and house dust on a daily basis, which may aggravate their allergy symptoms.
A recent study concluded the effect of early life exposure to antimicrobial agents on the risk of asthma and eczema in children [30,31].
Therefore, the authors believe that the use of antimicrobials in daily life should be risk managed by balancing the benefits of disinfection with the disadvantages of allergy induction.
Conclusion
In a recent epidemiologic study, the authors found that the frequency of paraben use was significantly higher in allergic patients, raising concerns about the negative effects of parabens on the gut microbiota [32]. To understand this mechanism, we administered parabens to mice and confirmed that parabens, like triclosan, have an exacerbating effect on allergies. Thus, the microbiome and asthma were found to be closely linked, suggesting that proper maintenance and management of the microbiome may lead to asthma prevention and treatment. According to the authors and their research team, the overuse of antimicrobials and preservatives in current daily life risks further increasing the number of allergic patients. The authors believe it is time to rethink this lifestyle.
- Fujita K. Why have allergic diseases increased? An examination of the merits and demerits of cleanliness (in Japanese). The Japanese Association of Rural Medicine. 2015; 63(6):910-3.
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989 Nov 18;299(6710):1259-60. doi: 10.1136/bmj.299.6710.1259. PMID: 2513902; PMCID: PMC1838109.
- Tantoco JC, Elliott Bontrager J, Zhao Q, DeLine J, Seroogy CM. The Amish have decreased asthma and allergic diseases compared with old order Mennonites. Ann Allergy Asthma Immunol. 2018 Aug;121(2):252-253.e1. doi: 10.1016/j.anai.2018.05.016. Epub 2018 May 23. PMID: 29802980; PMCID: PMC7744242.
- Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. 2008;32(3):603-11.
- Pawankar R, Canonica GW, Holgate ST, Lockey RF. World Allergy Organization (WAO) white book on allergy: update 2013. . Milwaukee, WI: World Allergy Organization, 2013 https://wwwworldallergyorg/UserFiles/file/WhiteBook2-2013-v8pdf. 2013.
- Okano M, Fujieda S, Gotoh M, Kurono Y, Matsubara A, Ohta N, Kamijo A, Yamada T, Nakamaru Y, Asako M, Sakurai D, Terada T, Yonekura S, Sakashita M, Okubo K. Executive summary: Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2023 Jan;72(1):41-53. doi: 10.1016/j.alit.2022.11.003. Epub 2022 Dec 9. PMID: 36509676.
- Ministry of the Environment Japan. Information on fine particulate matter (PM2.5) (in Japanese). 2018 http://www.env.go.jp/air/osen/pm/info.html#ABOUT.
- Bourdrel T, Bind MA, Béjot Y, Morel O, Argacha JF. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. 2017 Nov;110(11):634-642. doi: 10.1016/j.acvd.2017.05.003. Epub 2017 Jul 21. PMID: 28735838; PMCID: PMC5963518.
- Hirota R, Kang Y, Nakamura H, Uesaka S, Sakurai K, Narongpon D. The new materials for the filter to prevent allergic asthma caused by diesel exhaust: amorphous iron hydroxide and activated carbon. J Prev Med. 2012; 7:95-102.
- Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014 Aug 23;384(9944):691-702. doi: 10.1016/S0140-6736(14)61136-3. PMID: 25152271; PMCID: PMC4166502.
- Spanier AJ, Fausnight T, Camacho TF, Braun JM. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc. 2014 Nov-Dec;35(6):475-81. doi: 10.2500/aap.2014.35.3803. PMID: 25584915; PMCID: PMC5554376.
- Hirota R, Nakamura H, Bhatti SA, Ngatu NR, Muzembo BA, Dumavibhat N, Eitoku M, Sawamura M, Suganuma N. Limonene inhalation reduces allergic airway inflammation in Dermatophagoides farinae-treated mice. Inhal Toxicol. 2012 May;24(6):373-81. doi: 10.3109/08958378.2012.675528. PMID: 22564095.
- Hirota R, Ngatu NR, Miyamura M, Nakamura H, Suganuma N. Goishi tea consumption inhibits airway hyperresponsiveness in BALB/c mice. BMC Immunol 3594. 2011; 12:45.
- Hirota R, Nakamura H, Bhatti SA, Ngatu NR, Muzembo BA, Dumavibhat N, Eitoku M, Sawamura M, Suganuma N. Limonene inhalation reduces allergic airway inflammation in Dermatophagoides farinae-treated mice. Inhal Toxicol. 2012 May;24(6):373-81. doi: 10.3109/08958378.2012.675528. PMID: 22564095.
- Hirota R, Ngatu NR, Miyamura M, Nakamura H, Suganuma N. Goishi tea consumption inhibits airway hyperresponsiveness in BALB/c mice. BMC Immunol. 2011 Aug 11;12:45. doi: 10.1186/1471-2172-12-45. PMID: 21831323; PMCID: PMC3173379.
- Hirota R, Muzembo BA, Suganuma N. Japanese Loquat (Eriobotrya Japonica) Seed Extract, a Rich Source of Beta-Sitosterol Inhibits Airway Hyperresponsiveness in BALB/C Mice. International Journal of Research Studies in Biosciences 2015; 3(3):43-52.
- Tamura M, Shikina T, Morihana T, Hayama M, Kajimoto O, Sakamoto A, Kajimoto Y, Watanabe O, Nonaka C, Shida K, Nanno M. Effects of probiotics on allergic rhinitis induced by Japanese cedar pollen: randomized double-blind, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2007;143(1):75-82. doi: 10.1159/000098318. Epub 2006 Dec 29. PMID: 17199093.
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of Action of Probiotics. Adv Nutr. 2019 Jan 1;10(suppl_1):S49-S66. doi: 10.1093/advances/nmy063. Erratum in: Adv Nutr. 2020 Jul 1;11(4):1054. PMID: 30721959; PMCID: PMC6363529.
- Uwaezuoke SN, Ayuk AC, Eze JN, Odimegwu CL, Ndiokwelu CO, Eze IC. Postnatal probiotic supplementation can prevent and optimize treatment of childhood asthma and atopic disorders: A systematic review of randomized controlled trials. Front Pediatr. 2022 Aug 19;10:956141. doi: 10.3389/fped.2022.956141. PMID: 36061384; PMCID: PMC9437454.
- Atarashi K, Tanoue T, Umesaki Y, Honda K. Regulation of Th17 cell differentiation by intestinal commensal bacteria. Benef Microbes. 2010; 1(4):327-34.
- Harada N, Atarashi K, Murata Y, Yamaji R, Nakano Y, Inui H. Inhibitory mechanisms of the transcriptional activity of androgen receptor by resveratrol: Implication of DNA binding and acetylation of the receptor. J Steroid Biochem Mol Biol. 2011 Jan;123(1-2):65-70. doi: 10.1016/j.jsbmb.2010.11.002. Epub 2010 Nov 10. PMID: 21073951.
- Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003-2006. Environ Health Perspect. 2011 Mar;119(3):390-6. doi: 10.1289/ehp.1002883. Epub 2010 Nov 29. PMID: 21062687; PMCID: PMC3060004.
- Bergstrom KG. Update on antibacterial soaps: the FDA takes a second look at triclosans. J Drugs Dermatol. 2014 Apr;13(4):501-3. PMID: 24719072.
- U.S.Food & Drug Administration. FDA issues final rule on safety and effectiveness of antibacterial soaps. FDA news release. 2016;September 02, 2016.
- Hirota R, Ohya Y, Yamamoto-Hanada K, Fukutomi Y, Muto G, Ngatu NR, Nakamura T, Nakamura H. Triclosan-induced alteration of gut microbiome and aggravation of asthmatic airway response in aeroallergen-sensitized mice. Allergy. 2019 May;74(5):996-999. doi: 10.1111/all.13639. Epub 2019 Feb 1. PMID: 30353933; PMCID: PMC6590208.
- Liu J, Tao Y, Haikun W, Lanfang Y, Jingyi L, Ping Y. Triclosan exposure induced disturbance of gut microbiota and exaggerated experimental colitis in mice. BMC Gastroenterol. 2022 Nov 18;22(1):469. doi: 10.1186/s12876-022-02527-z. PMID: 36401221; PMCID: PMC9675201.
- Kashiwagi I, Morita R, Schichita T, Komai K, Saeki K, Matsumoto M, Takeda K, Nomura M, Hayashi A, Kanai T, Yoshimura A. Smad2 and Smad3 Inversely Regulate TGF-β Autoinduction in Clostridium butyricum-Activated Dendritic Cells. Immunity. 2015 Jul 21;43(1):65-79. doi: 10.1016/j.immuni.2015.06.010. Epub 2015 Jun 30. PMID: 26141582.
- Li L, Fang Z, Liu X, Hu W, Lu W, Lee YK, Zhao J, Zhang H, Chen W. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS One. 2020 Apr 21;15(4):e0231865. doi: 10.1371/journal.pone.0231865. PMID: 32315360; PMCID: PMC7173794.
- Sadrifar S, Abbasi-Dokht T, Forouzandeh S, Malek F, Baharlou R. The impact of multistrains of probiotics on Th17-related cytokines in patients with asthma: a randomized, double-blind, placebo-controlled trial. J Asthma. 2022 Dec 13:1-10. doi: 10.1080/02770903.2022.2144353. Epub ahead of print. PMID: 36332136.
- Jackson-Browne MS, Henderson N, Patti M, Spanier A, Braun JM. The Impact of Early-Life Exposure to Antimicrobials on Asthma and Eczema Risk in Children. Curr Environ Health Rep. 2019 Dec;6(4):214-224. doi: 10.1007/s40572-019-00256-2. PMID: 31745828; PMCID: PMC6923583.
- Quirós-Alcalá L, Hansel NN, McCormack MC, Matsui EC. Paraben exposures and asthma-related outcomes among children from the US general population. J Allergy Clin Immunol. 2019 Mar;143(3):948-956.e4. doi: 10.1016/j.jaci.2018.08.021. Epub 2018 Sep 5. PMID: 30194988; PMCID: PMC6691726.
- Mitsui-Iwama M, Yamamoto-Hanada K, Fukutomi Y, Hirota R, Muto G, Nakamura T, Yoshikawa T, Nakamura H, Mikami M, Morioka I, Ohya Y. Exposure to paraben and triclosan and allergic diseases in Tokyo: A pilot cross-sectional study. Asia Pac Allergy. 2019 Jan 21;9(1):e5. doi: 10.5415/apallergy.2019.9.e5. Erratum in: Asia Pac Allergy. 2021 Oct 28;11(4):e47. PMID: 30740353; PMCID: PMC6365653.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
