International Journal of Veterinary Science and Research
Potential for silvopastoral systems to control nematode burden in livestock farming in winter rainfall areas of South Australia, Australia
Hannah Griffiths* and Amanda (Mandi) N. Carr
Cite this as
Griffiths H, N Carr AM (2022) Potential for silvopastoral systems to control nematode burden in livestock farming in winter rainfall areas of South Australia, Australia. Int J Vet Sci Res 8(3): 118-126. DOI: 10.17352/ijvsr.000124Copyright License
© 2022 Griffiths H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Gastrointestinal nematode infections cause significant production losses in ruminants. In southern Australia, the estimated annual cost of internal parasites in sheep, cattle and goats are $436million, $82m and $2.54m, respectively. An over-reliance on anthelmintic treatments has resulted in anthelmintic resistance becoming an increasing concern for producers. Reducing the need for chemical anthelmintics is desirable to lower producer costs and limit the development of anthelmintic resistance. Condensed tannins found in many trees and forages are a plant secondary compound receiving considerable interest as an alternative anthelmintic strategy. Direct anthelmintic effects from condensed tannins are associated with the binding of larval proteins; slowing egg hatching, larval development and exsheathment. Indirect effects are associated with improvements to protein metabolism and immune function within the animal. Several native Australian tree extracts have demonstrated anthelmintic properties, but further research is required to assess their suitability for silvopastoral systems in South Australia.
Lay Summary
Gastrointestinal nematodes or ‘worms’, significantly lower both the physical well-being and economic profitability of livestock such as cattle, sheep and goats, in South Australia. There are concerns about parasite resistance with current chemical treatments and consumers are demanding chemical-free, sustainably produced food. Silvopastoral systems, where animals are grazed underneath or alongside trees, have many potential benefits to the animals and the environment. This review explores the potential effects of condensed tannins on worm burden and protein metabolism. Condensed tannins are found in the leaves of several types of plants, including trees and show considerable potential to reduce nematode burden in ruminant livestock by slowing parasite lifecycle, with a particular focus on egg and larval stages. Condensed tannins may also improve livestock protein metabolism or excretion. Several native Australian trees contain condensed tannins with the potential to reduce nematode burdens when used in silvopastoral systems.
Teaser Text
Condensed tannin ingestion by allowing access to tree forage in silvopastoral systems, has the potential to reduce gastrointestinal nematode burden in livestock by slowing the parasite lifecycle.
Abbreviations
AH: Anthelmintic; AR: Anthelmintic Resistance; CT: Condensed Tannins; DM: Dry Matter (of diet); FEC: Faecal Egg Count; GIN: Gastrointestinal Nematode; PSC: Plant Secondary Compounds; SPS: Silvopastoral Systems
Introduction
Gastrointestinal nematode (GIN) infections represent a significant cost on livestock production systems worldwide, imposing costs due to reduced growth and poor animal welfare, as well as treatment costs, which have been estimated at over half the animal therapeutic expense globally [1-3]. In southern Australia, the estimated annual cost of internal parasites in sheep, cattle, and goats are $436million, $82m, and $2.54m, respectively [4]. These costs are primarily a loss in production, rather than the cost of treatment [4,5]. The disease is most common in young stock for all three species, and in pregnant or lactating ewes [4]. Gastrointestinal nematodes of concern in South Australia namely: Trichostrongylus vitrinus/ Tricostrongylus colubriformis, Teladorsagia circumcincta, and Nematodirus spp in sheep and goats; and Cooperia spp and Ostertagia ostertagi in cattle, can be managed with various strategies such as breeding for nematode resistance, optimising grazing practices to reduce parasite load, and administration of chemical anthelmintics (AH) [6-9]. Unfortunately, when the chemical AH was first introduced, its popularity led to over-reliance on chemicals without concurrent use of the non-chemical nematode control methods described above. These chemicals were used at low dosages, which facilitates selection between resistant and susceptible worm populations and at high frequency, ensuring that subsequent generations are the progeny of resistant individuals [10]. These practices resulted in anthelmintic resistance (AR) becoming a noticeable problem within years of the first chemical AH being introduced in Australia [5,10]. Concerns over the increasing levels of AH resistance and consumer concerns regarding chemical use and residues in food encourage the development of alternative control measures [2,11 ]. A particularly popular area in this field is the analysis of the effects of bioactive plants on increasing host resistance to parasites or altering GIN biology, aiming to develop multiple strategies to control worm populations, rather than relying on one method [12]. Plant-based AH strategies may represent a lower cost alternative to chemicals, reduce the potential for the development of AH resistance and increase productivity Lane, et al. [4,13] showed a 50% reduction in disease severity would result in a $146m gain for the sheep industry in Australia.
Past research into AH effects of plants has focussed on cultivated forages, such as sainfoin (Onobrychis viciifolia) and bird’s foot trefoil (Lotus corniculatus), which fit easily into conventional pasture-based farming systems, but do not provide the economic or environmental benefits supplied by trees in silvopastoral systems [1,14-18]. Trees or perennial shrubs are incorporated into farms as a method of securing green, high protein forage during seasons when conditions are harsh or feed availability is low; or as a secondary source of income from timber or fuel [19-23]. The inclusion of these plants into an agricultural system can have many benefits in addition to their economic or AH properties such as increased biodiversity and soil health, protection from weather, and reduced methane production [21,23]. Positive environmental and economic effects have been observed in low-moderate rainfall mixed cropping regions of Australia with mixtures of perennial shrubs, mainly wattle and saltbush [24,25]. Similarly in northern parts of Australia, benefits have been observed with forage trees such as Leucaena leucocephala and Chamaecytisus palmensis, and with hardwoods grown for pulp or timber [26,27]. Comparatively, little interest has been shown in the cooler, winter rainfall areas of South Australia, despite the common occurrence of a seasonal feed gap in late summer and significant issues and costs associated with GIN in wetter seasons.
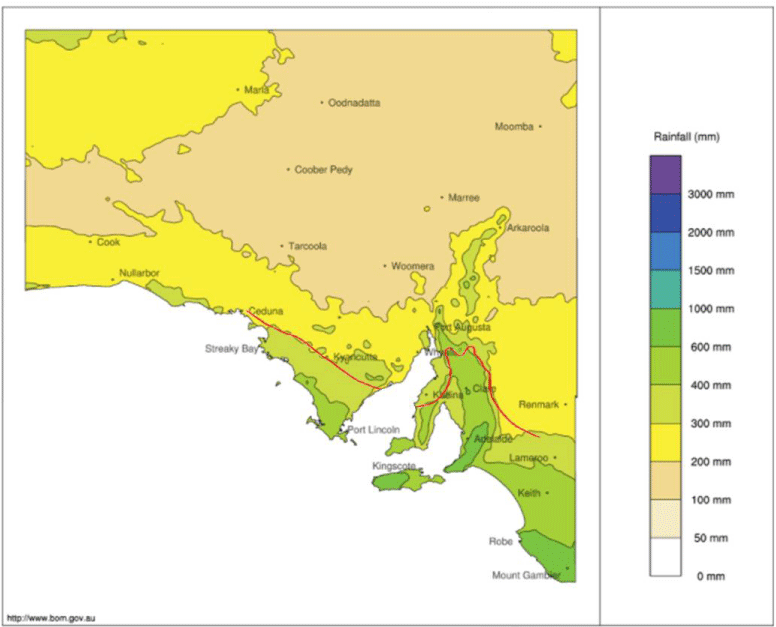
South Australia’s climate is highly variable across the state, with northern areas receiving small amounts of rain, while southern areas experience a Mediterranean climate of warm, dry summers and wet winters [28]. Typically, in South Australia, agriculture is more successful and livestock are more intensively reared in regions receiving over 300mm annual rainfall, often referred to as ‘below Goyder’s line’ (Figure 1) [29,30 ]. In these southern winter rainfall regions, the most concerning GIN in sheep and goats are Trichostrongylus vitrinus/Tricostrongylus colubriformis, Teladorsagia circumcincta and Nematodirus spp; and Cooperia spp and Ostertagia ostertagi in cattle [6-9]. In the winter rainfall areas of South Australia, worm burdens are generally low during the dry summer and highest around June and August [6]. Haemonchus spp can cause occasional issues when summer rainfall is above average [6,7].
While South Australia is not a major meat producer on a national scale, accounting for approximately 14% of the nation’s sheep and only 4% of cattle, red meat production represents 23% of the state’s agricultural revenue [31]. Much (53.1% cattle and 26.7% sheep, by value) of this, is produced in the higher rainfall South East region where GIN is problematic [31]. Moderate rainfall regions account for most of the remaining revenue with northern, urban and peri-urban regions only making a small contribution [31]. Meat and Livestock Australia has committed to improving the environmental impacts of red meat production, creating a push towards increased biodiversity and reduced chemical use, notwithstanding the costs of GIN-related disease experienced by individual farmers [32]. It is estimated that severe disease can cost producers up to $11.94 per cow or $28.29 per sheep in preventatives, treatments, and lost production [4]. Silvopastoral systems (SPS), or the use of trees within a livestock rearing system, have been proposed to reduce disease associated with GIN in two main ways: through the effects of plant secondary compounds (PSC) such as tannins, saponins, non-protein amino acid and phytohaemagglutanins, either directly on parasite life cycle or indirectly by improving host immunity and by altering the environmental conditions in which the stock and the nematodes must survive [19,22].
This review explores the effects of major PSC, specifically condensed tannins (CT), in altering parasite biology and host response, environmental considerations, formulation of alternative AH therapies and the potential for positive impacts and successful implementation in temperate, winter rainfall regions of South Australia.
Plant secondary compounds
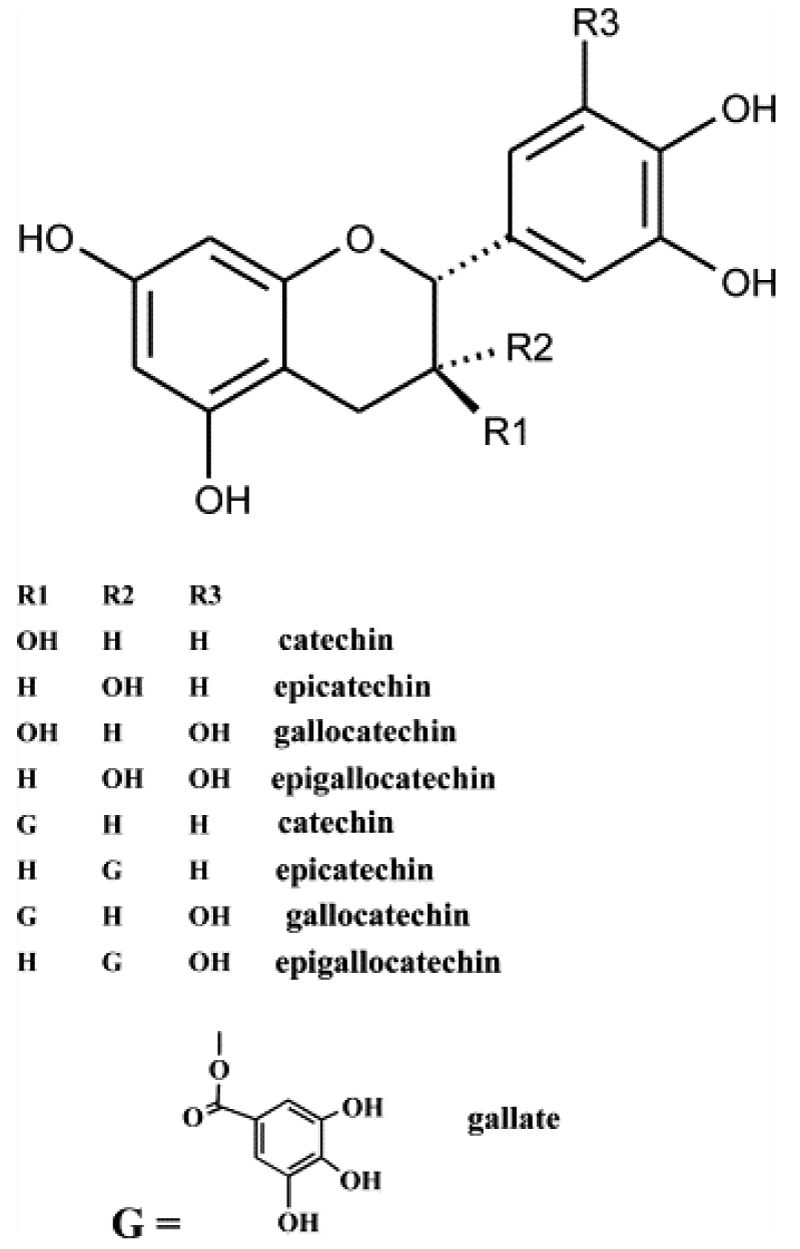
The category of PSC that has received the most attention regarding AH properties is Condensed Tannins (CT). CT are flavan-3-ol polymers, which are numerous and varied (Figure 2) [16,33]. Different classes of CT exist based on the structure of their monomers. These classes differ in AH efficacy; with prodelphinidin, containing galloyl derivatives of flavan-3-ols being more effective than procyanidin polymers [13,17,33]. The activity of CTs against GIN is divided into direct effects such as inhibition of egg hatching, larval development and adult viability, and indirect effects like improving protein metabolism or host immunity [34]. A major factor in determining the suitability of using plants containing CT as part of an AH protocol is the potentially detrimental effects of CT on feed intake and digestibility [16,20,21]. There is significant agreement that CTs at low concentrations improve protein metabolism and rumen health, but at high concentrations reduce voluntary feed intake and impair the functions of proteins, enzymes, and microbiota in the rumen, thus reducing productivity [3,16,19-21]. There is debate over the ideal levels of CT in the diet, studies in this review recommend concentrations ranging from 20-55g/kg Dry Matter of diet (DM) [3,16,19]. Inconsistencies between studies may be influenced by differences in nematode or host species or confounded by differences in CT preparation or testing method, but concentrations of approximately 30g kg/DM appear beneficial [1,3,13,16,19,35]. Both the positive and the negative effects of plants containing CT are hypothesized to be due to the CT binding to the proteins, either in the parasite or the host [35].
Several studies have used tannin-binding agents such as Polyvinylpolypyrrolidone or Polyethylene glycol to inhibit the actions of CT in their test compounds to assess the presence and effect of non-CT PSCs [1,21]. In some studies, the treatment groups that received tannin-inhibitors closely resembled the negative controls, indicating that CTs were the sole, or the major, bioactive component [1,15,17]. Other studies found that the AH effects of the plant extract tested were only partially negated by tannin inhibitors, indicating the need for further research into their bioactive components [18,21,36,]. Furthermore, each of these papers used different methods to extract the bioactive compounds, potentially increasing variability between experiments. Plant preparation methods and the use of water, temperature, acetone and ethanol for different time periods may alter the quantity and profile of PSC extracted [1,15,17,18,21,36].
Identifying plants with anthelmintic properties
Plants with AH properties can be identified in two main ways: screening plants with known bioactive PSC for AH effects; or evaluation of plants with AH effects according to traditional farming knowledge to identify bioactive PSC [13]. An exploration of available literature evaluating the effects of CT from different livestock forage plants on nematode burden was based on the use of a variety of plants used internationally. Appendix 1 contains the list of plants reviewed and the native location. One study by Kotze, et al. [21] evaluated 85 native Australian species for AH properties which are listed separately in Appendix 2. Some studies used commercial tannins or derivatives rather than plants or extracts [33,37,38]. The majority of plants are cultivated forages rather than trees and are found in climates different from southern Australia [23]. Most of the Australian native plants evaluated were for low-moderate rainfall areas, so further research is required to identify appropriate candidates for the higher rainfall southern regions [21]. Acacia spp shows good potential and endemic species are found across the southeast of South Australia, allowing appropriate candidates to be selected to reduce weed potential [20,21,39,40]. Trees from higher rainfall may have lower CT concentrations as the plant is under less stress [20]. Plant reproductive status and growing location altered the anthelmintic activity of some species by creating different PSC profiles [21]. These effects may explain differences in AH efficacy between the same species from different sites.
Direct effects on parasite biology
The majority of direct AH effects by plants containing CT targeted nematode biology, inhibiting egg hatching and larval development [15,17,18,21,23,33,35,41,42]. Sporadic effects against adult worm viability have been observed [1,3,23,35]. Appendix 3 summarizes the assays that have been used to assess CT effects on nematode activity. Six out of the seven in vitro tested Acacia spp reduced larval development to less than 40% of the untreated, including A. pycnantha, A. saligna and A. myrtifolia, which are naturally found in the southeast of South Australia[21]. However, no research has concentrated on the in vivo effects of many of these natives in grazing systems. The majority of tested CTs showed dose-dependent inhibition, identifying the need to consider the browsing behaviors of different ruminant species and the levels of CTs found naturally in digesta [1,35].
The primary proposed AH mechanism of action is binding to larval proteins [41]. Gastrointestinal nematode lifecycle processes, particularly exsheathment, are time-dependent [15]. Therefore, any delay may represent a critical disruption in parasite establishment [15]. A study by Castandea-Ramirez, et al. [43] indicated that Acacia pennatula extracts were more effective at inhibiting exsheathment of younger larvae (1-4wks) than older larvae (7wks), suggesting paddock management and larval contamination levels must be considered as part of a holistic AH protocol [43]. Unfortunately, time delays can also work against CT efficacy. Ad libitum feeding alters gastrointestinal transit times, reducing the time that the CTs remain in the abomasum, thus reducing their efficacy [23,43] found that larval culture from faeces takes a different amount of time in warmer climates compared to temperate, as such the lifespan and vitality may also differ. In Australia, nematode populations vary greatly across the livestock-raising regions due to interactions between temperature and rainfall [44]. Differences in nematode isolates may also cause inconsistencies between studies with Chan-Pérez, et al. [18] finding that H. contortus from different geographical locations were not equally resistant to each compound, in effect, the isolate that required the highest levels of Acacia pennatula extract did not need the highest level of Onobrychis viciifolia extract). Additionally, the primary worm species of concern in winter rainfall areas of South Australia have not received much attention from previous research [6,8].
In addition to the beneficial effects observed on individual worm burdens, CTs also improve GIN epidemiology. Several studies found that CT ingestion was associated with reduced Faecal Egg Count (FEC), thus reducing the larval load on pasture and the risk of reinfection [2,3,14,16,33,36-38,41,42,47]. The mechanism of this reduction may be the inhibition of larval establishment, the passage of CT through the rumen and subsequent concentration in the faeces lowering the hatchability, reduced adult vitality and fecundity, or a combination of these effects [3,35,41]. A study by Mupeyo, et al. [3] found that faeces containing willow had poorer egg recovery by FEC than the control, suggesting that CT could mask the presence of viable eggs, making conventional FEC unreliable. Newer, more sensitive methods are becoming more popular in accurately identifying worm burdens than the glass counting chambers that traditional FEC rely on [48,49]. These higher sensitivity methods are highly important for worm species with lower fecundity, such as Ostertagia ostertagi, where a low FEC may still suggest clinical disease [44,45]. It is also important to consider worm counts on a dry faeces basis, as faecal consistency in parasitized animals is variable [37].
Stocking rate is another key variable in larval load on pasture and reinfection. Oliveira et al. [50] found that the SPS stocking rate was higher without increasing the FEC or decreasing weight gain, indicating that the SPS may have had lower FECs if stocking rates were consistent. With livestock farming enterprises aiming to be as productive as possible, and stocking rate a key determinant of profitability any benefit in the ability to increase the carrying capacity of the enterprise needs to be investigated.
Indirect effects
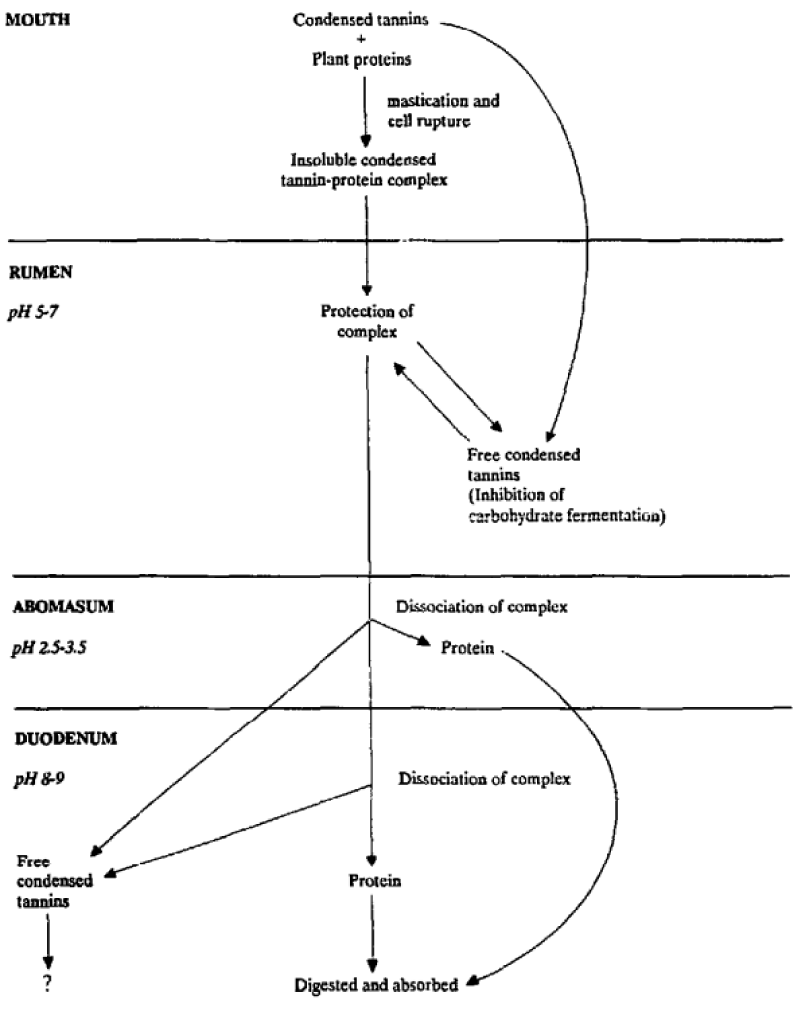
a) Altered protein metabolism: Gastrointestinal nematode infection reduces voluntary feed intake and increases protein loss by damaging the gut, particularly the abomasum [11]. As a result, more dietary protein is needed in the presence of infection, to maintain bodily functions such as growth, reproduction, or fiber production [11]. Poor performance, for example, reduced live weight gain and wool growth, is significant in parasitized animals on a low plane of nutrition [4,37,46]. Animals also require a high level of dietary protein to optimize immune function [11]. Therefore, it is critical to understand the effects of CT on metabolizable protein, which in ruminants consists of rumen microbial protein, undegraded feed proteins, and endogenous protein [40]. Several authors found that CT ingestion was associated with reduced ruminal protein digestion, lower blood urea concentrations, and increased faecal nitrogen (N) excretion [20,36,39,40,47]. In some cases, this led to the conclusion that protein was not digested and thus wasted [20,47]. However, overall feed digestibility was less severely impacted than apparent rumen digestibility, indicating a shift towards intestinal digestion of protein, due to the protection of protein in the rumen (Figure 3) [40,51]. This shift towards intestinal digestion is due to the dissociation of the CT-protein complexes formed in the rumen when they reach the acidic abomasum and is associated with benefits to liver health and reduced methane production [16,37,40,51]. By shifting protein digestion, CT may lead to greater protein availability [51].
Multiple studies have shown that, while faecal N was increased, urinary N was decreased, resulting in either improved or unchanged body N retention [39-41]. This switch in N excretion is better for the environment as the N is less volatile Butter, et al. [37,40] found that the positive effects of CT on GIN burden were maximized when dietary protein was low but did not observe an improvement in live weight gain improvement due to CT and suggested no increased protein availability. Lisonbee, et al. [38] observed higher daily gains in the non-parasitized group eating CT, suggesting the increased post-ruminal digestion of protein was beneficial and methane production was reduced. Moderate levels of CT in the diet also reduced the risks of ruminal bloat [52].
b) Improved host immunity: There is limited evidence to support that CTs directly affect the immune system of the animal. A study by Niezen, et al. [34] found inconsistent effects of CT on host immunity, possibly due to a limited study time frame and Butter, et al. [33] found that either CT or increased protein improved eosinophilic performance similarly. Nevertheless, an increase in available protein does have an indirect, protective effect against parasitism, and is associated with better expression of innate immunity, by increasing the numbers of key immune cells such as eosinophils and mast cells in the gut wall [11,12,16,37]. Plants high in tannins are also often high in both protein and micronutrients, which may contribute to improved immune response [11,12]. Under natural conditions, livestock is constantly exposed to parasites and eventually builds sufficient immunity to self-regulate their infections [53,54]. However young animals are particularly susceptible, and immunity may be decreased in the case of pregnancy or other physiological stressors [12,53,54]. Independent of treatment, lower FEC is seen in older animals, and immunity to parasitism develops over time [12,22]. As such, decreasing levels of GIN infection are to be expected in long term in vivo studies, and it is inappropriate not to provide control data as seen in Salem, et al. and Soca, et al. [55,56].
Both sheep and goats have been shown to preferentially eat tannin-rich food despite low nutritional content when parasitized compared to un-parasitized animals [38,47]. They are more likely to eat tannin-rich forages when they have been previously exposed to them, and they moderate their intake of these feeds depending on the severity of the parasite burden [38,47]. Voluntary ingestion of higher levels of CT was associated with a reduction in FEC, although this was not associated with higher weight gains [38,47]. Self-medicating behavior may allow the negative nutritional effects of ingesting high levels of CT to be restricted to parasitized animals, without adversely affecting healthy individuals, benefiting the production system.
c) Environmental benefits: There are many environmental benefits of SPS such as control of grass and weed growth, nutrient recycling, and reduction of the environmental impacts of farming, but these benefits have raised concerns about changes to parasite growth and survival [50,57]. Both Oliveira, et al. (2016) [50] and de Mendonca, et al. [22] discovered a balance of factors between the SPS encouraging nematode growth due to reduced solar exposure, moderate temperature, and increased humidity, and discouraging nematode growth due to increased fungal or insect predators and better animal health and productivity due to protection from the weather. In the study by Oliveira et al. (2016)[50] GIN levels in SPS were significantly higher than in conventional farms; however, blood-packed cell volume and weight gains did not differ. de Mendonca. et al. [22] did not find a difference in weight gain or GIN incidence between SPS or conventional farms. Interestingly, cattle in both these studies were unable to access the tree foliage, preventing them from receiving any possible benefits from ingesting PSC [22,50].
Considerations for implementing alternative anthelmintics
The use of plant-based alternative AH therapies can be divided into two broad categories: nutraceuticals, which are feed sources with known animal health benefits such as the CT containing plants administered as forage; and phytotherapeutic drugs, which are plant extracts that are isolated or synthesized and then administered as medication [13].
Identification of medicinal plants, and extraction and refinement of their bioactive PSCs into safe and effective phytotherapeutic drugs is an area of considerable ongoing research in human medicine [58]. The physiological effects of tannins identified in humans are quite broad ranging from beneficial effects on blood pressure and blood lipid concentration to action to improve certain cancers and benefits for HIV treatments [58,59]. However, research into the effects of tannins in ruminant livestock has largely focussed on parasitism, and rumen methane production [60]. The use of tannin-containing plants in nematode control in livestock, particularly goats, is an area of ongoing interest and research for key industry bodies [61]. Goat production is a growing industry in Australia, consequently increasing problems with GIN-related disease and production losses in goats are being observed. Goats are a good candidate for SPS systems due to a natural preference for above-ground browsing and high tolerance to tannin-rich feed [61].
Both nutraceuticals and phytotherapeutic drugs are currently poorly defined and difficult to standardize worldwide due to different growing conditions producing different concentrations of PSC, and an incomplete understanding of their functions [13]. Phytotherapeutic drugs have a greater potential for standardization and mass production, however, the environmental benefits of SPS described above would be lost and production costs would increase [13,22,50].
Economic and environmental benefits have been identified for SPS in the northern cattle grazing regions and the arid/semi-arid pastoral country of Western Australia, South Australia, and New South Wales [25,27]. Silvopastoral systems have not been similarly proven in higher rainfall areas of South Australia. However, A. saligna has been identified as a potentially valuable agroforestry crop for the production of biomass, timber, wood pulp, and fodder in southern Australia [62]. Further research is required to select appropriate trees for SPS in higher rainfall areas of South Australia and to ensure that they express sufficient CT in field conditions [21]. Traditionally trees have been removed from grazing areas for ease of management and due to concerns about pasture productivity [63]. Considerable time and effort would need to be dedicated to educating producers about the potential benefits. Tree planting for SPS may have the potential to work with government-funded carbon farming and drought mitigation projects.
Limitations
The lack of in vivo field relevant studies available makes drawing definitive conclusions difficult. Most research into the effects of Australian native perennials on GIN activity has been performed in vitro, where they demonstrated considerable effects of plant preparation and extraction methods on the activity of PSCs [21]. The activity of these PSCs may be different when ingested under field conditions. Furthermore, concerns over palatability may mean that insufficient quantities of CT will be voluntarily ingested [64]. Nonetheless, the identified effects of CT on GIN and the economic and environmental benefits of SPS systems encourage further experimentation in this area.
Conclusion
It is clear that PSCs are an area of considerable scope for research, with convincing evidence that CT can improve GIN infection dynamics and protein metabolism in ruminant species [3,33]. Nevertheless, to improve the use of these natural AHs we must standardize preparation methods between studies, better identify and understand what compounds are present and effective and perform long-term in vivo trials to assess their positive and negative effects [13]. Further study is needed to validate the key native species appropriate to the winter rainfall areas of South Australia, which will provide AH effects while benefiting the environment and potentially providing a secondary crop or income.
- Paolini V, Fouraste I, Hoste H. in vitro effects of three woody plant and sainfoin extracts on 3rd-stage larvae and adult worms of three gastrointestinal nematodes. Parasitology. 2004 Jul;129(Pt 1):69-77. doi: 10.1017/s0031182004005268. PMID: 15267113.
- Tariq KA, Chishti MZ, Ahmad F, Shawl AS, Tantray MA. Evaluation of anthelmintic activity of Iris hookeriana against gastrointestinal nematodes of sheep. J Helminthol. 2008 Jun;82(2):135-41. doi: 10.1017/S0022149X08912360. Epub 2008 Feb 5. PMID: 18252019.
- Mupeyo B, Barry TN, Pomroy WE, Ramírez-Restrepo CA, López-Villalobos N, Pernthaner A. Effects of feeding willow ( Salix spp.) upon death of established parasites and parasite fecundity. Animal Feed Science and Technology. 2011; 164(1):8-20. doi: 10.1016/j.anifeedsci.2010.11.015
- Lane JGPL, Jubb T, Shephard R, Webb-Ware J, Fordyce G. Priority list of endemic diseases for the red meat industries Meat and Livestock Australia, North Sydney, NSW. 2015
- Playford MC, Smith AN, Love S, Besier RB, Kluver P, Bailey JN. Prevalence and severity of anthelmintic resistance in ovine gastrointestinal nematodes in Australia (2009-2012). Aust Vet J. 2014 Dec;92(12):464-71. doi: 10.1111/avj.12271. PMID: 25424758.
- Maxwell D, Carmichael I, Trengove C, Johnsson G, Ellis S. WormBoss worm control program: South Australian winter rainfall. 2012. http://www.wormboss.com.au/sheep-goats/programs/sheep/sa.php (Accessed 01/07/2022)
- Roeber F, Jex AR, Gasser RB. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance - an Australian perspective. Parasit Vectors. 2013 May 27;6:153. doi: 10.1186/1756-3305-6-153. PMID: 23711194; PMCID: PMC3679956.
- Taylor L, Hodge A. Descriptive findings from analysis of a large database of cattle worm egg count and larval culture results collected across Australia between 2002 and 2012. Vet Parasitol. 2014 Aug 29;204(3-4):269-78. doi: 10.1016/j.vetpar.2014.04.029. Epub 2014 May 10. PMID: 24933468.
- Lyndal-Murphy M, Baxendell S, Kahn L, Maxwell D, Carmichael I, Trengove C, Johnsson G, Ellis S. Worm Control Program: South Australia. The University of New England, Armidale, NSW. 2016.
- Besier RB, Love SCJ. Anthelmintic resistance in sheep nematodes in Australia: the need for new approaches. Australian Journal of Experimental Agriculture. 2003; 43(12):1383-1391. doi: 10.1071/EA02229
- Coop RL, Kyriazakis I. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends Parasitol. 2001 Jul;17(7):325-30. doi: 10.1016/s1471-4922(01)01900-6. PMID: 11423375.
- Athanasiadou S, Houdijk J, Kyriazakis I. Exploiting synergisms and interactions in the nutritional approaches to parasite control in sheep production systems. Small Ruminant Research. 2008; 76(1):2-11. doi: 10.1016/j.smallrumres.2007.12.016
- Hoste H, Torres-Acosta JF, Alonso-diaz MÁ, Brunet S, Sandoval-Castro C, Adote SH. Identification and validation of bioactive plants for the control of gastrointestinal nematodes in small ruminants. Trop Biomed. 2008 Feb;25(1 Suppl):56-72. PMID: 18414378.
- Niezen JH, Robertson HA, Waghorn GC, Charleston WA. Production, faecal egg counts and worm burdens of ewe lambs which grazed six contrasting forages. Vet Parasitol. 1998 Dec 15;80(1):15-27. doi: 10.1016/s0304-4017(98)00202-7. PMID: 9877067.
- Brunet S, Aufrere J, El Babili F, Fouraste I, Hoste H. The kinetics of exsheathment of infective nematode larvae is disturbed in the presence of a tannin-rich plant extract (sainfoin) both in vitro and in vivo. Parasitology. 2007 Aug;134(Pt 9):1253-62. doi: 10.1017/S0031182007002533. Epub 2007 Mar 8. PMID: 17346358.
- Häring DA, Scharenberg A, Heckendorn F, Dohme F, Lüscher A, Maurer V, Suter D, Hertzberg H. Tanniferous forage plants: Agronomic performance, palatability and efficacy against parasitic nematodes in sheep. Renewable Agriculture and Food Systems. 2008; 23(1):19-29. doi: 10.1017/S1742170507002049
- Novobilský A, Mueller-Harvey I, Thamsborg SM. Condensed tannins act against cattle nematodes. Vet Parasitol. 2011 Dec 15;182(2-4):213-20. doi: 10.1016/j.vetpar.2011.06.003. Epub 2011 Jun 13. PMID: 21726942.
- Chan-Pérez JI, Torres-Acosta JF, Sandoval-Castro CA, Hoste H, Castañeda-Ramírez GS, Vilarem G, Mathieu C. in vitro susceptibility of ten Haemonchus contortus isolates from different geographical origins towards acetone:water extracts of two tannin rich plants. Vet Parasitol. 2016 Feb 15;217:53-60. doi: 10.1016/j.vetpar.2015.11.001. Epub 2015 Nov 4. PMID: 26827861.
- Nguyen TM, Binh DV, Ørskov ER. Effect of foliages containing condensed tannins and on gastrointestinal parasites. Animal Feed Science and Technology. 2005; 121(1):77-87. doi: 10.1016/j.anifeedsci.2005.02.013
- Krebs GL, Howard DM, Dods K. The effects of feeding Acacia saligna on feed intake, nitrogen balance and rumen metabolism in sheep. Asian-Australasian Journal of Animal Sciences. 2007; 20(9):1367-1373.
- Kotze AC, O'Grady J, Emms J, Toovey AF, Hughes S, Jessop P, Bennell M, Vercoe PE, Revell DK. Exploring the anthelmintic properties of Australian native shrubs with respect to their potential role in livestock grazing systems. Parasitology. 2009 Aug;136(9):1065-80. doi: 10.1017/S0031182009006386. Epub 2009 Jun 15. PMID: 19523255.
- de Mendonca RMA, Leite RC, Lana AMQ, Costa JO, Toth G. Parasitic helminth infection in young cattle raised on silvopasture and open-pasture in southeastern Brazil. Agroforestry Systems. 2013; 88(1):53-62. doi: 10.1007/s10457-013-9655-4
- Oliveira LM, Macedo IT, Vieira LS, Camurça-Vasconcelos AL, Tomé AR, Sampaio RA, Louvandini H, Bevilaqua CM. Effects of Mimosa tenuiflora on larval establishment of Haemonchus contortus in sheep. Vet Parasitol. 2013 Sep 23;196(3-4):341-6. doi: 10.1016/j.vetpar.2013.04.014. Epub 2013 Apr 16. PMID: 23643453.
- Emms J, Revell DK. Perennial forage shrubs - from principles to practice for Australian farms Future Farm Industries CRC. 2014.
- Revell, D. K., N. Phillips, A. C. Kotze, H. Norman, J. Emms, S. Hughes, P. E. Vercoe, Z. Durmic, P. Jessop, T. Kliminster, and M. Bennell. 2014. Perennial forage shrubs providing profitable and sustainable grazing: Key practical findings from the Enrich project. Future Farm Industries CRC.
- Lefroy EC, Dann PR, Wildin JH, Wesley-Smith RN, McGowan AA. Trees and shrubs as sources of fodder in Australia. Agroforestry Systems. 1992; 20:117-139.
- Donaghy P, Bray S, Gowen R, Rolfe J, Stephens M, Williams S, Hoffman M, Stunzer A. The bioeconomic potential for agroforestry in northern cattle grazing systems: an evaluation of tree alley scenarios in central Queensland. Rural Industries Research and Development Corporation Canberra, ACT. 2009.
- Bureau of Meteorology. 2020. Average annual, seasonal and monthly rainfall. http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall/index.jsp (Accessed 28/09/21.
- Jeffery J. Goyder's Line. 2014. https://sahistoryhub.history.sa.gov.au/subjects/goyders-line (Accessed 20/07/2022.
- Fielke, S., and D. K. Bardsley. 2015. A breif political history of South Australian agriculture Rural History. 26:101-125. doi: 10.1017/S095679331400017X
- Australian Bureau of Statistics. 2010. Feature Article: Beefing up our economy: Meat production in South Australia. https://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/1345.4Feature%20Article1Aug%202010 (Accessed 01/07/2022.
- MLA. Cattle, sheep and sustainability. 2016. https://www.mla.com.au/about-mla/Cattle-sheep-goat-industries/cattle-sheep-industry-information/cattle-sheep-and-sustainability/ May 2019).
- Molan AL, Meagher LP, Spencer PA, Sivakumaran S. Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylus colubriformis. Int J Parasitol. 2003 Dec;33(14):1691-8. doi: 10.1016/s0020-7519(03)00207-8. PMID: 14636684.
- Niezen JH, Charleston WA, Robertson HA, Shelton D, Waghorn GC, Green R. The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lamb parasite burdens and development of immunity to gastrointestinal nematodes. Vet Parasitol. 2002 May 2;105(3):229-45. doi: 10.1016/s0304-4017(02)00014-6. PMID: 11934463.
- Moreno-Gonzalo J, Manolaraki F, Frutos P, Hervás G, Celaya R, Osoro K, Ortega-Mora LM, Hoste H, Ferre I. in vitro effect of heather extracts on Trichostrongylus colubriformis eggs, larvae and adults. Vet Parasitol. 2013 Nov 8;197(3-4):586-94. doi: 10.1016/j.vetpar.2013.07.032. Epub 2013 Jul 30. PMID: 23948558.
- Landau S, Azaizeh H, Muklada H, Glasser T, Ungar ED, Baram H, Abbas N, Markovics A. Anthelmintic activity of Pistacia lentiscus foliage in two Middle Eastern breeds of goats differing in their propensity to consume tannin-rich browse. Vet Parasitol. 2010 Oct 29;173(3-4):280-6. doi: 10.1016/j.vetpar.2010.07.006. Epub 2010 Jul 22. PMID: 20705396.
- Butter NL, Dawson JM, Wakelin D, Buttery PJ. Effect of dietary tannin and protein concentration on nematode infection (Trichostrongylus colubriformis) in lambs. The Journal of Agricultural Science. 2000; 134(1):89-99. doi: 10.1017/S0021859699007315
- Lisonbee LD, Villalba JJ, Provenza FD, Hall JO. Tannins and self-medication: Implications for sustainable parasite control in herbivores. Behav Processes. 2009 Oct;82(2):184-9. doi: 10.1016/j.beproc.2009.06.009. Epub 2009 Jul 2. PMID: 19576969.
- Carulla JE, Kreuzer M, Machmüller A, Hess HD. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Australian Journal of Agricultural Research. 2005; 56(9):961-970. doi: 10.1071/AR05022
- Orlandi, T., G. V. Kozloski, T. P. Alves, F. R. Mesquita, and S. C. Ávila. 2015. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Animal feed science and technology 210:37-45. doi: 10.1016/j.anifeedsci.2015.09.012
- Iqbal Z, Sarwar M, Jabbar A, Ahmed S, Nisa M, Sajid MS, Khan MN, Mufti KA, Yaseen M. Direct and indirect anthelmintic effects of condensed tannins in sheep. Vet Parasitol. 2007 Mar 15;144(1-2):125-31. doi: 10.1016/j.vetpar.2006.09.035. Epub 2006 Nov 13. PMID: 17097807.
- Bachaya HA, Iqbal Z, Khan MN, Sindhu ZU, Jabbar A. Anthelmintic activity of Ziziphus nummularia (bark) and Acacia nilotica (fruit) against Trichostrongylid nematodes of sheep. J Ethnopharmacol. 2009 Jun 22;123(2):325-9. doi: 10.1016/j.jep.2009.02.043. Epub 2009 Mar 9. PMID: 19429379.
- Castañeda-Ramírez GS, Mathieu C, Vilarem G, Hoste H, Mendoza-de-Gives P, González-Pech PG, Torres-Acosta JFJ, Sandoval-Castro CA. Age of Haemonchus contortus third stage infective larvae is a factor influencing the in vitro assessment of anthelmintic properties of tannin containing plant extracts. Vet Parasitol. 2017 Aug 30;243:130-134. doi: 10.1016/j.vetpar.2017.06.019. Epub 2017 Jun 23. PMID: 28807282.
- Smeal MG, Fraser GC, Robinson GG. Seasonal changes in the structure of nematode populations of cattle in New South Wales in relation to inhibited larval development. Aust Vet J. 1980 Feb;56(2):80-6. doi: 10.1111/j.1751-0813.1980.tb05630.x. PMID: 7436895.
- Sutherland I, Scott I. Gastrointestinal nematodes of sheep and cattle: Biology and control. John Wiley & Sons, Chichester, UK. 2010
- Smeal, M., P. Nicholls, R. Webb, I. Hotson, F. Doughty, and W. Harding. 1981. The effect of anthelmintic treatments on growth of beef cattle in New South Wales. Australian Journal of Agricultural Research 32(5):813-823. doi: https://doi.org/10.1071/AR9810813
- Amit M, Cohen I, Marcovics A, Muklada H, Glasser TA, Ungar ED, Landau SY. Self-medication with tannin-rich browse in goats infected with gastro-intestinal nematodes. Vet Parasitol. 2013 Dec 6;198(3-4):305-11. doi: 10.1016/j.vetpar.2013.09.019. Epub 2013 Sep 25. PMID: 24140164.
- Santos LL, Salgado JA, Drummond MG, Bastianetto E, Santos CP, Brasil BSAF, Taconeli CA, Oliveira DAA. Molecular method for the semiquantitative identification of gastrointestinal nematodes in domestic ruminants. Parasitol Res. 2020 Feb;119(2):529-543. doi: 10.1007/s00436-019-06569-3. Epub 2019 Dec 13. PMID: 31834492.
- Amadesi A, Bosco A, Rinaldi L, Cringoli G, Claerebout E, Maurelli MP. Cattle gastrointestinal nematode egg-spiked faecal samples: high recovery rates using the Mini-FLOTAC technique. Parasit Vectors. 2020 May 6;13(1):230. doi: 10.1186/s13071-020-04107-0. PMID: 32375871; PMCID: PMC7204292.
- Oliveira MCS, Nicodemo MLF, Pezzopane JRM, Gusmão MR, Chagas ACS, Giglioti R, Bilhassi TB, Santana CH, Gonçalves TC, Rabelo MD, Néo TA. Gastrointestinal nematode infection in beef cattle raised in silvopastoral and conventional systems in São Paulo state, Brazil. Agroforestry Systems. 2016; 91(3):495-507. doi: 10.1007/s10457-016-9950-y
- D'Mello JPF. Chemical constraints to the use of tropical legumes in animal nutrition. Animal Feed Science and Technology. 1992; 38(2):237-261. doi: https://doi.org/10.1016/0377-8401(92)90105-F
- Min BR, Barry TN, Attwood GT, McNabb WC. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Animal Feed Science and Technology. 2003; 106(1):3-19. doi: https://doi.org/10.1016/S0377-8401(03)00041-5
- de Veer MJ, Kemp JM, Meeusen EN. The innate host defence against nematode parasites. Parasite Immunol. 2007 Jan;29(1):1-9. doi: 10.1111/j.1365-3024.2006.00910.x. PMID: 17187650.
- McRae KM, Stear MJ, Good B, Keane OM. The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 2015 Dec;37(12):605-13. doi: 10.1111/pim.12290. PMID: 26480845; PMCID: PMC4744952.
- Salem AZM, Elghandour MMY, Kholif AE, López S, Pliego AB, Cipriano-Salazar M, Chagoyán JCV, de Oca Jiménez RM, Alonso MU. Tree leaves of Salix babylonica extract as a natural anthelmintic for small-ruminant farms in a semiarid region in Mexico. Agroforestry systems. 2016; 91(1):111-122. doi: 10.1007/s10457-016-9909-z
- Soca M, Simon L, Soca M, Garcia E. Las nematodosis gastrointestinales de los bovinos jovenes en sistemas silvopastoriles comerciales. I. Empresa Pecuaria "El Cangre". Pastos y forrajes. 2003; 26(1)
- Francisco I, Arias M, Cortiñas FJ, Francisco R, Mochales E, Sánchez JA, Uriarte J, Suárez JL, Morrondo P, Sánchez-Andrade R, Díez-Baños P, Paz-Silva A. Silvopastoralism and autochthonous equine livestock: analysis of the infection by endoparasites. Vet Parasitol. 2009 Oct 14;164(2-4):357-62. doi: 10.1016/j.vetpar.2009.06.018. Epub 2009 Jun 24. PMID: 19632049.
- Kumar S, Egbuna C. Phytochemistry: An in-silico and in-vitro Update Advances in Phytochemical Research. 1st ed. 2019. ed. Springer Singapore, Singapore. 2019.
- Murthy HN, Paek KY. Bioactive compounds in underutilized vegetables and legumes. Springer, Cham, Switzerland. 2021
- Adejoro FA, Hassen A, Thantsha MS. Preparation of acacia tannin loaded lipid microparticles by solid-in-oil-in-water and melt dispersion methods, their characterization and evaluation of their effect on ruminal gas production in vitro. PLoS One. 2018 Oct 25;13(10):e0206241. doi: 10.1371/journal.pone.0206241. PMID: 30359451; PMCID: PMC6201938.
- MLA. Plants play a role in parasite control. 2022. https://www.mla.com.au/news-and-events/industry-news/plants-play-a-role-in-parasite-control/?utm_campaign=339963_Goats%20on%20the%20Move%20%E2%80%93%20Sep%202022&utm_medium=email&utm_source=Meat%20%26%20Livestock%20Australia&dm_i=4PKB,7ABF,12EGVT,S8ZE,1 (Accessed 08/09/2022.
- Millar MA. Acacia saligna as a sustainable agroforestry crop for southern Australia: a genetic assessment. 2008.
- Benavides R, Douglas GB, Osoro K. Silvopastoralism in New Zealand: review of effects of evergreen and deciduous trees on pasture dynamics. Agroforestry Systems. 2009; 76(2):327-350. doi: 10.1007/s10457-008-9186-6
- Krebs GL. The value of Acacia saligna as a source of fodder for ruminants : a report for the Rural Industries Research and Development Corporation. RIRDC, Barton, A.C.T. 2003.
- MacKown CT, Brown MA, Walker EL. Tannin rich peanut skins lack anthelmintic properties. Small Ruminant Research. 2011; 96(2-3):195-200. doi: 10.1016/j.smallrumres.2010.11.015.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
