Open Journal of Orthopedics and Rheumatology
Prospective Evaluation of Surgery vs. Low Dose External Beam Radiotherapy for Painful Thumb Carpometacarpal Osteoarthritis – A Study Protocol
Mike Rüttermann1,2, Alexander Kaltenborn3,4, Annika Trillmann3, Andre Gutcke3 and Robert Michael Hermann5,6*
2Plastic and Reconstructive Surgery, University Medical Center, Groningen, Netherlands
3Department of Hand, Trauma and Orthopedic Surgery, Federal Armed Forces Hospital Westerstede, Westerstede, Germany
4Core Facility Quality Management and Health Technology Assessment in Transplantation, Integrated Research and Treatment Center Transplantation (IFB-Tx), Hannover Medical School, Hannover, Germany
5Center for Radiotherapy and Radiooncology Bremen and Westerstede, Westerstede, Germany
6Department of Radiotherapy and Special Oncology, Hannover Medical School, Hannover, Germany
Cite this as
Rüttermann M, Kaltenborn A, Trillmann A, Gutcke A, Hermann RM (2016) Prospective Evaluation of Surgery vs. Low Dose External Beam Radiotherapy for Painful Thumb Carpometacarpal Osteoarthritis – A Study Protocol. Open J Orthop Rheumatol 1(1): 015-017. DOI: 10.17352/ojor.000004We report the protocol of a prospective observational study on surgical and conservative therapies for painful thumb carpometacarpal (CMC) osteoarthritis.
150 consecutive patients who are referred to our centers for surgery or LD-EBRT of CMC arthrosis will be offered the participation in this trial.
Four examinations are planned: 1) baseline (after given informed consent, before treatment), 2) at three months, 3) at 12 months, and 4) at 24 months after therapy.
Primary endpoint is symptom reduction by means of amelioration of pain (measured by visual analogue scale), improvement of motion (active range of motion, Kapandji-index), changes in grip strength (Jamar dynamometer, Pinch Gauge Meter), and subjective response to therapy (no change, partial remission, complete remission). Secondary endpoints are changes in Quality of Life measured by standardized questionnaires (DASH, PRWE). Further endpoints are the incidences of complications after therapy.
The aims of the proposed study are:
1) to provide evidence on the efficacy of LD-EBRT regarding pain reduction, of function, and of the quality of life after short- (3 months) and long-term (one and two years) follow-up. To our knowledge, this has never been done before.
2) to compare these results with the same endpoints in patients treated with surgery.
Abbreviations
AROM: Active Range of Motion; CMC: Carpometacarpal; DASH: Disabilities of Arm, Shoulder and Hand; LD-EBRT: Low Dose External Beam Radiotherapy; PRWE: Patient-Rated Wrist Evaluation; VAS: Visual Analogue Scale
We report the protocol of a prospective observational study on surgical and conservative therapies for painful thumb carpometacarpal osteoarthritis (in the following called „CMC arthrosis“).
Background
Osteoarthritis is one of the most common diseases in the elderly patient. The World Health Organizations expects osteoarthritis to become the fourth leading cause of severe disability by the year 2020 [1]. Degenerative joint disease of the hand is the third frequent localization of osteoarthritis [2]. Prevalence of CMC arthrosis is about 15% of the adult population, about one-third of the postmenopausal women are thought to suffer from it [3]. It is primarily affecting the quality of life since the hands represent the tools of daily living.
Only symptomatic therapies are available up to now. The backbone of conservative treatments are splinting, oral NSAID, steroids, or physiotherapeutic interventions. However, long-term use of NSAID is associated with gastrointestinal bleeding, kidney failure, or other side effects.
In patients suffering from persistent pain or functional impairment and who are not responding to the therapies mentioned above, surgery of the base of the thumb is indicated. Different surgical techniques have been established like trapeziectomies with or without ligament reconstruction with or without tendon interposition, or with interposition arthroplasty, arthrodesis, joint resurfacing or silicone spacer joint replacement. In a recent Cochrane review, insufficient evidence was found due to the low quality of most published randomized studies to define the most beneficial technique regarding pain reduction, functional outcome, or harms [4]. Post-surgical complications like weakness, restriction of motion, or pain syndrome have been reported in up to 20% of the patients after surgery [5]. In our clinics widely used surgical intervention for this indication are tapeziectomy alone or combined with tendon suspension, in primary cases generally without drilling through the first the metacarpal bone.
In Germany and Central Europe low-dose external beam radiotherapy (LD-EBRT) for painful degenerative diseases of the joints and tendons is widely adopted [6]. It has been established in the therapy of painful CMC arthrosis, too [7]. Protocols comprise an RT series of two to three fractions weekly with 0.5 – 1Gy single dose up to a total dose of 3 – 6Gy. The lifetime risk of a fatal radiation-induced cancer is negligible (lower than 0.3 per 1000) as most of the patients are older than 50 years [8]. In a collective of 84 patients (101 joints) treated with LD-EBRT, 70% reported amelioration of symptoms up to one year after therapy [7].
The aims of the proposed study are
1) to provide evidence on the efficacy of LD-EBRT regarding pain reduction, of function, and of the quality of life after short- (3 months) and long-term (one and two years) follow-up. To our knowledge, this has never been done before.
2) to compare these results with the same endpoints in patients treated with surgery.
Study type
The study is planned as an observational study. In our opinion randomization between the two therapies is not feasible, as most of the patients and the referring/treating physicians regard LD-EBRT not as an alternative to surgery, but as a treatment to delay a surgical intervention. Only in patients who are functional inoperable due to comorbidities or who are not willing to risk an operation LD-EBRT is offered as an alternative therapeutic option. This is why we will not be able to exclude bias in patient selection. However, will believe that the results will help to counsel patients on treatment efficacy and sequencing of different therapeutic options.
150 consecutive patients who are referred to our centers for surgery or LD-EBRT of CMC arthrosis will be offered the participation in this trial. We estimate the period to recruit the necessary number of patients over two years.
We suppose a response to therapy of about 70% [5,7] for both investigated treatments. The sample size calculations revealed a necessary minimum study population of 61 patients to be recruited for each therapy for a p-value pf 0.05 and 80% power, respectively (analysis with GPower 3.1 according to Faul [9]. To avoid effects of lose-to-follow-up a patient number of at least 75 patients per group is planned. Although the treatment groups are not randomized, we outlined the study in analogy according to the CONSORT – guidelines as a comparative non-inferiority trial [10].
Inclusion criteria
Patients suitable for the study must meet the following criteria: clinical symptomatic and radiological proof of CMC arthrosis, older than 39 years, and given informed consent for study participation. Exclusion criteria are age younger than 40 years, secondary arthrosis due to a trauma of the respective joints, multiple (more than 2) other joints of the affected hand with symptomatic degenerative changes, and patient not suitable for anaesthesia (regional as well as general).
Endpoints
Primary endpoint is symptom reduction by means of amelioration of pain (measured by visual analogue scale VAS), improvement of motion (active range of motion AROM, Kapandji-index), changes in grip strength (Jamar dynamometer, Pinch Gauge Meter), and subjective response to therapy (no change, partial remission, complete remission).
Secondary endpoints are changes in Quality of Life measured by standardized questionnaires (Disabilities of Arm, Shoulder and Hand DASH [11], and Patient-rated Wrist Evaluation PRWE [12]). Further endpoints are the incidences of complications after therapy.
Four examinations are planned: 1) baseline (after given informed consent, before treatment), 2) at three months, 3) at 12 months, and 4) at 24 months after therapy.
Study examinations
Baseline examination comprises the anamnesis with documentation of pain history, prior therapies, various risk factors and comorbidities, and of the working life of the patients.
At every time point VAS, AROM, Kapandji-index, grip strength, DASH, and PRWE are measured and documented. The subjective response to therapy, possible side-effects or toxicities, and the application of further therapies (no study interventions) are recorded at each time point after treatment (Figure 1).
Surgery
Surgery includes the resection of the trapzoid through a palmar incision, under protection of all small branches of the superficial radial nerve. After opening of the joint capsule the trapezoid as well as all exophytes are removed. A strip of approximately 50% of the APL or FCR tendon is dissected distally based and afterwards fixed to remnants of capsule and/or periosteum at the radial (APL) or ulnar (FCR) base of the first metarcapal in an abducted and opposed position. After irrigation a hemostasis the CMC I joint capsule en the skin are closed. A sterile dressing is applied followed by a cast dorsoradial to the thumb in abduction/opposition of the first metacarpal.
LD-EBRT
Patients who are scheduled for LD-EBRT will be treated twice weekly, with a single dose of 0.5Gy amounting to a total dose of 3Gy. This fractionation schedule has been shown equal efficacy with 6 x 1Gy in two randomized trials on calcaneodynia [13,14] and has been widely adopted since.
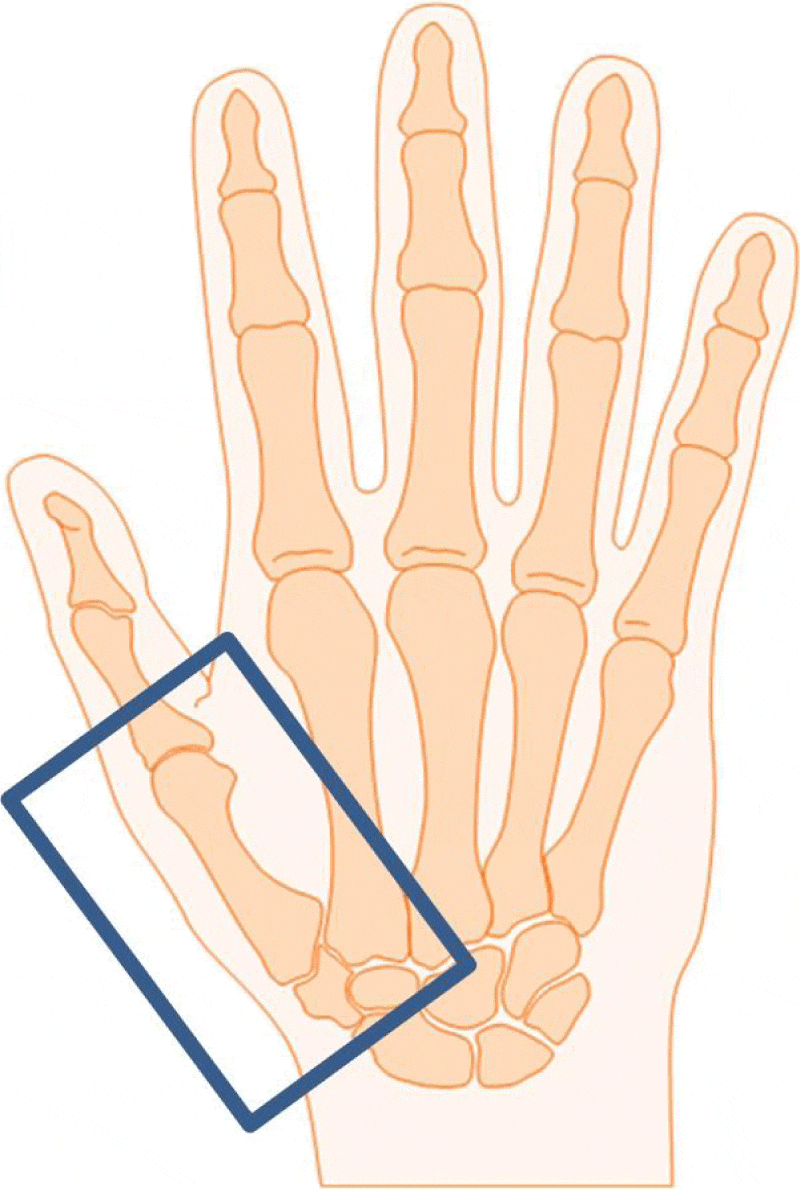
Treatment will be administered as 6MVX photons with a linear accelerator. The patient stands beside the treatment table and places the affected arm on this table. One stationary field ensuring a source–skin distance of 100cm will be irradiated from a 0° gantry position. The treatment portal will be defined according to the reported pain location with a field size of at least 6 x 4cm (Figure 2).
- Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81: 646–656. Link: https://goo.gl/FAaUtz
- Keller S, Müller K, Kortmann RD, Wolf U, Hildebrandt G, et al. (2013) Efficacy of low-dose radiotherapy in painful gonarthritis: experiences from a retrospective East German bicenter study. Radiat Oncol 8: 29. Link: https://goo.gl/HLTizb
- Berger AJ, Meals RA (2015) Management of Osteoarthrosis of the Thumb Joints. J Hand Surg Am 40: 843-850. Link: https://goo.gl/6SQ5oo
- Wajon A, Vinycomb T, Carr E, Edmunds I, Ada L (2015) Surgery for thumb (trapeziometacarpal joint) osteoarthritis. Cochrane Database Syst Rev 2:CD004631. Link: https://goo.gl/soHPjX
- Cook GS, Lalonde DH (2008) Management of Thumb Carpometacarpal Joint Arthritis. Plastic Recon Surg 121: 1-9.
- Hermann RM, Bulling E, Kaltenborn A, Carl UM, Nitsche M (2016) Efficacy of percutaneous high-voltage radiotherapy in painful degenerative diseases of the skeleton and tendons: clinical experiences in a general patient population. Nuklearmediziner 39: 57-63. Link: https://goo.gl/MYVFfI
- Kaltenborn A, Bulling E, Nitsche M, Carl UM, Hermann RM (2016) The field size matters: low dose external beam radiotherapy for thumb carpometacarpal osteoarthritis. Strahlenther Onkol 192: 582-588. Link: https://goo.gl/iZIu1L
- Jansen JT, Broerse JJ, Zoetelief J, Klein C, Seegenschmiedt HM (2005) Estimation of the carcinogenic risk of radiotherapy of benign diseases from shoulder to heel. Radiother Oncol 76: 270–277. Link: https://goo.gl/tgNPs0
- (13) Faul F, Erdfelder E, Lang A, Buchner A (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavioral Research Methods 2: 175-191. Link: https://goo.gl/YigW6P
- (14) Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group (2012) Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 308: 2594-2604. Link: https://goo.gl/hX5Vui
- Kennedy CA, Beaton DE, Solway S, McConnell S, Bombardier C (2011) Disabilities of Arm, Shoulder and Hand (DASH). The DASH and QuickDASH Outcome Measure User’s Manual, 3rdEdn. Toronto, Institute for Work & Health. Link: https://goo.gl/31GZoL
- Hemelaers L, Angst F, Drerup S, Simmen BR, Wood-Dauphinee S (2008) Reliability and validity of the German version of "the Patient-rated Wrist Evaluation (PRWE)" as an outcome measure of wrist pain and disability in patients with acute distal radius fractures. J Hand Ther 21: 366-376. Link: https://goo.gl/FbGzIl
- Heyd R, Tselis N, Ackermann H, Röddiger SJ, Zamboglou N (2007) Radiation therapy for painful heel spurs: results of a prospective randomized study. Strahlenther Onkol 183: 3–9. Link: https://goo.gl/POiwQo
- Ott OJ, Jeremias C, Gaipl US, Frey B, Schmidt M, et al. (2014) Radiotherapy for benign calcaneodynia: long-term results of the Erlangen Dose Optimization (EDO) trial. Strahlenther Onkol 190: 671–675. Link: https://goo.gl/YVPkiu
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
