Archives of Community Medicine and Public Health
Prevalence of smear positivity, hiv co-infection, trend and treatment outcome among tuberculosis patients accessing care at faith alive foundation, Jos, Nigeria
Agbo HA1*, Banwat ME1, Isichei M2 and Isichei Chris3
2Department of Surgery, Jos University Teaching Hospital/University of Jos, Nigeria
3Department of Chemical Pathology, Jos University Teaching Hospital/University of Jos, Nigeria
Cite this as
: Agbo HA, Banwat ME, Isichei M, Chris I (2021) Prevalence of smear positivity, hiv co-infection, trend and treatment outcome among tuberculosis patients accessing care at faith alive foundation, Jos, Nigeria. Arch Community Med Public Health 7(2): 133-137. DOI: 10.17352/2455-5479.000152Background: Tuberculosis (TB) in the African sub-region, is still a public health problem, heightened by its synergy with HIV. Sputum smear positivity which is diagnostic indicator often gives a lower prevalence of the disease that may be give a false burden of the disease thus leading to varied treatment outcomes that may add to the vicious cycle of the magnitude of the disease. Thus, the prevalence of sputum positivity and co-infection with HIV; trend and treatment outcomes of TB were assessed.
Methods: Secondary data of TB patients from 2012 (commencement of TB care at this centre) - 2018 at Faith Alive Foundation Jos, a renowned faith-based hospital with approximately with a large monthly patient turn-out rate. Software SPSS version 18 was used for data analysis.
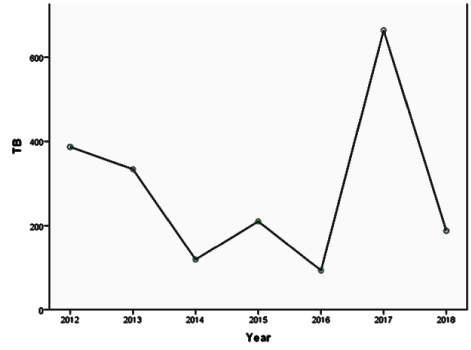
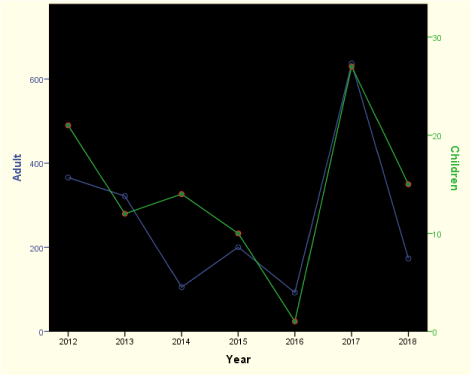
Results: Females adults were more affected; prevalence of sputum positivity/HIV co-infection were 15.1% (14% in adult; 1.1% in children) / 62.0% (57.4% in adults; 4.6% in children) respectively. The 7-year trend spiked in year 2015 and 2017, with an observed downward pattern in 2018 in all the ages. Cure after treatment was 6.3% (5.7% in adults and 0.6% in children).
Conclusion: Efforts will need to be put into TB diagnosis considering the low sensitivity of sputum smear microscopy in instituting treatment. TB is still a leading opportunistic infection in persons infected with HIV.
Introduction
Tuberculosis (TB) is amongst diseases that have affected mankind from time immemorial, whose causative agents and cure were initially obscured thus contributing significantly to morbidity and mortality [1-3]. Compared with diseases caused by a single infectious agent, it is the second biggest cause of mortality globally [4,5]. A third of the world’s population has been exposed and being infected with the organism with varying degrees of severity[6]. Globally, TB is currently responsible for more years of healthy life lost (2.5 percent of all disability-adjusted life years, or DALYs) than any other infectious disease [7], primarily infecting the lungs. The TB may be at the latent or active phase among persons infected [8].
Efforts have been made to curtail TB such as the Bacillus Calmette Guerin (BCG) vaccine administered to babies at birth [9,10]. In addition, the World Health Organization (WHO) designates a day annually to create awareness aimed at minimizing its global burden. Despite all these efforts, TB burden is still a problem in the African sub-region. Now with the advent of Human Immunodeficiency Virus (HIV) infection, there is a dramatic resurgence of TB with more than 8 million new cases each year worldwide and more than 2 million persons dying from it [11]. The emergence of HIV has made all the battles aimed at reducing these predisposing factors to TB infection fruitless. Mainly because TB is the most common opportunistic infections of persons infected with HIV. Additionally, the sub-Saharan countries have barely curtailed the double burden of diseases imposed by the infectious diseases (diarrhoea disease, measles, etc.) and non-infectious diseases (diabetes, hypertension, obesity, cancers due to epidemiologic transition) then the advent of HIV and some re-emerging diseases, thus creating a triple burden on the health system and a further weakening of the already frail system. The synergy of TB and HIV co-infections poses particular diagnostic and therapeutic challenges e.g. infection with HIV is the most powerful known predisposing risk factor for Mycobacterium tuberculosis infection and progression to active disease, which increases the risk of latent TB reactivation to about 20-folds. Tuberculosis on the other hand is the most common cause of AIDS-related death in persons infected with HIV. Thus, they act in synergy, accelerating the decline of immunological functions and leading to subsequent death if untreated though the mechanisms behind the breakdown of the immune defense of the co-infected individual are not well known [12]. The HIV like many diseases affecting mankind in a pandemic proportion has not spared any age group and has brought a lot of setbacks [13-17], and the Nigerian health system had suffered several infectious disease outbreaks year after year [18,19], which has continuously weakened the system and contributing to the increasing trends of diseases despite numerous health interventions. It is estimated that each day there are about 1,000 new infections of HIV in Nigeria and 2011 WHO report on other health problems in the country has shown that the number of reported cases of malaria is on the increase [20]. In spite of the various reforms to increase the provision of health to the Nigerian people, many still find it extremely difficult to afford the high cost of health care Figure 1 [21].
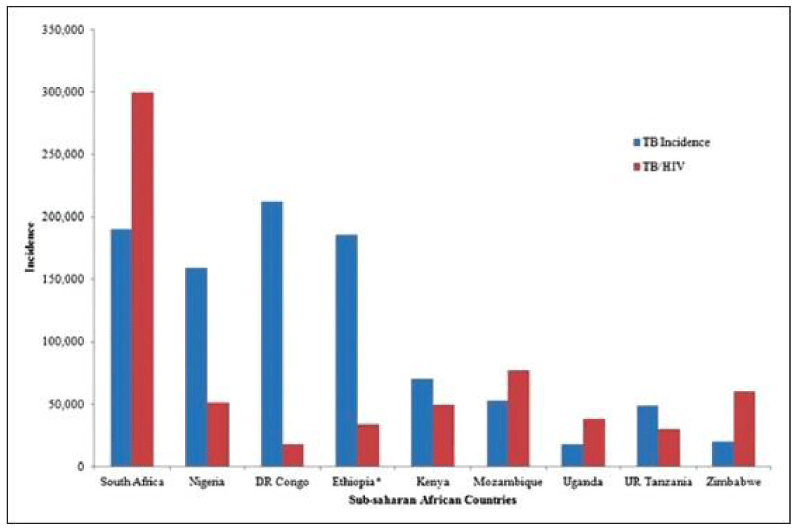
Among the 30 high TB burden countries who accounted for 87% of the new cases; Nigeria is one of them. Ending the TB epidemic by 2030 is among the health targets of the United Nations Sustainable Development Goals (SDGs)[23]. According to the WHO, mortality from TB is seen among HIV-negative people and additionally from HIV-associated TB [24]. More than 95% of TB deaths occurred in low and middle-income countries [23,25]. The two synergetic partners (TB and HIV) pose a threat to a desired healthy population especially in sub-Saharan Africa which provides other enabling factors to these diseases Figure 2.
The World Health Organization estimates that 9 million people a year get sick with TB, with 3 million of these “missed” by health systems [22,27]. The contribution of TB to child mortality is undetermined, particularly in TB endemic areas and a substantial number of children dying from meningitis, presumed sepsis, HIV/AIDS, or severe malnutrition may have had TB as its underlying cause [28], thus, the real burden of childhood TB in Sub-Saharan Africa is not known. A Malawi study found that 12% of all registered TB cases were children less than 15 years of age[9,29]. In the United States, a total of 9,582 cases of TB were reported in 2013, of which 485 (5%) cases were among children less than 15 years of age. In high TB burden settings outside of the United States, children account for an estimated 15–20% of TB cases [29], Children with latent infections become reservoirs for future transmission following disease reactivation in adulthood, thus fueling future epidemics [30].
The study determined the prevalence; trend and treatment outcome of patients who received TB treatment at the health centre.
The study was framed on the Donabedian health care model with a framework for examining health services and evaluating the quality of health care based on organizational structure, process and outcomes [31].
Materials and methods
Secondary data of TB patients seen from 2012 (commencement of TB care at this centre) - 2018 at Faith Alive Foundation Jos, a faith-based hospital with approximately 10,000 patients seen monthly were assessed. A minimum sample size of 300 was calculated [32].
N = Z2pq/d2; Where N = minimum sample size
Z = alpha level corresponding to the 95% confidence interval = 1.96
P = Proportion of TB cases co-infected with HIV in African countries was 39% = 0.39[26,33].
q = 1-p
d = maximum acceptable error margin = 5% or 0.05
N = (1.96)2 X 0.39 X 0.5/ (0.05)2
= 3.8416 x 0.195/0.0025
= 299.64
= 300
Inclusion criteria
Data of all registered TB patients was assessed.
Ethical consideration
Written consent was sought for and obtained from the Health Research Ethics Committee of Faith Alive Centre, Jos (Appendix I).
Limitation of the study
Treatment outcome is usually influenced by factors that may affect the external validity of the data collected.
Results
Discussion
Many problems have been attributed to the spread of TB in the sub-region; such are immune suppressive conditions like HIV/AIDS, malnutrition, overcrowding, non/poor utilization of health facilities, etc. Smear positivity in making a working diagnosis of TB is still of upmost concern; even in HIV negatives, this may be high[34]. The burden of TB was observed more among adults than children with a smear positivity of (14%) in the adults and (1.1%) in the children, similar (14%); [35], lower than that obtained from a pooled meta-analysis with prevalence of sputum smear positive TB of (52%) in adults and (6.8%) in children; [36] whose findings may have being influenced by the massive data pooled from 14 countries. Lower prevalence were compared with an Ethiopian study among homeless adults (2.6 %) [37]; (5.4%) [38] in another Ethiopian study (10%) [39] TB infection was higher among the female gender as against the male preponderance from other studies, this may be due to the peculiarities in most Africans where cultural restrictions on nutritious foods may predispose more females to lower immunity with a higher risk of TB than her male counterpart. Polygamy which may fuel the spread of HIV (the synergistic partner of TB) may indirectly account for the female prevalence and the higher TB/HIV co-infection seen in more than half of the adult population. Heterogeneity of smear positivity was recorded from the different study assessed with marked disparity among age groups and gender [39-42] in addition, the disparities might have been influenced by the presence of lung cavities seen more in adults, ease of sample collection/diagnosis, rapid progression from infectivity to disease, factors related to skilled manpower, low case finding in children due to misconceptions etc.
TB/HIV is the most common co-infection which still carries high mortality and morbidity worldwide [43,44]. The TB/HIV co-infection was (57.4%) in adults and (4.6%) in children similar to (55.5%) [37] HIV positive individual are more susceptible to TB infection and disease [38,45]. All the HIV positive (1145) had TB, the HIV/TB co-infection brought about by the immune suppressive state of HIV and the reactivation of latent TB infection has totally reversed the gains of programs aimed at reducing/eliminating the scourge of tuberculosis especially in Africa. The impact of these diseases on one another is bidirectional, with HIV increasing risk of TB infection and disease progression and TB slowing CD4 recovery and increasing progression to AIDS and death among the HIV infected [46].
The downwards pattern signifying a reduction may be attributed to better detection techniques that may have coincided with the introduction of more sensitive means of detecting smear positivity. The existence of a good TB Prevention and Control Program is essential to fight against TB [44]. The trend will provide a basis for effective monitoring and supervision, and an indirect audit of the many efforts that is/has been put into reducing the disease in the state through regional and national efforts.
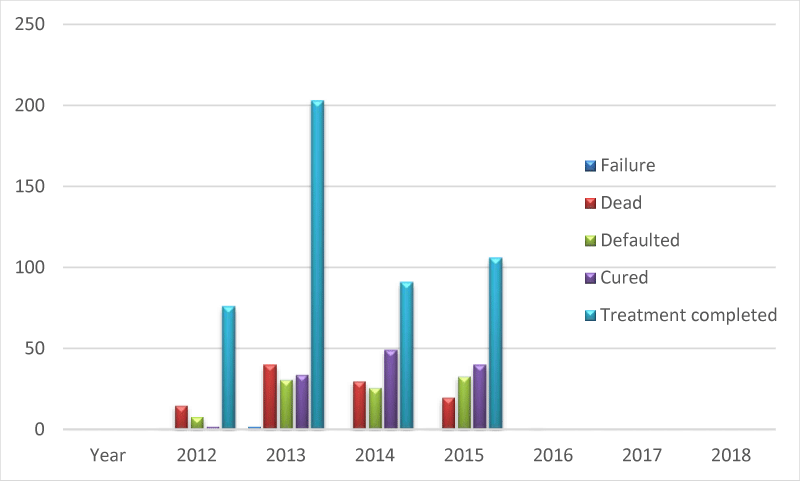
The desired treatment outcome is a complete cure; this wasn’t the case in the 7 years under review. TB-HIV co-infected people are facing multifaceted problems like high lost to follow up rates, poor treatment adherence, high TB recurrence rate, and high mortality risk [47]. The observed treatment outcomes with defaults, treatment failures, dead; has re-emphasized the fact that TB/HIV co-infection as seen among the patients had a higher chance of unsuccessful treatment outcome [48], despite the accessibility of the treatment centre and its affordability.
Conclusion
This retrospective review of data over seven years has shown that TB and its synergy with HIV infection is still a public health problem and more awareness will need to be created on case detection and treatment.
Recommendations
TB diagnosis by health workers should not only be placed on sputum smear results due to its low sensitivity.
Programmes aimed at TB control should be inclusive of control of HIV by stakeholders in health due to their synergetic association.
- World Health Organization (2020) WHO Information Note Tuberculosis and COVID-19. Considerations for tuberculosis (TB) care. Link: https://bit.ly/3x0NNPP
- Centre for Disease Control (2020) Tuberculosis. Link: https://bit.ly/3A4fl8R
- McIntosh J (2020) All you need to know about tuberculosis (TB). Medical News Today. Link: https://bit.ly/3w5zUOZ
- Centre for Disease Control (2017) Global epidemiology of tuberculosis and progress towards achieving global targets-2017. CDC Morbidity and Mortality Weekly Report 68: 263–266. Link: https://bit.ly/3xTEktI
- African Union/Africa CDC (2019) Tuberculosis. Link: https://bit.ly/3xWRshO
- Cedars Sinai (2021) What is tuberculosis? 2021. Link: https://ceda.rs/3y0WWrW
- Disease World Health Organization. Global tuberculosis report 2018 (2018) Link: https://bit.ly/3A7Jm7I
- World Health Organization (2020) Preventive treatment for tuberculosis infection. Link: https://bit.ly/3qwc265
- Avanya M (2019) History of tuberculosis. Link: https://bit.ly/3A5uMh9
- VBI Vaccines (2018) Louis Pasteur and the development of attenuated vaccines. Link: https://bit.ly/3AfI6zM
- Seddon JA, Shingadia D (2014) Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist 7: 153-165. Link: https://bit.ly/3w1C5Dh
- Christopher D, Anthony DH, Dermot MS, Mehran H, Wilfred N, et al. (2017) Disease and mortality in sub-Saharan Africa. Link: https://bit.ly/3qtrf87 .
- Boutayeb A (2009) The impact of HIV/AIDS on human development in African countries. BMC Public Health 9: S3. Link: https://bit.ly/3qtq5JP
- Andrzej P, Marianne J, Markus S, Martin ER, Gunilla K (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8: e1002464. Link: https://bit.ly/35TGca5
- Nicoll A, Timaeus I, Kigadye RM (2018) The impact of HIV-1 infection on mortality in children under 5 years of age in sub-Saharan Africa: a demographic and epidemiologic analysis. AIDS 8: 995-1005. Link: https://bit.ly/35TTvHq
- Walker N, Schwartländer B, Bryce J (2012) Meeting international goals in child survival and HIV/AIDS. Lancet 360: 284-289. Link: https://bit.ly/3w1fMOj
- Newell ML, Brahmbhatt H, Ghys PD (2014) Child mortality and HIV infection in Africa: a review. AIDS 18: 27-34. Link: https://bit.ly/3h97wq4
- Marston M, Zaba B, Salomon JA (2018) Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. J Acquir Immune Defic Syndr 38: 219-227. Link: https://bit.ly/3qtdjuA
- Olumade TJ, Adesanya OA, Fred-Akintunwa IJ, Babalola DO, et al. (2020) Infectious disease outbreak preparedness and response in Nigeria: history, limitations and recommendations for global health policy and practice. AIMS Public Health 7: 736-757. Link: https://bit.ly/3A4zhse
- Muhammad F, Abdulkareem JH, Chowdhury AA (2017) Major Public Health Problems in Nigeria: A review. Southeast Asia Journal of Public Health ISSN: 2220-9476ISSN: 2313-531X (Online). Southeast Asia Journal of Public Health 7: 6-11. Link: https://bit.ly/3h1UnAh
- Nwankwor C, Aneke C, Henry-Arize I, Okoronkwo I (2018) Perceived Utilization of National Health Insurance among Staff of University of Nigeria Teaching Hospital (UNTH), Ituku –Ozalla, Enugu, Southeast Nigeria. Int J Med Health Dev 23: 255-260. Link: https://bit.ly/3xWMe5C
- Welcome MO (2011) The Nigerian health care system: Need for integrating adequate medical intelligence and surveillance systems. J Pharm Bioall Sci 3: 470‑478. Link: https://bit.ly/3jlyBJ2
- Ogbo FA, Ogeleka P, Okoro P (2018) Tuberculosis disease burden and attributable risk factors in Nigeria, 1990–2016. Trop Med Health 46: 34. Link: https://bit.ly/2Su9OHM
- WHO (2018) Tuberculosis. Link: https://bit.ly/3A3GtF6
- Kwan CK, Ernst JD (2011) HIV and Tuberculosis: a Deadly Human Syndemic. Clin Microbiol Rev 24: 351–376. Link: https://bit.ly/3y1TQ78
- Jang SY, Kim MJ, Cheong H (2020) Estimating Disability-Adjusted Life Years due to Tuberculosis in Korea through to the Year 2040. Int J Environ Res Public Health 17: 5960. Link: https://bit.ly/3wW7w3f
- Sarmento E (2017) Transmission. Link: https://bit.ly/35TZOuy
- Global Plan to End TB. (2017) The paradigm shift 2016-2020. Link: https://bit.ly/35V4cJE
- World Health Organization (2017) Mother to child transmission of HIV. Link: https://bit.ly/3A7Im3s
- Moss WJ, Clements CJ, Halsey NA (2013) Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ 81: 61-70. Link: https://bit.ly/3havW2q
- Seddon JA, Jenkins HE, Liu L, Cohen T, Black RE, et al. (2015) Counting Children with Tuberculosis: Why Numbers Matter. Int J Tuberc Lung Dis 19: 9–16. Link: https://bit.ly/3xTDmO6
- Ogbonna C (2017) Sample size determination. In: The basics in Biostatistics, Medical Informatics and Research Methodology. Yakson Printing Press
- Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J (2002) Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J 21: 1053–1061. Link: https://bit.ly/3y4E77f
- Zaman K, Yunus M, Arifeen SE, Baqui AH, Sack DA, et al. (2006) Prevalence of sputum smear-positive tuberculosis in a rural area in Bangladesh. Epidemiol Infect 134: 1052-1059. Link: https://bit.ly/3gW6mPD
- Kunkel A, Wiesch PA, Nathavitharana RR, Marx FM, Jenkins HE, et al. (2016) Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC Infect Dis 16: 282. Link: https://bit.ly/3dkCLgN
- Semunigus T, Tessema B, Eshetie S, Moges F (2016) Smear positive pulmonary tuberculosis and associated factors among homeless individuals in Dessie and Debre Birhan towns, Northeast Ethiopia. Ann Clin Microbiol Antimicrob 15: 50. Link: https://bit.ly/3qtzlO1
- Berju A, Haile B, Nigatu S, Mengistu A, Birhan G (2019) Smear-Positive Tuberculosis Prevalence and Associated Factors among Pregnant Women Attending Antinatal Care in North Gondar Zone Hospitals, Ethiopia. International Journal of Microbiology 2019: 9432469. Link: https://bit.ly/3gWaFKS
- Nugussie DA, Mohammed GA, Anteneh Tesfaye Tefera AT (2017) Prevalence of Smear-Positive Tuberculosis among Patients Who Visited Saint Paul's Specialized Hospital in Addis Ababa, Ethiopia. Biomed Res Int 2017: 6325484. Link: https://bit.ly/3gYrWmw
- Mekonnen A (2014) Smear-positive pulmonary tuberculosis and AFB examination practices according to the standard checklist of WHO’s tuberculosis laboratory assessment tool in three governmental hospitals, Eastern Ethiopia. BMC Res Notes 7: 295. Link: https://bit.ly/3dkcaQU
- Gashaw F, Bekele S, Mekonnen Y (2019) High helminthic co-infection in tuberculosis patients with undernutritional status in northeastern Ethiopia. Infect Dis Poverty 8: 88. Link: https://bit.ly/3h78VNI
- Chinnakali P, Selvaraj K, Thekkur P, Ramasamy G, Thulasingam M, et al. (2014) Age and Sex Differences in Sputum Smear Microscopy Results for Acid Fast Bacilli in a Tertiary Care Centre, South India. Journal of Respiratory Medicine 2014: 674942. Link: https://bit.ly/3h1jjHY
- Gunda DW, Maganga SC, Nkandala I, Kilonzo SB, Mpondo BC, et al. (2018) Prevalence and Risk Factors of Active TB among Adult HIV Patients Receiving ART in Northwestern Tanzania: A Retrospective Cohort Study, Canadian Journal of Infectious Diseases and Medical Microbiology 2018: 1346104. Link: https://bit.ly/3qD0Qos
- Millet JP, Moreno A, Fina L, del Baño L, Orcau A, et al. (2013) Factors that influence current tuberculosis epidemiology. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 22: 539–548. Link: https://bit.ly/3qwg6U7
- FMOH (2013) Guidelines for Clinical and Programmatic Management of TB, TB/HIV and Leprosy in Ethiopia, FMOH, Addis Ababa, Ethiopia, 5th edition, 2013. Link: https://bit.ly/3hfFIQy
- Tornheim JA, Dooley KE (2017) Tuberculosis Associated with HIV Infection. Microbiol Spectr 5. Link: https://bit.ly/3gYqL6A
- Tola A, Mishore KM, Ayele Y (2019) Treatment Outcome of Tuberculosis and Associated Factors among TB-HIV Co-Infected Patients at Public Hospitals of Harar Town, Eastern Ethiopia. A five-year retrospective study. BMC Public Health 19: 1658. Link: https://bit.ly/3w7bAww
- Abdulkader M, van Aken I, Nigus S (2019) Treatment outcomes and their trend among tuberculosis patients treated at peripheral health settings of Northern Ethiopia between 2009 and 2014: a registry-based retrospective analysis. BMC Res Notes 12: 786. Link: https://bit.ly/3w5xiAF
- Feldacker C, Tweya H, Keiser O, Weigel R, Kalulu M, et al. (2012) Characteristics of adults and children diagnosed with tuberculosis in Lilongwe, Malawi: findings from an integrated HIV/TB clinic. Trop Med Int Health 17: 1108–1116. Link: https://bit.ly/3vVeZOI
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
