International Journal of Veterinary Science and Research
Nutritional Intervention with Bacillus subtilis strain PB6 in Early Days, enhances Performance without affecting Carcass Characteristics of Broiler Chickens
Nabila Fathima*, Rajendra Moorthy Rajendran, Ravichandran Mani, Srinivasan Balaji and Santosh Vyas
Cite this as
Fathima N, Rajendran RM, Mani R, Balaji S, Vyas S (2022) Nutritional Intervention with Bacillus subtilis strain PB6 in Early Days, enhances Performance without affecting Carcass Characteristics of Broiler Chickens. Int J Vet Sci Res 8(3): 100-109. DOI: 10.17352/ijvsr.000121Copyright License
© 2022 Fathima N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The objective of the study was to evaluate the combinational effect of Bacillus subtilis strain PB6 along with vitamins (V), minerals (M), and amino acids (A) on performance, growth, and carcass characteristics of broiler chickens during the early days and compare with commercially available combinations of V+A, and M+A without probiotics. An in vivo trial was conducted for a period of 35 days with day 1 Cobb 430 broiler chicks, randomly allotted to one control and four treatment groups namely T1 (PB6+VMA-1 g/L), T2 (PB6+VMA-2 g/L), T3 (V+A-1 mL/L) and T4 (M+A-2 mL/L) using a completely randomized design. Each group had 7 replicates and 12 birds per replicate. The performance parameters such as body weight (BW), and feed conversion ratio (FCR) were monitored throughout the trial. At the end of 7 days, BW was significantly higher for T2 (174.71 g) in comparison with T1 (173.99 g), T3 (174.41 g), T4 (173.39 g), and control (173.35 g, p < 0.05). However, no difference in FCR was observed (p > 0.05). Similarly, at the end of 35 days, T2 (1842.15 g) showed the highest BW compared to control (1818.36 g), T1 (1839.39 g), T3 (1833.20 g), and T4 (1816.73 g) and significantly least FCR (1.53, p < 0.05) in comparison with control (1.55), T1 (1.54), T3 (1.57) and T4 (1.56). At the end of 35 days, carcass characteristics such as carcass, breast meat, and organ yield were evaluated and no significant difference between the groups was observed (p > 0.05). The gut health of the birds was assessed by evaluating the dysbacteriosis and total mean lesion score at the end of 35 days and a score of less than one was observed for all the groups. Furthermore, return on investment (ROI) was analyzed and T1 showed an ROI of 2.21:1, followed by T2 which showed an ROI of 1.72:1, and no ROI was seen for T3 and T4. The results from this study suggest that supplementation of PB6 along with essential nutrients has a positive impact on the performance of broiler chickens, without affecting gut health and helps poultry producers for profitable farming.
Introduction
The performance of broiler chickens from the chick stage to the adult stage decides the overall health and productivity of the birds [1]. The prime factors that govern performance characteristics in broilers are body weight (BW), feed intake (FI), hatchability, mortality, and carcass characteristics. In order to achieve maximum performance in birds for better profitability, it is essential that these factors are monitored and maintained optimally throughout their life cycle [2].
After hatching, the chicks are pulled and processed for various purposes, like sorting, vaccinating, and counting; and subsequently are transported to broiler farms [3]. All these processes lead to deprivation of feed and water in chicks, affecting the availability of nutrition, which is known to be detrimental to the birds’ development and health [4]. During this period, chicks are provided with immediate energy and protein by the yolk sac, as absorption of essential nutrients and maternal antibodies becomes critical for the survival of the newly hatched chick [5]. However, the nutrition provided by the yolk sac alone remains insufficient for the birds.
After post-hatch, the first week is the most critical, because chicks undergo a series of morphological, physiological, and functional changes during this period [6]. Hence, the supply of nutrients in the early days of chicks is essential to increase intestinal mechanical activity, faster intestinal development, greater assimilation of feed, and development of immunity [7].
The importance of providing early nutrition has been extensively studied over the years, which provides an elaborate overview of the effects of early nutrient supplementation on the growth parameters of newly hatched chicks [5]. It has been proved that the BW of broiler chickens at a later stage is linearly proportional to their BW in the first week [4]. Leeson, 2008 [8] suggests that nutritional intervention in the early stages of a chick’s life shows a positive effect on the overall growth of birds.
Pre-starter diets are usually the most expensive form of diet, due to their complex and intense formulation containing a higher concentration of nutrients and highly digestible ingredients [9]. However due to the poorly developed digestive system of chicks at this early stage, the fullest utilization of nutrients is not achieved, therefore growth promoters are added to nutritionally balanced diets to enhance the efficiency of poultry production. Growth promoters are generally essential nutrient substances, which provoke a response toward the maximum utilization of the genetic potential of birds, in terms of health, growth, and improvement in feed conversion efficiency [7]. These growth promoters are of different kinds which include probiotics, prebiotics, vitamins, minerals, oils, and amino acids among the widely used nutrients [10-13].
Vitamins (V) are a group of organic compounds, that are only required in small amounts for poultry birds but are essential in several metabolic and physiological processes in the growth of the animal. Fat-soluble vitamins like vitamin A, E, and D3 are involved in several metabolic processes, that positively influence birds’ performance [14-16].
One area of early nutrition that needs more attention is the requirement of trace minerals (M) during incubation and early performance of poultry birds [17]. Zinc is essential for the development of young hatchlings, as it is involved in the regulation of DNA transcription, which controls the differentiation of many cell types like T–lymphocytes and myeloid precursor cells [18]. Copper is also known for its role in blood pigmentation and to withstand any mechanical stress in cardiovascular or skeletal systems [19]. Manganese is an essential trace mineral involved in bone formation [20] and the activation of metalloenzymes that contribute to the metabolism of carbohydrates, lipids, and amino acids [21]. Chromium is an essential mineral for improving product performance and carcass yield in poultry and also plays a major role in enhancing the metabolic action of insulin, regulating energy production, muscle tissue deposition, and fat metabolism [22].
Metabolic functions of dietary essential amino acids (A) used by intestinal tissues influence their availability for growth and their requirement. The amino acids ingested by animals are metabolized by the intestinal mucosa to provide energy, which reiterates their importance for intestinal epithelial cells, and a lower percentage is used for mucosal protein synthesis [23]. Another importance of amino acids is their role in maintaining the intestinal integrity and health of the animals [10].
Probiotics are direct-fed microbial that when administered in adequate amounts have a beneficial effect on the immunity and intestinal health of the host [24]. Besides, these microorganisms are responsible for the production of vitamins of the B complex and digestive enzymes, for stimulation of intestinal mucosa immunity, and for increased protection against toxins produced by pathogenic microorganisms [25]. Along with essential nutrients like vitamins, minerals, and amino acids, probiotics play a major role in enhancing the performance of birds from the early stage onwards as they are involved in several processes that positively influence growth, along with maintaining intestinal health and integrity [26]. Bacillus subtilis strain PB6 (PB6) is a natural probiotic isolated from a healthy chicken gut that is known to produce antimicrobial substances with broad activity against various strains of Campylobacter and Clostridium species [27]. In a study conducted on Cobb 400 birds orally infected with C. perfringens, PB6 supplementation at 500 g/ton of feed reduced the FCR and intestinal C. perfringens counts significantly (p < 0.05) compared with the infected control group [28]. In addition, PB6 is known to improve overall performance in broilers compared to antibiotics bacitracin methylene di salicylate (BMD) and adriamycin and is a potential AGP replacement in the poultry industry [29].
Commercially, the combination of either vitamin and amino acids (V+A) or minerals and amino acids (M+A) in drinking water are used as growth promoters for broiler chickens. However, modern poultry producers realize that the potential of broiler chickens can be further improved. Therefore, we have hypothesized that enhancing the gut health and alleviating the stress of the birds through supplementation of PB6+VMA during the first week in drinking water will have a positive effect on the performance of broiler chickens. Also, the early supplementation effect of PB6+VMA on broiler birds has not been studied.
The objective of the work was to evaluate the effect of supplementation of PB6+VMA on the growth, performance, and carcass traits of the birds given in their early days and compare it with commercially available growth promoter formulations like V+A and M+A combinations.
Materials and methods
Study design
An in vivo trial was conducted for a period of 35 days, using a complete randomized design with Cobb 430 male broiler chicks, purchased from Komarla Hatcheries, Pollachi Taluk, India. Altum™ dry (PB6+VMA) having the combination of Bacillus subtilis strain PB6 along with vitamins, minerals, and amino acids (V+M+A) was received from Kemin Industries South Asia Pvt. Ltd., India. Bacillus subtilis subsp. subtilis (Ehrenberg), Cohn, ATCC PTA-6737 (PB6) was received in the powder form having specifications of ~1 × 10-11 spores per gram of the material from Kemin Industries South Asia Pvt. Ltd., India.
Experimental setup
The trial was conducted with a total of 420 1-d old chicks, that were allotted to five different groups. The groups were segregated into one control and four treatment groups namely T1, T2, T3, and T4, with seven replicates per group housed with 12 birds per replicate. The control group birds were supplemented with normal, untreated drinking water throughout the trial period. T1 group was supplemented with PB6+VMA at 5 grams for 100 birds, with an effective concentration of 1 g/L based on the birds’ water consumption from day 1 to 7. T2 was supplemented with PB6+VMA at 10 grams for 100 birds or 2 g/L of drinking water from day 1 to 7. T3 was supplemented with a combination of V+A at the recommended dose of 1 mL/L of drinking water from day 1 to 7 of the birds’ age and the T4 group was supplemented with a combination of M+A at a 2 mL/L dosage on days 12 – 14, 24 – 26 and 30 – 32 based on the usage recommendation.
Product application in drinking water
The products containing active ingredients, PB6+VMA in T1 and T2 treated groups, V+A in T3, and M+A in the T4 group was added to drinking water at different periods of the birds’ life span based on the recommended dosage and application periods. The water required for each replicate in different treatment groups was collected, and the respective products were added to the collected water at the given dosage levels. The treated water was then placed in respective pens of the treatment groups in bell type drinker system. This procedure of product application in water was followed twice a day at 12-hour intervals. At the end of 12 hours, any unconsumed water in the bell drinker was discarded and replaced with freshly treated water.
Feed composition and nutrient specifications
Breeder manual recommends feeding the chicken in three different phases namely pre-starter, starter, and finisher stage [30]. The feed components and the composition varies between these stages are listed in Table 1. The nutrient specifications for the diets are given in Table 2. The pre-starter, starter, and finisher feed were given from day 0 to day 14, day 15 to day 28, and day 29 to day 35 respectively. All birds were fed with ad libitum feed, containing the base composition of corn and soybean in mash form, according to the requirements for the pre-starter, starter, and finisher stage of broiler birds [30]. The ingredients such as maize, soy, rice polish, mustard de-oiled cake, crude rice bran oil, DL-methionine, L-lysine, L-threonine, sodium chloride, and sodium bicarbonate were purchased from Pooja agencies, Namakkal, India. Dicalcium phosphate was purchased from Sree Annam Chemicals Private Limited, Namakkal, India. Calcium carbonate was purchased from Sree Sakthi Industries, Coimbatore, India. Toxfin™ 360 Dry, Kemtrace® Broiler, AcidLAC™ Dry, and Phytase (5000 phytase unit – FTU) were received from Kemin Industries South Asia Private Ltd., India.
Farm conditions and bird management
Temperature and ventilation conditions were monitored throughout the trial and were appropriate to the age as recommended by the breeder manual [31]. Standard management and husbandry practices were followed throughout the trial. During the study duration, birds were provided with the following light schedule 1 – 14 days: 23 h light and 1 h dark; 15 – 35 days: 24 h optimal light. All the birds were vaccinated with the live freeze-dried vaccine against Newcastle Disease (Nobilis® ND Clone 30, MSD Animal Health, India) and live virus of intermediate strain against Infectious Bursal Disease (IBD intermediate plus, Venky’s®, India) on different days of the birds’ life span through intraocular route and drinking water. The details of the vaccination schedule are given in Table 3. Throughout the trial, the percentage of mortality of the birds was monitored.
Parameters studied for the trial
Water analysis: The drinking water was sourced from the groundwater available on the farm. Water quality was analyzed for its physical and microbial parameters, to determine the suitability for consumption by birds according to poultry drinking water standards [32]. The primary physical parameters assessed were pH, total dissolved solids (TDS), and hardness. Six samples were taken during the trial period and tested for the physical parameters. The TDS present in the water samples was measured using the AquaPro digital water tester AP-1, HM Digital, USA, and the pH was measured using the Seven Compact pH meter S220, Mettler Toledo, USA. The hardness of the water was measured using the Aquasol total hardness test kit 50 – 1000 mg/L, Rakira Biotech System Private Limited, Navi Mumbai, India.
Microbial analysis: The microbial analysis was carried out for feed from all the stages and water samples collected during the trial. The samples were analyzed for commonly found microbes in poultry feed and drinking water samples [13]: Escherichia coli, Salmonella enterica, Staphylococcus aureus, Pseudomonas aeruginosa, Clostridium perferingens, Mold, and Enterobacteriaceae. The microbial load in the feed and water samples was analyzed by enumeration of the samples using the pour plate technique [33]. For the microbial enumeration of the feed, 25 grams of the respective samples were added to 225 mL of 0.9 % sterile saline and serially diluted till 10-5 dilution and plated on specific media. For the water samples, one mL of the water sample was added aseptically in a sterile tube containing 0.9% saline solution and serially diluted till 10-5 dilution and placed on specific media for the various microbes. The selective growth media listed in Table 4 were purchased from HiMedia Laboratories, India. The results were expressed as colony-forming units (CFU).
Body weight: Birds’ BW in all the groups was measured on a weekly basis on days 7, 14, 28, and 35 [34]. The BW of birds on day 0 was also noted, and each bird was allocated with a wing band number, prior to their placement in the individual pens. The weighing apparatus for measuring the BW was a digital scale (Shimadzu UW2200H, Shimadzu Analytical Private Limited, Mumbai, India) and was calibrated on a regular basis, before recording the measurements. The average weight of the birds in the individual pen determined the average weight of the corresponding replicate group.
Weight gain: The average weight gain (AWG) of birds in each treatment group was determined from days 0 – 7, 7 – 21, and 22– 35, and the overall weight gain during the trial period was also calculated from days 0 – 35 [34]. The AWG was determined by measuring the difference between the average BW of the birds in each replicate group, on the corresponding days of weight gain measurement.
Feed consumption: The feed intake (FI) of the birds was calculated daily, and the average FI was determined from day 0 to 7, 7 to 21, and 22 to 35 along with the overall average FI from day 0 to 35 [34]. The FI was the cumulative value of the feed consumed by the birds, which was averaged for each pen from all the groups.
Feed conversion ratio: The feed conversion ratio (FCR) was calculated on a weekly basis on days 7, 14, 28, and 35 of the trial [34]. FCR was calculated by determining the ratio of the average FI of the birds in each pen and the average BW by the birds in the corresponding pen, which was done for the individual replicates in each treatment group.
Carcass characteristics: At the end of the trial, carcass yield, breast meat yield, and organ yield were calculated for all the groups as per the calculations described by Van Hoeck, et al. 2020 [22]. On day 36, 2 birds were randomly selected from each replicate, euthanized, and directly taken for weight measurements of the total carcass, breast meat, and organ meat (cumulative weight of heart, liver, and gizzard).
Intestinal lesion scoring: The birds that were utilized for determining the carcass characteristics were also subjected to lesion scoring for Eimeria tenella, E. maxima, E. acervulina, and dysbacteriosis. The lesion scoring for Eimeria species was performed as per the method described by Xue, et al. 2017 [35]. Briefly, the entire length of the small intestine of the sample birds was observed for individual lesion scoring for Eimeria tenella, Eimeria acervulina, and Eimeria maxima. The sum of the average of the mean lesion scoring of all the three species was determined as the Total Mean Lesion Score (TMLS) for Eimeria species. In the same way, birds were subjected to the monitoring of lesions for dysbacteriosis and the dysbacteriosis score was done as per Teirlynck, et al. 2011 [36].
Return on investment (ROI): The ROI was calculated at the end of the trial for all the treatment groups in comparison with the control group [37]. The economics was done based on the final body weight, total feed consumption, FCR, total chick and feed cost, total treatment cost of the products, and the benefit difference between the control and treatment groups.
The Statistical Analysis System from STATGRAPHICS® Centurion XVI software, Version 16.2.04 (Stat Graphics Technologies, Inc., Virginia, USA) was used for performing the statistical analyses. Mean, standard deviation, and pooled standard errors (SEM) were calculated for each variable. Data were analyzed with one-way ANOVA at a 95 % confidence level. No data points were excluded from the analysis. Each replicate was considered as the experimental unit for FCR. A p-value of less than 0.05 was considered statistically different.
Results
Feed quality analysis
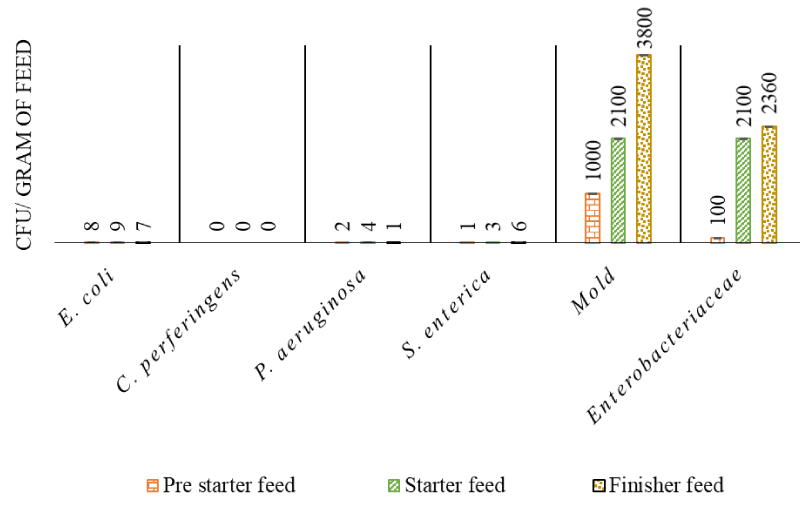
The feed samples used at different stages namely the pre-starter, starter, and finisher stages, were assessed for commonly found microbes, and the results are shown in Figure 1. It was observed that the feed samples in all phases showed < 10 CFU/g of E. coli, P. aeruginosa, C. perferingens, and S. enterica. However, Enterobacteriaceae and mold were found to be between 102 and 103 colonies in all phases of the feed.
Water quality analysis
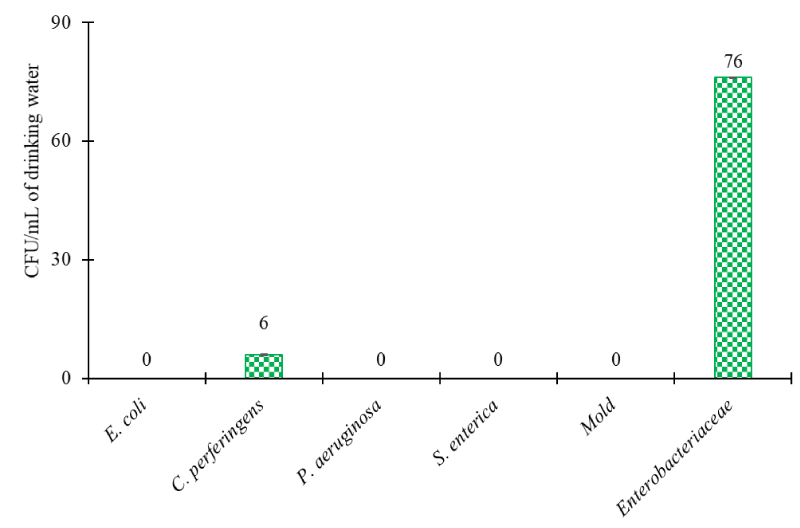
The water used for birds’ consumption was tested for TDS, hardness, and pH, and the results are given in Table 5. The TDS, hardness, and pH were found to be 189 ppm, 150 ppm, and 8.21, respectively. The presence of microbes in drinking water was also evaluated and the results are shown in Figure 2. The water samples showed less than 2 log colonies for the tested microbes.
Effect on the performance of broiler birds
Body weight: The cumulative BW of birds in all the groups was monitored on a weekly basis and the results are given in Table 6. At the end of the first week, T2 showed significantly higher BW when compared to control and other treatment groups (p = 0.009). This was also reflected at the end of the fifth week, on day 35, where the T2 group also showed significantly higher BW than the other groups (p = 0.021). Also, a dose-dependent increase of BW was observed in T1 and T2 treated groups (Table 6).
Body weight gain: The weekly BWG for all the groups was calculated and given in Table 7. T2 showed the highest BWG among the tested groups for the first 7 days and at different stages of the growth period (Table 7). For the overall rearing period (0 – 35 days), the T2 group showed significantly higher BWG (p = 0.02) than the control and other tested groups.
Feed intake: The average weekly FI was calculated during the trial and the results are given in Table 8. No significant difference in FI was noticed for any of the groups (p > 0.05).
Feed conversion ratio: The FCR of all the groups was calculated on a weekly basis and the results are given in Table 9. At the end of days 7 and 28, no significant difference was seen in the FCR of any of the groups (Table 9, p > 0.05). At the end of day 35, T1 and T2 showed the least FCR and were found to be statistically different from all the tested groups (p = 0.012).
Return on investment (ROI): The ROI for the treatment groups was calculated in comparison with the control group, at the end of the trial and the calculation is shown in Table 10. Among the groups, T1 and T2 treated groups showed a positive ROI of 2.21:1 and 1.72:1 respectively over the control group. In this trial, no cost-benefit was seen in T3 and T4 treatment groups (Table 10).
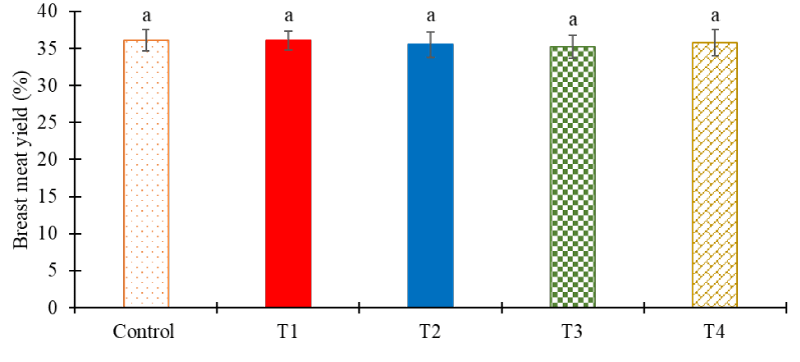
Carcass characteristics: The carcass yield, breast meat yield, and organ weight were calculated after the completion of the trial and the results are shown in Figures 3,4. Among the tested groups, no significant difference was seen in the carcass yield among the groups (Figure 3, p > 0.05). No significant difference was seen in the breast meat yield of any of the tested groups (Figure 4, p > 0.05) and T2 showed the least organ yield among all the tested groups (Figure 5, p = 0.022).
Intestinal lesion scoring for Eimeria species and dysbacteriosis: The intestinal lesion scores for Eimeria species represented as TMLS was evaluated and the results are shown in Table 11. All the groups including the control had a score of less than one, and no significant difference was observed in T3 and T4 treated groups (Table 11, p > 0.05). The dysbacteriosis lesion score for all the groups was also found to be less than one (Table 12, p = 0.031).
Discussion
The presence and occurrence of the high amount of total viable bacterial and fungal species in the feed and feed materials indicate health hazards in terms of direct consumption of such contaminated feed or their toxins by poultry birds [38]. The most common bacterial pathogens found in poultry feed belong to the Enterobacteriaceae family, specifically E. coli and Salmonella species, in addition to Staphylococcus and Pseudomonas species. The fungal pathogens present in the feed belong to the Mold genera [39]. In the poultry feed, the acceptable limits of the total Enterobacteriaceae count are 103 CFU/g of feed and the Mold count should be less than 105 CFU/g of feed [40]. In the present study, the microbial content of these specific organisms was evaluated in the pre-starter, starter, and finisher feed (Figure 1). Overall, the microbial load was found to be well within the acceptable limits of the total microbial count for poultry feed [40].
The assessment and maintenance of drinking water for poultry consumption holds as much importance as maintaining the feed quality. The drinking water quality is primarily assessed by measuring the physical and microbiological parameters comprising measurement of TDS, hardness, pH, and microbial estimation. Under ideal conditions, poultry drinking water must contain hardness of less than 500 ppm, neutral pH with total coliform counts of less than 100 colonies, and absence of Mold colonies [41]. In this study, water quality was assessed in terms of both physical parameters (Table 6) and quantification of microbial presence (Figure 2). The tested parameters showed that the water used in the trial had all the values within the acceptable range [41] and that the water was of optimum quality for poultry consumption. Since the feed and water quality were acceptable to the standard quality, any effects raised in the performance parameters must be attributed to the supplementation.
The early nutritional effect of PB6 in combination with V+M+A was studied by assessing the performance parameters such as mortality, BW, average BWG, FI, and FCR, particularly in the first week. Throughout the trial period, no mortality was observed in any of the groups, indicating that the nutrient combinations were safe for the animal. At the end of the first week, a significant improvement was seen in the BW of the birds in PB6+VMA supplemented groups (Table 6, p = 0.009), when compared to the control and other nutritional supplemented groups. A linear effect of PB6+VMA supplementation was also seen in the BW of birds, where a significant improvement was seen in the groups treated with 10 grams of PB6+VMA when compared to 5 grams dosage (Table 6, p = 0.009). Jha, et al. 2019 [7] reported that the digestive system is poorly developed in the first week of a broiler chicken’s life which could be due to insufficient utilization of ingredients when supplemented through the feed. Sugiharto, et al. 2018 [42] studied the effect of multi probiotic strains (Bacillus cereus strain SIIA_Pb_E3, Bacillus licheniformis strain FJAT-29133, Bacillus megaterium strain F4-2-27 and Bacillus sp. 11CM31Y12) along with minerals and vitamins on the growth parameters of broilers added in feed as on top application. In their study, the combination of multi probiotics at 0.1 %, 0.5 % and 1 % along with vitamins and minerals did not yield any significant difference in the body weight when compared to the control group. These results are contradictory to the results of the present study as the combination of PB6+VMA resulted in significantly higher BW at the end of the first week. The observed positive effect could be correlated to the supplements which were well utilized by birds due to the higher bioavailability of the ingredients present in the formulation.
In this trial, improvement in the first week of BW has been seen clearly in the PB6 + VMA groups (Table 6, p = 0.021), indicating the positive effect of the combination of Bacillus subtilis PB6 and essential nutrients in the early nutrition of broiler life cycle. This improvement in the first week BW was also seen at the end of the fifth week, which confirms that the BW gain in the first week is very crucial to reaching maximum BW at the end of 5 weeks and supports the findings of Simon, et al. 2015 [4]. Also, PB6+VMA treated group showed a significant increase in BW compared to other treated groups (Table 6, p = 0.021). Such 1st week BW translation to final BW agrees with a study done by Selvam, et al. 2015 [13] who reported that groups supplemented with liquid multivitamin and amino acids through drinking water showed higher BW in the first week and the same increase in BW was observed at the end of the trial in broiler birds.
The average BWG from 0 – 35 days showed significantly higher WG in T2 (PB6+VMA at 10 g dose) when compared to the control group, T3 and T4 groups (Table 7, p = 0.02). Such a trend in WG was observed throughout the trial. The additional WG could be attributed to the inclusion of probiotics (PB6) in T1 and T2. These findings were similar to studies done by Zhang, et al. 2014 [43] and Kim, et al. 2012 [44] where supplementation of probiotics in broiler chickens showed higher BW gain when compared to the control and antibiotics treated group.
No significant difference was seen in the overall feed consumption in any of the groups including the control group (Table 8, p > 0.05). These results were similar to findings by Sugiharto, et al. 2018 [42] in which the supplementation of probiotic preparation in combination with vitamins and minerals did not yield a significant difference in the overall feed intake of the broiler chickens at the end of 42 days.
At the end of the first week, no significant difference was seen in the FCR of any of the groups (Table 9, p > 0.05). However, on day 35, T2 showed the least FCR among the treated groups, and T3 showed the highest FCR (Table 9, p = 0.012). A similar finding was also reported by Yang, et al. 2016 [45] in which chromium enriched probiotic treated group supplemented through feed showed a significant improvement in FCR of broiler birds. Peric, et al. 2010 [46] reported no improvement in FCR at the end of six weeks in broiler birds supplemented with probiotics and phytogenic compound combinations. Gajula, et al. 2011 [47] studied the effect of zinc and manganese on the performance of broiler chickens and reported no significant effect of the tested compounds on the FCR of birds at 35 days of age. Considering the reports by Yang, et al. 2016, Peric, et al. 2010, Gajula, et al. 2011 [45-47] and the results observed in this study, the positive effect on FCR could be correlated to the presence of PB6 in PB6+VMA.
Further ROI for the treatment groups was calculated in comparison with the control group. Among the treated groups, T1 showed an ROI of 2.21:1 over the control group, followed by T2 which showed an ROI of 1.72:1 (Table 10). In the present study, no cost-benefit was seen in the T3 and T4 groups which contradicts the ROI of 24:1 reported by Selvam, et al. 2015 [13] studied with VA combination. In another study done by Lokapirnasari, et al. 2017 [48] birds administered with 0.005 % probiotics in drinking water resulted in an ROI of 15 % over the control group.
At the end of five weeks, on day 36, additional performance parameters such as carcass meat yield, breast meat yield, and organ yield were also monitored in the tested groups. No significant difference was seen in the breast meat yield among any of the tested groups (Figure 3, p > 0.05). Likewise, Hossain, et al. 2015 [49] also observed no statistical difference in the breast yield of the birds treated with probiotics (Figure 4, p > 0.05). The organ yield percentage in VA-treated groups was the highest among the tested groups and no other groups showed any significant difference (Figure 5, p > 0.05).
The effect of the nutritional supplements on intestinal health in addition to performance was assessed through dysbacteriosis and Eimeria species lesion scoring. The intestinal lesion scoring of the birds in all the tested groups was found to be less than one for both Eimeria species (Table 11, p > 0.05) and dysbacteriosis lesions (Table 12, p = 0.031). Bozkurt, et al. 2014 [50] studied the effect of multienzymes, probiotics, prebiotics, and herbal essential oil mixture on the intestinal lesion improvement of Eimeria species upon induced challenge. In their study, all the treatment groups showed a reduction in the severity of intestinal coccidial lesions induced by the mixed Eimeria species. In the present study, no such challenge was induced in the birds, and hence the intestinal lesion scoring was found to be minimal for both Eimeria and dysbacteriosis. This confirms that Eimeria and bacterial pathogens did not affect the study results and the supplementation did not influence the growth of Eimeria species and pathogenic bacteria.
Conclusion
Early nutritional supplementation of PB6+VMA to broiler chicks in the first seven days of their life span can significantly improve the overall performance of birds. This positive impact on performance is attributed to the supplementation of these essential nutrients through drinking water, increasing their bioavailability without affecting the gut health of birds. This study generates an insight that the right nutritional intervention in the early days results in profitable farming.
Conflict of interest
The authors have no conflict of interest to declare. All co-authors have seen and agreed with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
The authors are grateful to the team members of R&D Poultry Farm, Kemin Industries South Asia Pvt. Ltd. for their help in conducting the trial.
Authors’ contribution
Nabila Fathima, S prepared the manuscript, processed the data, and conducted lab studies; Rajendra Moorthy, R involved in trial design and animal trial protocol, reviewed the manuscript, and interpreted the data; Ravichandran, M supervised the animal trial; Srinivasan, B conducted feed and water quality analysis; Santosh, V reviewed the manuscript.
Ethics approval statement
The studies carried out in this trial were conducted by authorized, qualified, and trained veterinarians, scientists, and technicians in compliance with the guidelines laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals.
- González-Ortiz G, Olukosi O, Bedford MR. Evaluation of the effect of different wheats and xylanase supplementation on performance, nutrient and energy utilisation in broiler chicks. Anim Nutr. 2016 Sep;2(3):173-179. doi: 10.1016/j.aninu.2016.06.005. Epub 2016 Jul 1. PMID: 29767098; PMCID: PMC5941034.
- Mebratie WS. The genetics of body weight and feed efficiency in broiler chickens. Wageningen University and Research. 2019.
- Jessen CT, Foldager L, Riber AB. Effects of hatching on-farm on performance and welfare of organic broilers. Poult Sci. 2021 Sep;100(9):101292. doi: 10.1016/j.psj.2021.101292. Epub 2021 May 27. PMID: 34298386; PMCID: PMC8322470.
- Simon K, de Vries Reilingh G, Bolhuis JE, Kemp B, Lammers A. Early feeding and early life housing conditions influence the response towards a noninfectious lung challenge in broilers. Poult Sci. 2015 Sep;94(9):2041-8. doi: 10.3382/ps/pev189. Epub 2015 Jul 17. PMID: 26188030.
- Taha-Abdelaziz K, Hodgins DC, Lammers A, Alkie TN, Sharif S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: A review. Vet Immunol Immunopathol. 2018 Jul;201:1-11. doi: 10.1016/j.vetimm.2018.05.001. Epub 2018 May 7. PMID: 29914674.
- Givisiez PEN, Moreira Filho ALB, Santos MRB, Oliveira HB, Ferket PR, Oliveira CJB, Malheiros RD. Chicken embryo development: metabolic and morphological basis for in ovo feeding technology. Poult Sci. 2020 Dec;99(12):6774-6782. doi: 10.1016/j.psj.2020.09.074. Epub 2020 Oct 7. PMID: 33248593; PMCID: PMC7705034.
- Jha R, Singh AK, Yadav S, Berrocoso JFD, Mishra B. Early Nutrition Programming (in ovo and Post-hatch Feeding) as a Strategy to Modulate Gut Health of Poultry. Front Vet Sci. 2019 Mar 21;6:82. doi: 10.3389/fvets.2019.00082. PMID: 30949488; PMCID: PMC6437089.
- Leeson S. Predictions for commercial poultry nutrition. Journal of Applied Poult Res, 2008; 17(2): 315-22.
- Barekatain MR, Swick RA. Composition of more specialised pre-starter and starter diets for young broiler chickens: a review. Anim Production Sci. 2016; 56(8): 1239-47.
- Bortoluzzi C, Rochell SJ, Applegate TJ. Threonine, arginine, and glutamine: Influences on intestinal physiology, immunology, and microbiology in broilers. Poult Sci. 2018 Mar 1;97(3):937-945. doi: 10.3382/ps/pex394. PMID: 29294123.
- Oladokun S, Adewole DI. In ovo delivery of bioactive substances: an alternative to the use of antibiotic growth promoters in poultry production—a review. J Appl Poult Res. 2020; 29(3): 744-63.
- Gadde U, Kim WH, Oh ST, Lillehoj HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017 Jun;18(1):26-45. doi: 10.1017/S1466252316000207. Epub 2017 May 9. PMID: 28485263.
- Selvam R, Saravanakumar M, Sureshbabu G, Prashanth D. A liquid multivitamin and amino acid supplement, Easygrow™ on growth performance in Vencobb 400 broiler chickens. Int J Adv Sci Technol Res. 2015; 5: 72-7.
- Espín S, Ruiz S, Sánchez-Virosta P, Salminen JP, Eeva T. Effects of experimental calcium availability and anthropogenic metal pollution on eggshell characteristics and yolk carotenoid and vitamin levels in two passerine birds. Chemosphere. 2016 May;151:189-201. doi: 10.1016/j.chemosphere.2016.02.074. Epub 2016 Mar 15. PMID: 26943740.
- Niu ZY, Liu FZ, Yan QL, Li WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci. 2009 Oct;88(10):2101-7. doi: 10.3382/ps.2009-00220. PMID: 19762862.
- Yair R, Shahar R, Uni Z. In ovo feeding with minerals and vitamin D3 improves bone properties in hatchlings and mature broilers. Poult Sci. 2015 Nov;94(11):2695-707. doi: 10.3382/ps/pev252. PMID: 26500269.
- Torres CA, Korver DR. Influences of trace mineral nutrition and maternal flock age on broiler embryo bone development. Poult Sci. 2018 Aug 1;97(8):2996-3003. doi: 10.3382/ps/pey136. PMID: 29788199.
- Huang L, Li X, Wang W, Yang L, Zhu Y. The Role of Zinc in Poultry Breeder and Hen Nutrition: an Update. Biol Trace Elem Res. 2019 Dec;192(2):308-318. doi: 10.1007/s12011-019-1659-0. Epub 2019 Feb 14. PMID: 30767181.
- Hu Y, Chen Z, Lu L, Zhang L, Liu T, Luo X, Liao X. Determination of dietary copper requirement by the monoamine oxidase activity in kidney of broilers from 1 to 21 days of age. Anim Nutr. 2022 Mar;8(1):227-234. doi: 10.1016/j.aninu.2021.05.013. Epub 2021 Nov 15. PMID: 34988304; PMCID: PMC8688862.
- Mwangi S, Timmons J, Ao T, Paul M, Macalintal L, Pescatore A, Cantor A, Dawson KA. Effect of manganese preconditioning and replacing inorganic manganese with organic manganese on performance of male broiler chicks. Poult Sci. 2019 May 1;98(5):2105-2113. doi: 10.3382/ps/pey564. PMID: 30590788; PMCID: PMC6448132.
- Haq Z, Jain RK, Khan N, Dar MY, Ali S, Gupta M, Varun TK. Recent advances in role of chromium and its antioxidant combinations in poultry nutrition: A review. Vet World. 2016 Dec;9(12):1392-1399. doi: 10.14202/vetworld.2016.1392-1399. Epub 2016 Dec 9. PMID: 28096611; PMCID: PMC5234053.
- Van Hoeck V, Sonawane M, Gonzalez Sanchez AL, Van Dosselaer I, Buyens C, Morisset D. Chromium propionate improves performance and carcass traits in broilers. Anim Nutr. 2020 Dec;6(4):480-487. doi: 10.1016/j.aninu.2020.03.005. Epub 2020 Apr 24. PMID: 33364464; PMCID: PMC7750789.
- Adebowale TO, Yao K, Oso AO. Major cereal carbohydrates in relation to intestinal health of monogastric animals: A review. Anim Nutr. 2019 Dec;5(4):331-339. doi: 10.1016/j.aninu.2019.09.001. Epub 2019 Sep 20. PMID: 31890909; PMCID: PMC6920401.
- Liao SF, Nyachoti M. Using probiotics to improve swine gut health and nutrient utilization. Anim Nutr. 2017 Dec;3(4):331-343. doi: 10.1016/j.aninu.2017.06.007. Epub 2017 Jul 8. PMID: 29767089; PMCID: PMC5941265.
- M'Sadeq SA, Wu S, Swick RA, Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim Nutr. 2015 Mar;1(1):1-11. doi: 10.1016/j.aninu.2015.02.004. Epub 2015 Mar 11. PMID: 29766984; PMCID: PMC5884463.
- Wang B, Gong L, Zhou Y, Tang L, Zeng Z, Wang Q, Zou P, Yu D, Li W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim Nutr. 2021 Sep;7(3):829-840. doi: 10.1016/j.aninu.2021.03.008. Epub 2021 Jul 24. PMID: 34466687; PMCID: PMC8384779.
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Villa RE, Wallace RJ, Wester P, Brozzi R, Saarela M. Safety and efficacy of Bacillus subtilis PB6 (Bacillus subtilis ATCC PTA-6737) as a feed additive for sows. EFSA J. 2017 May 26;15(5):e04855. doi: 10.2903/j.efsa.2017.4855. PMID: 32625503; PMCID: PMC7010055.
- Jayaraman S, Thangavel G, Kurian H, Mani R, Mukkalil R, Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult Sci. 2013 Feb;92(2):370-4. doi: 10.3382/ps.2012-02528. PMID: 23300303.
- Jayaraman S, Das PP, Saini PC, Roy B, Chatterjee PN. Use of Bacillus subtilis PB6 as a potential antibiotic growth promoter replacement in improving performance of broiler birds. Poult Sci. 2017 Aug 1;96(8):2614-2622. doi: 10.3382/ps/pex079. PMID: 28482065.
- NRC. Nutrient Requirements of Poultry, 9th rev. ed. Washington, DC: Natl Acad Pressn In Poult Sci. 1994; 2002: 74.
- Cobb Broiler Management Guide. 2018. https://www.cobb-vantress.com/assets/5c7576a214/Broiler-guide-R1.pdf [accessed 02 Jan 2021]
- Bügener E, Kump AW, Casteel M, Klein G. Benefits of neutral electrolyzed oxidizing water as a drinking water additive for broiler chickens. Poult Sci. 2014 Sep;93(9):2320-6. doi: 10.3382/ps.2014-03909. Epub 2014 Jul 18. PMID: 25037820.
- Altug G, Cardak M, Ciftci PS, Gurun S. The application of viable count procedures for measuring viable cells in the various marine environments. J Appl Microbiol. 2010 Jan;108(1):88-95. doi: 10.1111/j.1365-2672.2009.04411.x. PMID: 19566720.
- Roofchaei A, Rezaeipour V, Vatandour S, Zaefarian F. Influence of dietary carbohydrases, individually or in combination with phytase or an acidifier, on performance, gut morphology and microbial population in broiler chickens fed a wheat-based diet. Anim Nutr. 2019 Mar;5(1):63-67. doi: 10.1016/j.aninu.2017.12.001. Epub 2017 Dec 28. PMID: 30899811; PMCID: PMC6407079.
- Xue GD, Wu SB, Choct M, Swick RA. The role of supplemental glycine in establishing a subclinical necrotic enteritis challenge model in broiler chickens. Anim Nutr. 2017 Sep;3(3):266-270. doi: 10.1016/j.aninu.2017.05.004. Epub 2017 May 25. PMID: 29767149; PMCID: PMC5941231.
- Teirlynck E, Gussem MD, Dewulf J, Haesebrouck F, Ducatelle R, Van Immerseel F. Morphometric evaluation of "dysbacteriosis" in broilers. Avian Pathol. 2011 Apr;40(2):139-44. doi: 10.1080/03079457.2010.543414. PMID: 21500033.
- Wealleans AL, Li W, Romero LF, Mathis G, Lumpkins B. Performance, and cost-benefit improvements following supplementation with a combination of direct-fed microbials and enzymes to broiler chickens raised with or without ionophores. J Appl Poult Res. 2018; 27: 23-32.
- Danbappa AA, Alhassan KA, Shah MM. Isolation and identification of microbial contaminants associated with commercial poultry feeds. J Appl Adv Res. 2018; 3(5): 142-7.
- Maciorowski KG, Herrera P, Jones FT, Pillai SD, Ricke SC. Effects on poultry and livestock of feed contamination with bacteria and fungi. Anim Feed Sci Technol. 2007; 133(1-2): 109-36.
- Andreoletti O, Budka H, Buncic S, Colin P, Collins JD, De A, Noeckler BN, Maradona MP, Roberts T, Vagsholm I, Vanopdenbosch E. Microbiological risk assessment in feedingstuffs for food-producing animals Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008; 720: 1-84.
- Fairchild BD, Ritz CW. Poultry drinking water primer. 2009. https://athenaeum.libs.uga.edu/bitstream/handle/10724/12452/B1301.pdf?sequence=1&origin=publication_detail. [accessed 02 Jan 2021].
- Sugiharto S, Isroli I, Yudiarti T, Widiastuti E. The effect of supplementation of multistrain probiotic preparation in combination with vitamins and minerals to the basal diet on the growth performance, carcass traits, and physiological response of broilers. Vet World. 2018 Feb;11(2):240-247. doi: 10.14202/vetworld.2018.240-247. Epub 2018 Feb 24. PMID: 29657411; PMCID: PMC5891882.
- Zhang ZF, Kim IH. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult Sci. 2014 Feb;93(2):364-70. doi: 10.3382/ps.2013-03314. PMID: 24570458.
- Kim JS, Ingale SL, Kim YW, Kim KH, Sen S, Ryu MH, Lohakare JD, Kwon IK, Chae BJ. Effect of supplementation of multi-microbe probiotic product on growth performance, apparent digestibility, cecal microbiota and small intestinal morphology of broilers. J Anim Physiol Anim Nutr (Berl). 2012 Aug;96(4):618-26. doi: 10.1111/j.1439-0396.2011.01187.x. Epub 2011 Jun 23. PMID: 21699585.
- Yang J, Qian K, Zhang W, Xu Y, Wu Y. Effects of chromium-enriched Bacillus subtilis KT260179 supplementation on chicken growth performance, plasma lipid parameters, tissue chromium levels, cecal bacterial composition and breast meat quality. Lipids Health Dis. 2016 Nov 8;15(1):188. doi: 10.1186/s12944-016-0355-8. PMID: 27821122; PMCID: PMC5100260.
- Peric LI, Milosevic NI, Zikic DR, Bjedov SI, Cvetkovic DR, Markov SI, Mohnl M, Steiner T. Effects of probiotic and phytogenic products on performance, gut morphology and cecal microflora of broiler chickens. Arch Anim Breeding. 2010; 53(3): 350-9.
- Gajula SS, Chelasani VK, Panda AK, Mantena VL, Savaram RR. Effect of supplemental inorganic Zn and Mn and their interactions on the performance of broiler chicken, mineral bioavailability, and immune response. Biol Trace Elem Res. 2011 Feb;139(2):177-87. doi: 10.1007/s12011-010-8647-8. Epub 2010 Mar 3. PMID: 20198454.
- Lokapirnasari WP, Dewi AR, Fathinah A, Hidanah S, Harijani N, Soeharsono, Karimah B, Andriani AD. Effect of probiotic supplementation on organic feed to alternative antibiotic growth promoter on production performance and economics analysis of quail. Vet World. 2017 Dec;10(12):1508-1514. doi: 10.14202/vetworld.2017.1508-1514. Epub 2017 Dec 25. PMID: 29391694; PMCID: PMC5771178.
- Hossain MA, Dev S, Jahan I, Hossain MM. Growth performance, gut health, carcass yield traits and profitability of broiler chicken raised on compound diet supplemented with probiotics. Int J Agric Res, Innov Technol. 2020; 10(1): 28-34.
- Bozkurt M, Aysul N, Küçükyilmaz K, Aypak S, Ege G, Catli AU, Aksit H, Cöven F, Seyrek K, Cinar M. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poult Sci. 2014 Feb;93(2):389-99. doi: 10.3382/ps.2013-03368. PMID: 24570461.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
