Open Journal of Chemistry
Preparation of Aluminium dodecaboride (AlB12) powder by Self-propagating High-temperature Synthesis (SHS)
Chao Wang1*, Xiaoming Cao2, Mengge Dong3, Lu Zhang4, Jianxing Liu3, Xiaozhou Cao3 and Xiangxin Xue3*
2Institute of Metal Research, Chinese Academy of Science, Shenyang, Liaoning, 110016, China
3School of Metallurgy, Northeastern University, Shenyang, Liaoning, 110819, China
4School of Energy and Environment, Anhui University of Technology, Ma’anshan, 243002, China
Cite this as
Wang C, Cao X, Dong M, Zhang L, Liu J, et al. (2021) Preparation of Aluminium dodecaboride (AlB12) powder by Self-propagating High-temperature Synthesis (SHS). Open Journal of Chemistry 7(1): 025-028. DOI: 10.17352/ojc.000025Self-propagating High-temperature Synthesis (SHS) process is used to prepare Aluminium dodecaboride (AlB12). The phase analysis results of preparing AlB12 with Al and B2O3 as raw materials show that under air and argon conditions, the self-propagating and acid-washed self-propagating powders all have α-Al2O3 impurities when Mg, Al and B2O3 are used as raw materials. The phase analysis results of the preparation of AlB12 show that under argon conditions, the self-propagating and acid-washed, self-propagating powder has un-removable MgAl2O4 impurities, and the root cause of the low purity of AlB12 prepared by the self-propagating method is the presence of un-removable impurities.
Introduction
Most of the borides are crystals with high hardness and melting point [1-4]. Stable chemical properties and a wide range of applications make it widely used in composite materials, semiconductors, and in various areas of national defense, such as radiation protection [5-8]. Among them, AlB12 has a special electronic structure and bonding characteristics [9,10]. It can effectively adjust the conductivity of semiconductor materials, and thus is extensively employed in conductors and semiconductor materials. In addition to the above characteristics, the content of boron in AlB12 is extremely high, reaching 82.8%, which is very promising as neutron shielding material [11-13].
Ceramic powders are usually synthesized by traditional sintering methods [14-16]. However, the use of this method to synthesize ceramic powder takes a long time, consumes a great deal of energy and pollution [17]. Self-propagating high-temperature synthesis (SHS) is a unique technique for synthesizing materials by self-heating and self-conduction of high chemical reaction heat between reactants. This technology was first discovered by Merzhanov et al., in their research on the combustion of solid propellants in rockets and was announced in 1967. Compared with the conventional sintering method, the advantages of the SHS method can be summarized as follows: (1) It is time saving and makes full use of energy [18]. (2) It requires only simple equipment and processes [19]. (3) The high product purity and product conversion rate are close to 100% [20]. (4) It can not only produce ceramic powder, but if the proper amount of pressure is applied at the same time, high-density combustion products can also be produced [21]. (5) High output [22].
In previous studies, AlB12 powder was fabricated by using the SHS method [23-25]. The calculation results of preparing AlB12 with Mg, Al2O3 and B2O3 as raw materials show that the adiabatic temperature of the system is 2789.5K, which satisfies the self-propagating reaction conditions. Further, the phase analysis results show that there is no matter in air or argon, the self-propagating powder and the acid-washed self-propagating powder all have Mg0.4Al2.4O4 impurities, and the purity of the prepared AlB12 is not high.
Although AlB12 is produced, Mg0.4Al2.4O4 has not been removed and still exists. In this work, Al, B2O3 and Mg, Al, B2O3 were used as raw materials to conduct experimental studies on self-propagating synthesis of AlB12.
Experimental procedure
The starting materials used in this research were Al powder (purity>99% Al, average particle
size 50 µm, provided by Dandong Chemical Research Institute, Dandong, China), B2O3 powder (purity>99%, average particle size 96 µm, provided by Dandong Chemical Research Institute, Dandong, China), and Mg powder (purity>98%, average particle size 100 µm, provided by Dandong Chemical Research Institute, Dandong, China).
The steps used in the self-propagating process to synthesize AlB12 ceramic powder are as follows: (1) Weigh a certain amount (in proportion to the reaction equation) of the original material powder, place it in the ball milling tank, and mix the ball mill for 2 hours. (2) Intercept the resistance wire and connect it to the two poles of the self-propagating device and place the material in the atmosphere with one end close to the resistance wire. (3) Start the ignition device and slowly increase the current. When the pointer fluctuates sharply, reduce the current and keep the current increasing steadily. Finally, the resistance wire will reach a molten state when the material is induced to burn, and the current is turned off. (4) The reaction product is pulverized and sieved with 160 meshes, and samples under the sieve are sampled for detection and analysis.
The phase analysis of the synthesized powder was carried out using an X-ray diffractometer (XRD, X’Pert Pro MRD, Netherlands) with a Philips diffractometer using Cu Ka. The microstructure of powders and elements analysis were investigated using a scanning electron microscope with EDS detector (SEM, S-3400N, Japan).
This article focuses on the study of two reaction systems, system 1: Al and B2O3, and system 2: Mg, Al, and B2O3. Two experimental atmospheres are used in both systems (Table 1).
In the Al-B2O3 system, the following chemical reactions mainly occur:
In the Mg-Al-B2O3 system, the following chemical reactions mainly occur:
After the combustion synthesis, the extraneous components were leached out from the synthesized powder with 60° C in diluted HCl (pH value is 3).
Introduction
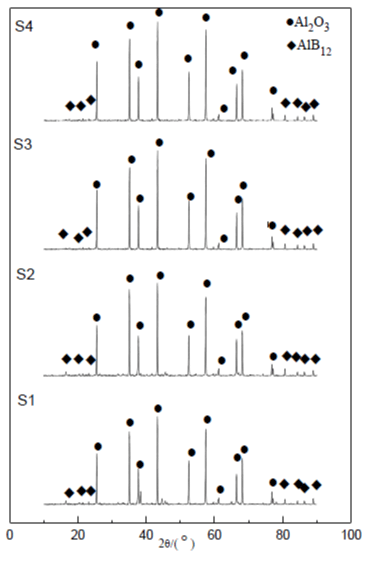
Figure 1 is the X-ray diffraction pattern of Al and B2O3 prepared under both air conditions (before and after pickling) and argon conditions (before and after pickling) respectively. It can be seen from the figure that in either air or argon conditions, irremovable Al2O is found in the bottom. Analysis of its crystal structure revealed that the α-Al2O3 is corundum, an extremely stable substance that is difficult to remove through physical and chemical reactions. Therefore, AlB12 prepared from Al and B2O3, contains a large amount of inseparable corundum, which contributes to the failure of the self-propagating preparation of AlB12 using Al and B2O3 as raw materials.
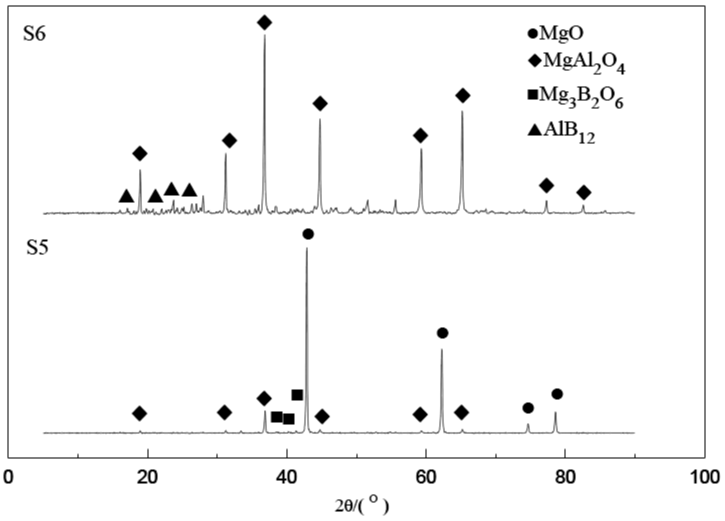
Figure 2 is the X-ray diffraction pattern of powder prepared with Mg, Al and B2O3 under argon conditions (before pickling). From the results of phase analysis, the main components of the coarse powder before pickling are MgO, Mg3B2O6, MgAl2O4, and AlB12, while in the powder after pickling, when MgO and Mg3B2O6 are removed, the main impurity is MgAl2O4. This shows that the purity of AlB12 is not high when prepared by self-propagating, self-propagation when the raw materials used are Mg, Al and B2O3.
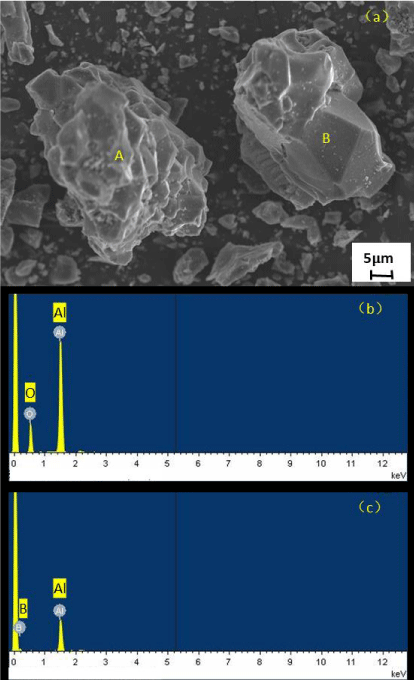
Figure 3 shows the microscopic morphology of powder prepared through use of the self-propagating method under argon conditions with Al and B2O3 as raw materials after pickling. From an analysis of the energy spectrum results, the A particles – with obvious layering phenomenon on the left are Al2O3 particles – while the B particles – with more obvious granular shape on the right – are AlB12. This situation shows that despite the pickling treatment, Al2O3 is still untreated. It also shows that the unremovable by-product Al2O3 uses Al and B2O3 as raw materials and is the biggest obstacle to self-propagating preparation of AlB12.
Table 2 shows the elemental analysis results of EDS analysis after pickling of Al and B2O3 as raw materials and self-propagating preparation of AlB12 with Mg, Al, and B2O3 as raw materials. From the results in the table, the acid wash product prepared with the use of Al and B2O3 as raw materials has the highest content of O element, followed by B and Al. Observing the test samples, there are still insoluble substances, so the test results are also relatively incomplete. This shows that under argon conditions, using Al and B2O3 as raw materials to prepare AlB12, and using the self-propagating method, the purity of AlB12 in the prepared product is low, and the B content is insufficient.
Summary
The phase analysis results of preparing AlB12 using Al and B2O3 as raw materials shows that there are α-Al2O3 impurities in the self-propagating powder regardless of either the air condition or the argon condition, and it cannot be removed. Consequently, the purity of the prepared AlB12 is not high. The phase analysis results of preparing AlB12 using Mg, Al and B2O3 as raw materials indicates that the self-propagating and acid-washed self-propagating powder has unremovable MgAl2O4 impurities under argon conditions, and the purity of the prepared AlB12 is not high, causing self-propagation. The fundamental reason for the low purity of AlB12 prepared by this method is the existence of impurities that cannot be removed. For future research work, it may be very promising to consume Al2O3 through aluminum electrolysis.
Notes
The authors declare that they have no competing financial interest.
This work was supported by the fundamental, scientific-research business resources of the central universities (award # N10060200).
- Wang C, Xue X, Cao X, Yang H (2012) Effect of BN Addition on Mechanical Properties and Microstructure of TiB2-Al Composites, Journal of Northeastern University (Natural Science) 19. Link: https://bit.ly/37nZLbx
- Cao X, Wang C, Shi L, Yang H, Xue X, et al. (2013) Effect of Ni addition on pressureless sintering of tungsten diboride. International Journal of Refractory Metals and Hard Materials 41: 597-602. Link: https://bit.ly/2NzrKhg
- Cao X, Wang C, Xue X, Yang H (2014) Preparation of tungsten boride ceramic by pressureless sintering. Journal of Inorganic Materials 29: 498-502. Link: https://bit.ly/3jUoYzk
- Matkovich VI (1977) Boron and refractory borides, Springer. Link: https://bit.ly/37oSA30
- Cao X, Wang C, Xue X, Cheng G (2015) Effect of ti addition on the residual aluminium content and mechanical properties of the B4C-al composites produced by vacuum infiltration. Archives of Metallurgy and Materials 60: 2493-2398. Link: https://bit.ly/3s4vdTY
- Dong M, Xue X, Yang H, Liu D, Wang C, et al. (2016) A novel comprehensive utilization of vanadium slag: as gamma ray shielding material. Journal of Hazardous Materials 318: 751-757. Link: https://bit.ly/3qsmAm7
- Qi D, Yong G, Zhiheng R, Xiaoming C, Chao W, et al. (2019) Preparation and Erosion Performance for Co-continuous Phase Composites of Si3N4/1Cr18Ni9Ti, Chinese Journal of Materials Research 33: 34-42. Link: https://bit.ly/3dkcH61
- Cao X, Wang H, Meng X, Wang C, Yang H, et al. (2011) High temperature electrochemical synthesis of tungsten boride from molten salt. Advanced Materials Research. 463-466. Link: https://bit.ly/3qt80L2
- Gosset D, Guery M, Kryger B (1991) Thermal properties of some boron‐rich compounds ("BnC" and AlB12). AIP Conference Proceedings American Institute of Physics 231: 380-383. Link: https://bit.ly/3pnQuX3
- Wang C, Cao X, Jiang T, Rong Y, Zhang J, et al. (2013) Research Progress on Aluminum-Boron Compounds (Al-B) and Its Composite Materials. Bulletin of the Chinese Ceramic Society 26. Link: https://bit.ly/3aq3eYV
- Higashi I (2000) Crystal chemistry of α-AlB12 and γ-AlB12. Journal of solid state chemistry 154: 168-176. Link: https://bit.ly/3s3wMls
- Mahesh V, Nair PS, Rajan T, Pai B, Hubli R (2011) Processing of surface-treated boron carbide-reinforced aluminum matrix composites by liquid–metal stir-casting technique. Journal of Composite Materials 45: 2371-2378. Link: https://bit.ly/3psSD43
- Mahmoudi M, Wang C, Moreno S, Burlison SR, Alatalo D, et al. (2020) Three-Dimensional Printing of Ceramics through “Carving” a Gel and “Filling in” the Precursor Polymer. ACS Appl Mater Interfaces 12: 31984-31991. Link: https://bit.ly/3ptAS4k
- Luo X, Wang Z, Hu X, Shi Z, Gao B, et al. (2009) Influence of metallic additives on densification behaviour of hot-pressed TiB2. Light Metals 1151-1155. Link: https://bit.ly/3bhouzk
- Wang C, Hossain Bhuiyan ME, Moreno S, Minary-Jolandan M (2020) Direct-Write Printing Copper–Nickel (Cu/Ni) Alloy with Controlled Composition from a Single Electrolyte Using Co-Electrodeposition. ACS Applied Materials & Interfaces 12: 18683-18691. Link: https://bit.ly/3asMa4J
- Chao W, Xiangxin X, Xiaozhou C, Lu Z, Jian Z, et al. (2013) A New Method of Fabricating AlN-TiB2 Composite Ceramics. Materials and Manufacturing Processes 28: 953-956. Link: https://bit.ly/37nfwiV
- Chao W, Xiangxin X, Xiaozhou C, He Y, Gongjin C (2013) The effect of Ti addition on the microstructure and fracture toughness of BN-Al composite materials synthesized by vacuum infiltration. Archives of Metallurgy and Materials 58: 509-512. Link: https://bit.ly/3qzcfVy
- Cao X, Xu L, Wang C, Li S, Wu D, et al. (2020) Electrochemical Behavior and Electrodeposition of Sn Coating from Choline Chloride–Urea Deep Eutectic Solvents. Coatings 10: 1154. Link: https://bit.ly/3dlvD4A
- Tao W, Wang Z, Chen G, Shi Z, Gao B, et al. (2009) Finite element analysis of thermo-electric coupled field in 400kA large-scale aluminum reduction cell. 2009 World Non-Grid-Connected Wind Power and Energy Conference. IEEE 1-4. Link: https://bit.ly/3jTrUfM
- Subrahmanyam J, Vijayakumar M (1992) Self-propagating high-temperature synthesis. Journal of Materials Science 27: 6249-6273. Link: https://bit.ly/3dm5bYf
- Merzhanov A (1995) History and recent developments in SHS. Ceramics International 21: 371-379. Link: https://bit.ly/3ppyY4N
- Yukhvid V (1992) Modifications of SHS processes. Pure Appl Chem 64: 977-988. Link: https://bit.ly/3qoHwKy
- Wang C, Ma B, Zhang L, Cao X, Yang H, et al. (2014) Elementary research on preparation of AlB12 powder by self-propagating high-temperature synthesis (SHS), Materials Science Forum. Trans Tech Publ 365-369. Link: https://bit.ly/3prLjWf
- Odawara O (2010) Mass-forced SHS technology of ceramic materials. Trans Tech Publ 302-311. Link: https://bit.ly/3qoHouy
- Huang KJ, Yan L, Kou HM, Xie CS (2010) Synthesis of Al2O3/AlB12/Al composite ceramic powders by a new laser-induction complex heating method and a study of their mechanical properties. Applied Mechanics and Materials 596-601. Link: https://bit.ly/2Zmne8u
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
