Journal of Cardiovascular Medicine and Cardiology
Quantitative nuclear cardiology and assessment of areas under risk via adenosine stress protocol-A pictorial assay for cardiologists and cardiovascular surgeons during era of COVID
Nese İlgin Karabacak*, Edanur Tuncay Ibıs, Salih Demir, Merve Uluç Balım, Bariş Kücükali and Rıdvan Yalcın
Cite this as
Karabacak Nİ, Ibıs ET, Demir S, Balım MU, Kücükali B, et al. (2022) Quantitative nuclear cardiology and assessment of areas under risk via adenosine stress protocol-A pictorial assay for cardiologists and cardiovascular surgeons during era of COVID. J Cardiovasc Med Cardiol 9(4): 042-048. DOI: 10.17352/2455-2976.000187Copyright License
© 2022 Karabacak Nİ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Coronary Artery Disease (CAD) is one of the most important causes of mortality and morbidity in the world. Nevertheless, Myocardial Perfusion Scintigraphy (MPS) using Single-Photon Emission Computed Tomography (SPECT) with radiopharmaceuticals is still widely used for the non-invasive diagnosis of obstructive CAD and functional imaging of the myocardium at stress.
MPS provides comprehensive information on myocardial perfusion, and regional and global left ventricular function that provides incremental diagnostic and prognostic information. MPS evaluates regional myocardial perfusion as well as gives information about functional parameters such as Transient Ischemic Dilation (TID), the extent of perfusion defect, etc. [1]. A normal stress MPS with adequate stress indicates a very good prognosis, with an annual myocardial infarction or death rate of less than 1% - 2%. Ischemic perfusion abnormalities usually remain undetected during rest, while stenosis of 50% or more is reliably identified with MPS under maximal myocardial stress. That is why MPS studies are performed with several stress test protocols. Exercise or pharmacological stress augments myocardial blood flow. Although with different mechanisms, myocardial blood flow in coronary vasculature without significant stenosis increases nearly 3-fold with exercise and 3 - to 5- fold with vasodilator stressors [2]. Exercise (treadmill or bicycle) is the preferred stress modality in patients who can exercise and achieve adequate exercise end-points. The most common mode of stress used in myocardial perfusion imaging is a multi-stage Exercise Treadmill Test (ETT) based on a Bruce or modified Bruce protocol. This pictorial review aims to focus on the specific exploration of the adenosine stress myocardial perfusion SPECT- a functional diagnostic tool for the screening and management of coronary artery disease.
For Myocardial Perfusion Imaging (MPI), the best test to evaluate hemodynamic changes during stress is an exercise treadmill test. It provides independent prognostic value, including evaluation of total exercise time, performance, and capacity; heart rate response during exercise, with ischemia and in recovery; blood pressure response; myocardial oxygen demand; and assessment of symptoms. Combining these exercise data with perfusion imaging provides the best prognostic value and risk stratification for patients. Although exercise stress testing accompanied by MPI is preferential, it is not always possible since an increasing number of patients cannot exercise to a maximal (symptom-limited) level. Further, there is much evidence in the literature demonstrating a suboptimal, non-symptom-limited (not achieving at least 4 min - 6 min or < 85% of maximum predicted heart rate) exercise test performed as part of an MPI study may result in a false-negative outcome. Therefore, pharmacologic stress agents provide an excellent alternative for those patients who cannot achieve an adequate heart rate response or adequately perform physical exercise.
Pharmacologic stress with adenosine, dobutamine and dipyridamole causes coronary hyperemia and increases myocardial workload allowing a successful myocardial perfusion study in patients who cannot perform or tolerate the adequate exercise, those with limited heart rate response due to β- blockers or calcium-channel blockers, those with a pacemaker rhythm, Wolff-Parkinson-White syndrome, a transient ventricular pacemaker or with left bundle-branch block. This option is suggested for particular patients in the guidelines. The sensitivity and specificity rates of MPS with pharmacological stress test study are reported to be comparable to that of maximal exercise studies, in the range of 85% to 90% [3]. A meta-analysis determined the sensitivity and specificity of adenosine SPECT imaging as 90% and 70%, respectively [4]. However, a treadmill exercise test is a primarily preferred method for MPS in most nuclear medicine clinics.
A treadmill test combined with Myocardial Perfusion Scintigraphy (MPS) is a commonly used technique in the assessment of coronary artery disease. There are many patients, however, who may not be able to undergo treadmill tests. Such patients would benefit from pharmacological stress procedures combined with MPS. The most commonly used pharmacological agents for cardiac stress are coronary vasodilators (adenosine, dipyridamol) and catecholamines.
Concomitant low-level treadmill exercise with adenosine pharmacologic stress (AdenoEX) during MPS has become commonly used in recent years. A number of studies have demonstrated the beneficial impact of the AdenoEX protocol.
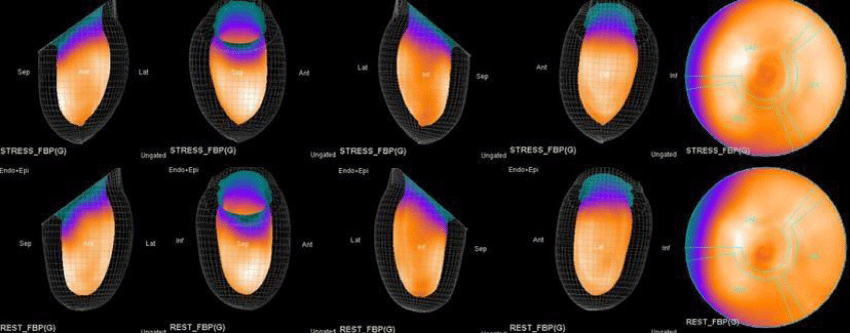
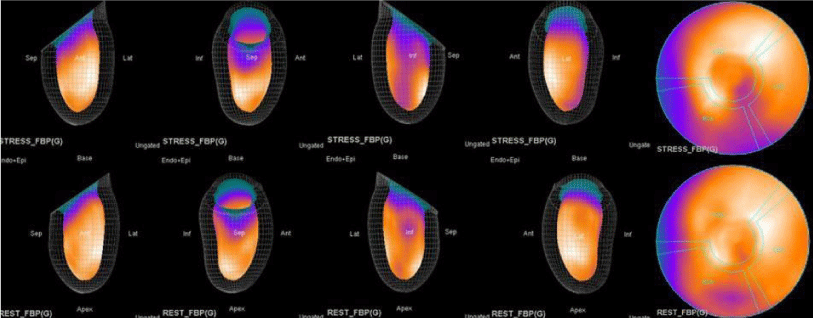
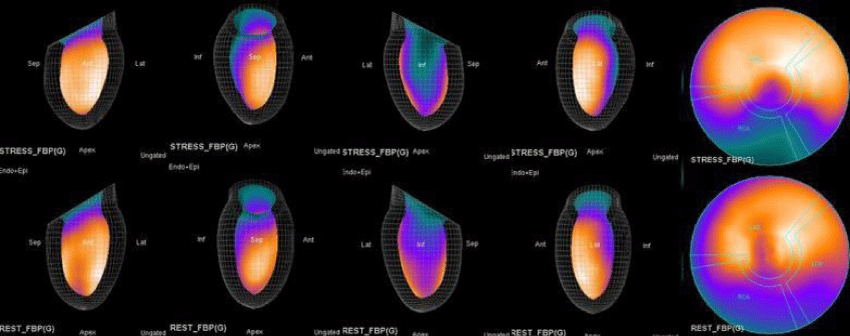
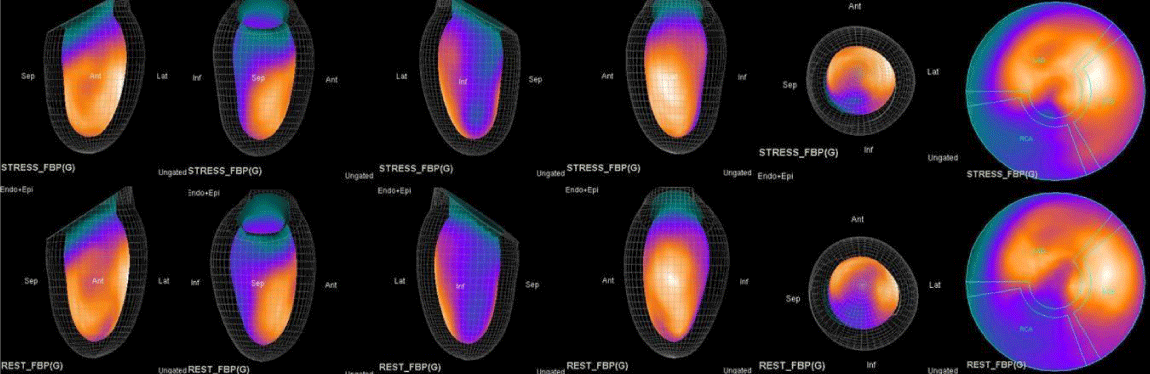
The main purpose of this case series was to establish and quantitate perfusıon defıcıt areas induced by adenosine infusion protocol during COVID pandemic era according to the established guidelines for stress MPS in our center for a population of 500 patients and compare the ^D perfusion areas to coronary angiographic data in selected cases. Our department previously used exercise for stress protocole and adenosine was only used for selected patients before. However, the COVID era changed our approach to routine adenosine stress protocole and this series identifies areas at risk ın demonstrative cases using quantitative analysis in 3D.
In general, SPECT studies are interpreted based on visual assessment of relative tracer uptake images. In clinical practice, imaging equipment, imaging protocols, stress protocols, reconstruction algorithm and filters, the patient’s body habitus, age and gender, artifacts from patient motion, display monitor, the physician’s vision and various other issues affect image evaluation by a nuclear medicine physician. However, automated analysis data from quantitative software tools can be used to assist visual analysis. Quantification is an extremely valuable tool in MPS, as it provides an objective assessment of the parameters under investigation.
Quantitative analysis of SPECT and PET has become a major part of nuclear cardiology practice. Current software tools can automatically segment the left ventricle, quantify function, establish myocardial perfusion maps and estimate global and local measures of stress/rest perfusion – all with minimal user input. State-of-the-art automated techniques have been shown to offer high diagnostic accuracy for detecting coronary artery disease, as well as predict prognostic outcomes.
Radionuclide Myocardial Perfusion Imaging (MPI) with SPECT or PET is the most widely used technique for detecting Coronary Artery Disease (CAD) in clinical practice.1 Currently, one of the main advantages of nuclear techniques over other modalities such as stress echocardiography or cardiac MRI, is the development of standardized methods for automated quantitation. Automated analysis of three-dimensional SPECT and PET images is now routine for both clinical and research purposes. Current software can automatically segment the left ventricle (LV), quantify Left Ventricular Ejection Fraction (LVEF), establish myocardial perfusion maps, and estimate global and local measures of stress/rest perfusion – all with minimal user input. These methods have demonstrated better reproducibility, and at least similar diagnostic accuracy as qualitative visual analysis by expert readers.
Gated Myocardial Perfusion Imaging (MPI) with SPECT or PET generates information on reversible perfusion defects, fixed perfusion defects, LV function, LV volumes, regional wall motion and thickening. Although visual interpretation for all these parameters is feasible, it is more time-consuming, less reproducible, and ultimately more dependent on the observer’s expertise than utilizing automated methods. It has been demonstrated that computer-based quantitation provides an important means of improving the consistency of interpretation [5]. A number of validated software packages are available for automated quantification (QPS-QGS, Emory Toolbox, 4D- MSPECT and Wackers-Liu CQ) [6–9] and are distributed by the main vendors of nuclear medicine imaging equipment. The basic principles are similar for each of these software packages: after segmentation of the LV, normalized relative radiotracer uptake in reconstructed slices is quantitatively compared against normal data files.
Both Attenuation-Corrected (AC) and non-attenuation-corrected (NC) images are obtained from SPECT/CT. MPS is currently interpreted as ischemia positive or negative according to the presence of a reversible perfusion defect. Alternatively, several scores obtained from scintigraphy images depict ischemia severity or extent, such as Summed Stress Score (SSS), Rest Score (SRS) and Differential Score (SDS); Total Perfusion Deficit (TPD); and extent of the defect [10-12]. While scores are calculated using automatic programs, each image requires comparison with its own normal data; therefore, scores from AC and NC images may differ.
Using the endocardial surfaces from LV segmentation, a volume curve spanning the cardiac cycle can be generated. From the volume curve data, LV End-Diastolic Volume (EDV), LV End-Systolic Volume (ESV), LV Ejection Fraction (LVEF), cardiac output, myocardial mass and diastolic function parameters (peak and time to peak filling and ejection rates) can then be calculated. Several studies confirm the strong agreement between gated MPI and reference standard measurements of quantitative LVEF and LV volumes [7,10-15]. This relationship is relatively independent of the isotope, protocol, standard, and algorithm used. Reproducibility and repeatability for LVEF and LV volumes have also been shown to be high [16,17] With regards to cross-algorithm reproducibility, a number of studies confirm a strong correlation between different approaches - but systematic differences in the measurements do exist and therefore normal limits for the specific imaging approach are required [15,18-20].
In this study, our first aim was to evaluate the diagnostic concordance and patterns of perfusion distribution observed in Myocardial Perfusion Scintigraphy (MPS) by Pharmacological Stress Test with adenosine (APST) with Coronary Angiography (CAG). Our second aim was to evaluate different patterns of perfusion for variants and anatomically rare defects. All patients underwent the two-day MPS protocol. A dose of ~20 - 30 mCi Tc - 99m sestamibi was injected intravenously for the stress study and ~20 mCi Tc-99m sestamibi was injected for the rest of the study. All data acquisition was performed with a double-head SPECT system (GE) equipped with a low-energy, high-resolution collimator. A protocol consisting of a 64x64 matrix, 30 projections per head, 25 - s projections over a 180° circular orbit and 8 frames per cycle was applied, with a 140 keV energy photopeak. A rotational arc of 180° was used, beginning at the 45° right anterior oblique position and ending at the 45° left posterior oblique position with 64 steps every 3° - 6°.
Image acquisition was done 15 - 30 minutes after ETT and 45 - 60 minutes after APST. The gated images were used to assess left ventricle volumes and EF. Gated data acquisition was done with 16 frames per cardiac cycle for the R-R interval length by using the forward-backward gating method.
According to the EANM guideline, the diagnostic performance of an MPS study is statistically independent of stress agents or modalities [20]. A meta-analysis that includes 24 studies and 14.918 patients showed that patients undergoing pharmacologic stress studies are at a higher risk for subsequent cardiac events like myocardial infarction and death from cardiac reasons [21]. In contrast, in their prospective study including 266 exercises (bicycle) stress testing and 65 APST, Hochgruber, et al. [22], stated that exercise stress but not adenosine stress results in an increase of cardiac wall stress, angina symptoms and ECG changes in patients with reversible ischemic changes on MPS. That is why the absence of these surrogates of myocardial ischemia suggests that adenosine stress does not induce acute myocardial ischemia, but rather displays relative perfusion differences [22]. According to American Heart Association data in a study on vasodilator stress in a cohort of 130 women who underwent APST imaging, there was a reported 91% sensitivity and 86% specificity for detecting significant coronary artery stenosis >50% [23]. Nevertheless, the same study reported that the sensitivity of MPS with ETT was 78-88% and the specificity was 64-91% [24,25].
Coronary Angiography (CAG) is the gold standard in diagnosing CAD. Using intracoronary pressure measurement as the reference standard, CT coronary angiography compared to invasive coronary angiography has a sensitivity of 80 % (95 % CI: 61 % to 92 %) versus 67 % (95 % CI: 51 % to 78 %), a specificity of 67 % (95 % CI: 47 % to 83 %) versus 75 % (95 % CI: 60 % to 86 %), an average positive likelihood ratio of 2.3 versus 2.6, and an average negative likelihood ratio 0.3 versus 0.4, respectively [26]. Compared to invasive coronary angiography, the average effective radiation dose of CT coronary angiography seems to be higher with retrospective electrocardiogram (ECG) gating and relatively similar to prospective ECG gating.
The health economic model using invasive coronary angiography as the reference standard showed that at a pretest probability of CHD of 50 % or lower, CT coronary angiography resulted in lower cost per patient with a true positive diagnosis. At a pretest probability of CHD of 70 % or higher, invasive coronary angiography was associated with a lower cost per patient with a true positive diagnosis. Using intracoronary pressure measurement as the reference standard, both types of coronary angiographies resulted in a substantially higher cost per patient with a true positive diagnosis.
A recent controlled clinical head-to-head comparative study revealed PET to exhibit the highest accuracy for the diagnosis of myocardial ischemia [27]. Furthermore, a combined anatomical and functional assessment does not add incremental diagnostic value but guides clinical decision-making in an unsalutary fashion. Both in PET and SPECT we observe quantıtatıvely the rea at rısk and significant stenosis in a coronary artery causes regional hypoperfusion in the myocardium supplied by that vessel. The greater the severity and extent of the perfusion defect in the myocardial segments supplied by any coronary artery observe that the more severe and more proximal the stenosis in the coronary artery.
Adenosine is an endogenous nucleoside that strongly impacts the cardiovascular system. Adenosine is released mostly by endothelial cells and myocytes during ischemia or hypoxia and greatly regulates the cardiovascular system via four specific G-protein-coupled receptors named A1R, A2AR, A2BR, and A3R. Among them, A2 subtypes are strongly expressed in coronary tissues and their activation increases coronary blood flow via the production of cAMP in smooth muscle cells. A2A receptor modulators are an opportunity for intense research by the pharmaceutical industry to develop new cardiovascular therapies. Most innovative therapies are mediated by the modulation of adenosine release and/or the activation of the A2A receptor subtypes.
Using the pharmacological activation stress model, we may discuss the phenomenon of -Use of Spare A2Rs Detection for the Diagnosis of Inducible Myocardial Ischemia in CAD Patients. The expression levels of adenosine A2Rs and their functional activity are therefore of paramount importance in coronary blood flow maintenance. In a previous review, Fenouillet, et al. described the concept of spare receptors where the receptor reserve concept was originally defined as the fraction of receptors not required to achieve maximal response by a full agonist [28,29]. The presence of spare receptors seems to be an adaptive mechanism to counterbalance a low level of agonist or a low level of receptors expression of both [30]. The spare receptor model can be designed as a signal amplification system in which the effectiveness of the response to different ligands, full or partial agonists, can be quite complex due to the dissociation between receptor activation level and biological effects. Intravenous adenosine infusion leads to increased myocardial blood flow in the normal myocardium but not the ischemic or infarcted segment. This difference in the resulting perfusion reactivity can be used to distinguish these two groups, as demonstrated previously in the proof-of-concept studies as well as the current validation study. While the blood flow increase distal to the stenotic coronary artery during adenosine stress is limited due to already compensated resistance vessels at rest, adenosine-induced vasodilation results in an increased and thus a greater chance of perfusion increase in the normal coronary artery territories.
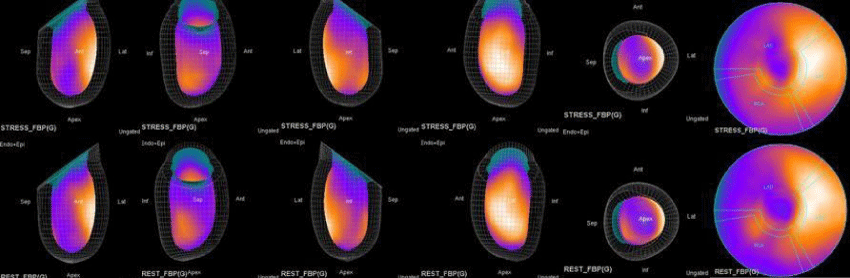
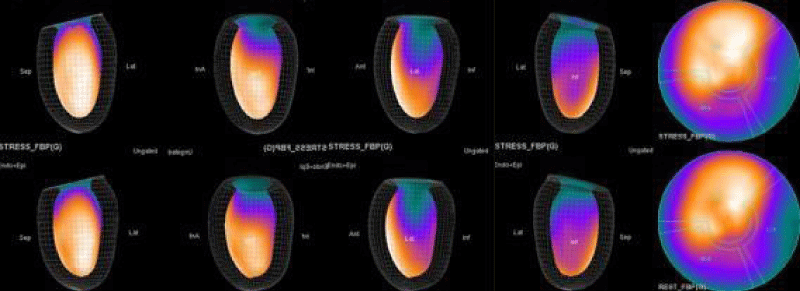
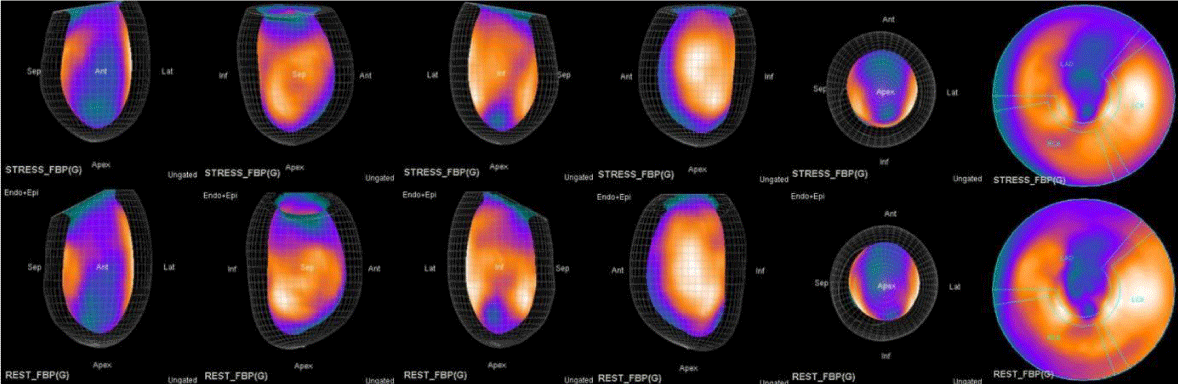
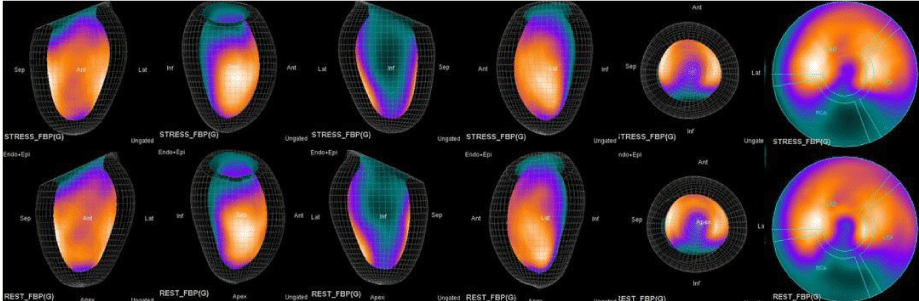
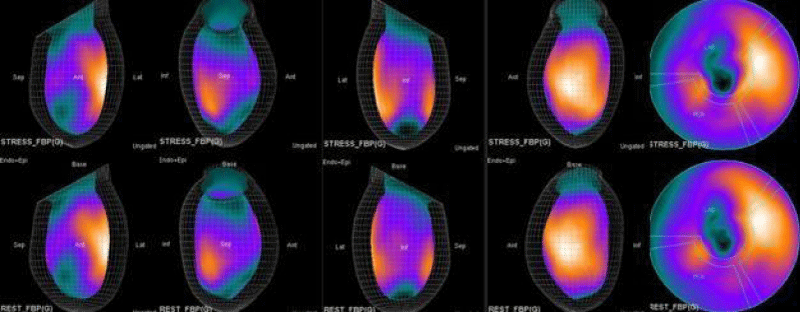
In summary, the approach of using adenosine stress and rest 3D mapping to differentiate myocardial tissue characteristics in CAD compared with coronary angiography in this subset of patients was re-validated in ischemic, infarcted and remote myocardium as well as cohort-matched controls where this pictorial series can be used as a reference for future trainees to benefit from the aggregated results of comparative anatomıc and functıonal methodology (Figures 1-10).
- Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009 Sep;2(5):412-24. doi: 10.1161/CIRCIMAGING.109.854893. PMID: 19808630.
- Xu Y, Kavanagh P, Fish M, Gerlach J, Ramesh A, Lemley M, Hayes S, Berman DS, Germano G, Slomka PJ. Automated quality control for segmentation of myocardial perfusion SPECT. J Nucl Med. 2009 Sep;50(9):1418-26. doi: 10.2967/jnumed.108.061333. Epub 2009 Aug 18. PMID: 19690019; PMCID: PMC2935909.
- Arsanjani R, Xu Y, Dey D, Vahistha V, Shalev A, Nakanishi R, Hayes S, Fish M, Berman D, Germano G, Slomka PJ. Improved accuracy of myocardial perfusion SPECT for detection of coronary artery disease by machine learning in a large population. J Nucl Cardiol. 2013 Aug;20(4):553-62. doi: 10.1007/s12350-013-9706-2. Epub 2013 May 24. PMID: 23703378; PMCID: PMC3732038.
- Arsanjani R, Dey D, Khachatryan T, Shalev A, Hayes SW, Fish M, Nakanishi R, Germano G, Berman DS, Slomka P. Prediction of revascularization after myocardial perfusion SPECT by machine learning in a large population. J Nucl Cardiol. 2015 Oct;22(5):877-84. doi: 10.1007/s12350-014-0027-x. Epub 2014 Dec 6. PMID: 25480110; PMCID: PMC4859156.
- Slomka P, Xu Y, Berman D, Germano G. Quantitative analysis of perfusion studies: strengths and pitfalls. J Nucl Cardiol. 2012 Apr;19(2):338-46. doi: 10.1007/s12350-011-9509-2. PMID: 22302181; PMCID: PMC3412547.
- Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: the Michigan method for quantitative nuclear cardiology. J Nucl Cardiol. 2007 Jul;14(4):455-65. doi: 10.1016/j.nuclcard.2007.06.006. PMID: 17679053.
- Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C. The increasing role of quantification in clinical nuclear cardiology: the Emory approach. J Nucl Cardiol. 2007 Jul;14(4):420-32. doi: 10.1016/j.nuclcard.2007.06.009. PMID: 17679051.
- Germano G, Kavanagh PB, Slomka PJ, Van Kriekinge SD, Pollard G, Berman DS. Quantitation in gated perfusion SPECT imaging: the Cedars-Sinai approach. J Nucl Cardiol. 2007 Jul;14(4):433-54. doi: 10.1016/j.nuclcard.2007.06.008. PMID: 17679052.
- Liu YH. Quantification of nuclear cardiac images: the Yale approach. J Nucl Cardiol. 2007 Jul;14(4):483-91. doi: 10.1016/j.nuclcard.2007.06.005. PMID: 17679055.
- Faber TL, Cooke CD, Folks RD, Vansant JP, Nichols KJ, DePuey EG, Pettigrew RI, Garcia EV. Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med. 1999 Apr;40(4):650-9. PMID: 10210225.
- Kumita S, Cho K, Nakajo H, Toba M, Uwamori M, Mizumura S, Kumazaki T, Sano J, Sakai S, Munakata K. Assessment of left ventricular diastolic function with electrocardiography-gated myocardial perfusion SPECT: comparison with multigated equilibrium radionuclide angiography. J Nucl Cardiol. 2001 Sep-Oct;8(5):568-74. doi: 10.1067/mnc.2001.116853. PMID: 11593221.
- Bax JJ, Lamb H, Dibbets P, Pelikan H, Boersma E, Viergever EP, Germano G, Vliegen HW, de Roos A, Pauwels EK, Van der Wall EE. Comparison of gated single-photon emission computed tomography with magnetic resonance imaging for evaluation of left ventricular function in ischemic cardiomyopathy. Am J Cardiol. 2000 Dec 15;86(12):1299-305. doi: 10.1016/s0002-9149(00)01231-5. PMID: 11113402.
- Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, Van Train KF, Berman DS. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995 Nov;36(11):2138-47. PMID: 7472611.
- Chua T, Yin LC, Thiang TH, Choo TB, Ping DZ, Leng LY. Accuracy of the automated assessment of left ventricular function with gated perfusion SPECT in the presence of perfusion defects and left ventricular dysfunction: correlation with equilibrium radionuclide ventriculography and echocardiography. J Nucl Cardiol. 2000 Jul-Aug;7(4):301-11. doi: 10.1067/mnc.2000.105279. PMID: 10958271.
- Faber TL, Vansant JP, Pettigrew RI, Galt JR, Blais M, Chatzimavroudis G, Cooke CD, Folks RD, Waldrop SM, Gurtler-Krawczynska E, Wittry MD, Garcia EV. Evaluation of left ventricular endocardial volumes and ejection fractions computed from gated perfusion SPECT with magnetic resonance imaging: comparison of two methods. J Nucl Cardiol. 2001 Nov-Dec;8(6):645-51. doi: 10.1067/mnc.2001.117173. PMID: 11725260.
- Nakajima K, Nishimura T. Inter-institution preference-based variability of ejection fraction and volumes using quantitative gated SPECT with 99mTc-tetrofosmin: a multicentre study involving 106 hospitals. Eur J Nucl Med Mol Imaging. 2006 Feb;33(2):127-33. doi: 10.1007/s00259-005-1916-7. Epub 2005 Sep 29. PMID: 16193310.
- Hyun IY, Kwan J, Park KS, Lee WH. Reproducibility of Tl-201 and Tc-99m sestamibi gated myocardial perfusion SPECT measurement of myocardial function. J Nucl Cardiol. 2001 Mar-Apr;8(2):182-7. doi: 10.1067/mnc.2001.112753. PMID: 11295696.
- Nichols K, Santana CA, Folks R, Krawczynska E, Cooke CD, Faber TL, Bergmann SR, Garcia EV. Comparison between ECTb and QGS for assessment of left ventricular function from gated myocardial perfusion SPECT. J Nucl Cardiol. 2002 May-Jun;9(3):285-93. doi: 10.1067/mnc.2002.121449. PMID: 12032476.
- Nakajima K, Higuchi T, Taki J, Kawano M, Tonami N. Accuracy of ventricular volume and ejection fraction measured by gated myocardial SPECT: comparison of 4 software programs. J Nucl Med. 2001 Oct;42(10):1571-8. PMID: 11585875.
- Verberne HJ, Acampa W, Anagnostopoulos C, Ballinger J, Bengel F, De Bondt P, Buechel RR, Cuocolo A, van Eck-Smit BL, Flotats A, Hacker M, Hindorf C, Kaufmann PA, Lindner O, Ljungberg M, Lonsdale M, Manrique A, Minarik D, Scholte AJ, Slart RH, Trägårdh E, de Wit TC, Hesse B; European Association of Nuclear Medicine (EANM). EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur J Nucl Med Mol Imaging. 2015 Nov;42(12):1929-40. doi: 10.1007/s00259-015-3139-x. Epub 2015 Aug 21. PMID: 26290421; PMCID: PMC4589547.
- Navare SM, Mather JF, Shaw LJ, Fowler MS, Heller GV. Comparison of risk stratification with pharmacologic and exercise stress myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2004 Sep-Oct;11(5):551-61. doi: 10.1016/j.nuclcard.2004.06.128. PMID: 15472640.
- Hochgruber T, Reichlin T, Wasila M, Vogler E, Twerenbold R, Sou SM, Roost K, Lee G, Fischer A, Freidank H, Osswald S, Zellweger MJ, Mueller C. Novel insights into the pathophysiology of different forms of stress testing. Clin Biochem. 2014 Apr;47(6):338-43. doi: 10.1016/j.clinbiochem.2014.02.018. Epub 2014 Feb 25. PMID: 24582696.
- Abu Daya H, Hage FG. Guidelines in review: ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease. J Nucl Cardiol. 2017 Oct;24(5):1793-1799. doi: 10.1007/s12350-017-1017-6. Epub 2017 Aug 23. PMID: 28836156.
- Mieres JH, Shaw LJ, Arai A, Budoff MJ, Flamm SD, Hundley WG, Marwick TH, Mosca L, Patel AR, Quinones MA, Redberg RF, Taubert KA, Taylor AJ, Thomas GS, Wenger NK; Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005 Feb 8;111(5):682-96. doi: 10.1161/01.CIR.0000155233.67287.60. Epub 2005 Feb 1. PMID: 15687114.
- Shaw LJ, Mieres JH, Hendel RH, Boden WE, Gulati M, Veledar E, Hachamovitch R, Arrighi JA, Merz CN, Gibbons RJ, Wenger NK, Heller GV; WOMEN Trial Investigators. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation. 2011 Sep 13;124(11):1239-49. doi: 10.1161/CIRCULATIONAHA.111.029660. Epub 2011 Aug 15. PMID: 21844080.
- Gorenoi V, Schönermark MP, Hagen A. CT coronary angiography vs. invasive coronary angiography in CHD. GMS Health Technol Assess. 2012;8:Doc02. doi: 10.3205/hta000100. Epub 2012 Apr 16. PMID: 22536300; PMCID: PMC3334923.
- Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, Knuuti J, Mäki M, Underwood RS, Min JK, Elmore K, Stuijfzand WJ, van Royen N, Tulevski II, Somsen AG, Huisman MC, van Lingen AA, Heymans MW, van de Ven PM, van Kuijk C, Lammertsma AA, van Rossum AC, Knaapen P. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017 Oct 1;2(10):1100-1107. doi: 10.1001/jamacardio.2017.2471. PMID: 28813561; PMCID: PMC5710451.
- Fenouillet E, Mottola G, Kipson N, Paganelli F, Guieu R, Ruf J. Adenosine Receptor Profiling Reveals an Association between the Presence of Spare Receptors and Cardiovascular Disorders. Int J Mol Sci. 2019 Nov 27;20(23):5964. doi: 10.3390/ijms20235964. PMID: 31783510; PMCID: PMC6928742.
- Bahreyni A, Avan A, Shabani M, Ryzhikov M, Fiuji H, Soleimanpour S, Khazaei M, Hassanian SM. Therapeutic potential of A2 adenosine receptor pharmacological regulators in the treatment of cardiovascular diseases, recent progress, and prospective. J Cell Physiol. 2019 Feb;234(2):1295-1299. doi: 10.1002/jcp.27161. Epub 2018 Aug 26. PMID: 30146778.
- 30. Peleli M, Fredholm BB, Sobrevia L, Carlström M. Pharmacological targeting of adenosine receptor signaling. Mol Aspects Med. 2017 Jun;55:4-8. doi: 10.1016/j.mam.2016.12.002. Epub 2017 Jan 12. PMID: 28088486.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
