International Journal of Agricultural Science and Food Technology
Natural bee bread positively regulates lipid metabolism in rats
Zhen Li1,2, Qiang Huang1,2, Yibo Liu1,2, Chengtao Peng1,2 and Zhijiang Zeng1,2*
2Jiangxi Province Key Laboratory of Honeybee Biology and Beekeeping, Nanchang 330045, China
Cite this as
Li Z, Huang Q, Liu Y, Peng C, Zeng Z (2021) Natural bee bread positively regulates lipid metabolism in rats. J Agric Sc Food Technol 7(3): 266-271. DOI: 10.17352/2455-815X.000118Natural bee bread contains various active ingredients, which can promote human health. However, the functional evaluation of bee bread is seldom reported. We conducted this study to evaluate the effect of natural bee bread on lipid metabolism using rats as model organism.
Rats were treated with three doses of bee bread (80 mg/kg, 400 mg/kg and 800 mg/kg). A blank control group and a high fat model control group also were set up. The protein and gene expression of Fatty Acid Synthase (FAS), Acetyl CoA carboxylase (ACC) and lipoprotein lipase (LPL) in the liver tissue of rats were measured by reagent kits and real time quantitative PCR (RT-qPCR), respectively. The results showed that bee bread treatment groups significantly decreased the concentration of FAS and ACC in the liver (P < 0.05). LPL was also reduced after bee bread treatment, even though the difference was not statistically significant from the high fat model group. The gene expression levels of FAS and ACC significantly declined (P < 0.05) in all treatment groups compared with the high fat model group. It was concluded that the natural bee bread has a positive effect on the regulation of rat lipid metabolism, indicating that it is a promising lipid metabolic regulator candidate to treat hyperlipidemia.
Practical applications: Our results show that it is a promising lipid metabolism regulator, and may be used to treat hyperlipidemia.
Introduction
Pollen is a male germ cell of flowering plants and contains abundant nutrients including protein, amino acids, vitamins, trace elements and flavonoids, etc [1]. Pollen is an essential food resource for pollinating insects. As an ancient pollinator, the honey bee has developed specific tissues and organs for collecting pollen through hundreds of millions of years of evolution, such as tarsus brush, pollex, bristles and pollen baskets. They can forage a large amount of fresh plant pollen as the basic nutrition source of protein needed for larvae and young bees [2]. However, pollen cannot be stored for a long time because of its high moisture content which makes it easy to deteriorate [3]. As a result, the honeybees collect and bite the pollen mass, wet it, add nectar and hypopharyngeal gland secretion, finally tamp it in the nest [4]. Perhaps this process leads to the production of bee bread.
During the fermentation of pollen turning into mature bee bread, the moisture content and pH value gradually decrease to a suitable condition for long-term storage [4]. Therefore, bee bread has an excellent bacteriostatic effect [2]. Many scholars believe that the nutritional value of pollen will increase greatly after it is fermented into bee bread. This may be due to the fact that, during fermentation, proteins are cut into small polypeptides by Bifidobacterium, which are easily absorbed by the body [2,5,6]. The level of total sugar, hydrolyzed amino acids, flavonoid compounds, polyphenols, unsaturated fatty acids and other nutrients were all increased [7,8]. Bee bread is a distinctive product, which is very important not only for bees, but also for humans. In our previous study, bee bread shows an extraordinary effect on reducing blood lipids [10]. More importantly, it has antioxidant, anti-tumor and anti-cancer effects, and can prevent and improve cardiovascular disease, cancer and gastrointestinal disease [4].
Hyperlipidemia is a disorder of lipid metabolism, and its clinical manifestation is that the levels of Triglycerides (TG), Total Cholesterol (TC) and Low Density Lipoprotein (LDL) are increased while the levels of High Density Lipoprotein (HDL) are decreased [9,10]. It is the ‘initiator’ of atherosclerosis, which can cause cardiovascular disease, fatty liver, cancer, Alzheimer’s Disease (AD), Parkinson’s Disease (PD), etc [11,12]. With the improvement of people’s living standards and changes of lifestyle, the incidence of hyperlipidemia is increasing yearly. There are many factors causing hyperlipidemia, including dietary habits, daily life, mental stress and social environmental factors [13]. Excessive lipid intake is one of the main factors causing hyperlipidemia. At present, hyperlipidemia in all age groups has an obvious rising trend, and has become an important public health problem, attracting extensive attention from scholars at home and abroad [14].
Recently, researchers have focused on the use of natural active ingredients, such as polysaccharides, flavonoids and polyphenols, to treat hyperlipidemia [15-17]. However, lipid metabolism in the liver is a very complex process, which is determined by many factors. Therefore, conducting a deep investigation of metabolic regulation mechanisms is the key to solve this problem. FAS and ACC are the key enzymes of fat synthesis. Overexpression of FAS results in the deposition of triglycerides in liver and blood [18]. ACC is a rate-limiting enzyme in the first step of fatty acid ab initio synthesis and the content of ACC restricts the rate of fatty acid synthesis [19]. Additionally, LPL catalyzes the hydrolysis of triglycerides in chyle particles and LDL. Deficiency of LPL leads to abnormal plasma metabolism [20].
In the current study, we quantified the effect of natural bee bread on liver lipid metabolism enzymes and relative gene expression in hyperlipidemia rats, to evaluate the potential for medical treatment of hyperlipidemia disease using bee bread as the active ingredient.
Materials and method
Production of natural bee bread
Natural bee bread was prepared with Natural Bee Bread Grain Producer from the fermentation of lotus pollen. Bee bread was stored in -18 0C refrigerator for experimental use. Then natural bee food was dissolved in pure water and used for intragastric gavage animals to ensure complete ingestion.
Animals and treatments
Fifty SPF rats (bought from Laboratory Animal Science and Technology Center of Jiangxi Chinese Medicine University, Certificate No. SCXK (GAN) 2018-0003) weighted 93.44±1.19 g was used in this experiment. The rats were fed in room temperature (20-23 0C) and humidity (45-55%) with SPF barrier system. The rats were fed adaptively for four days, during which time they were free to criterion forage and water. To induce hyperlipidemia, 42 rats were fed with a high fat fodder (88.9% basic chow, 10% butter, 1% cholesterol and 0.1% extract) for 20 days. At the same time, eight normal rats as the blank control, were fed with basic diet continuously. After 20 days, serum levels of TC, TG, HDL and LDL were quantified in rats after an overnight fast. According to the TG levels, 32 hyperlipidemia rats were randomly assigned to 4 groups: high fat model group (each rat was gavaged with 1 mL of water per day), three dose of bee bread (80 mg/kg·BW/d, 400 mg/kg·BW/d and 800 mg/kg·BW/d) were fed to the remaining three groups hyperlipidemia rats respectively. The three groups are referred to as LBG, MBG and HBG respectively. All test substance and reference material were took gavage of the hyperlipidemia treatment rats while the blank control group rats were continuously provided with a basic feed during the whole trial period, but high fat diet was still supplied to the treatment group and high fat model group. Intragastric gavage was continuously conducted for 8 weeks. All rats were anaesthetized and sacrificed after the trial ended. The liver was removed and stored in the refrigerator at -800C until it was used in the follow-up experiment. All of the experimental procedures outlined in this work were performed in accordance with current China laws on animal experimentation and ethical standards of animal ethics committee of Jiangxi Agricultural university (JXAULL-20180012).
Biochemical analysis
1 g liver tissue was taken and washed in pre-cooled PBS (0.01 mol/L, pH 7.2-7.4) to remove blood. Tissue blocks were moved into glass homogenizer, and 5-10 mL pre-cooled PBS was added to grind sufficiently. This process was carried out on ice. The mass-volume ratio of adipose tissue to PBS was 1:9. The obtained homogenate was frozen and thawed repeatedly for 2-3 times. The prepared homogenate was centrifuged for 5 min at 5000 r/min. The supernatant was carefully collected and the levels of FAS, ACC and LPL were measured according to the instructions of ELISA kit (Shanghai Enzyme Link Biotech).
RNA extraction and cDNA synthesis
Pools of 50-100 mg livers were homogenized in liquid nitrogen using a pestle, and then were used for isolation of total RNA with 1 mL Trizol Reagent according to the manufacturer’s instructions (Trans Gen Biotech, China). RNA was reverse-transcribed using the Primescript reagent kit (Takara Biotech, China). During the first step, 1μg of total RNA was mixed with 1μL gDNA Eraser (5×), 1μL gDNA Eraser and 6μL RNase-Free water to a final volume of 20μL and incubated at 420C for 5 min which aimed to remove DNA. Then the cDNA synthesis mixture contained of 10μL first step reaction liquid, 1μL Prime Script RT Enzyme Mix (1), 1μL RT Primer Mix, 4μL Prime Script Buffer (2), and 4 μL Rnase-Free water, so the finally total volume was 20μL. The mixture was incubated at 370C for 15 min, and 850Cfor 5 sec. The cDNA was kept at -200C until used for real-time quantification PCR.
Primer design and RT-qPCR assays
Quantification measurement of mRNA expression levels of the three specific genes was performed by RT-qPCR technology, using β-actin as an internal reference gene [21]. Primer sequences and GenBank number are summarized in Table 1.
The final volumes of all reactions in the system were 10μL, which were carried out in a 96-well plate. The reaction mixture includes 5μL TB Green Mix (Trans Gen Biotech, China), 0.4 μL forward primer (10μM), 0.4 μL reverse primer (10μM) 0.2 μL Rox fluorescent dyes, 3μL RNase-Free water and 1μL cDNA. The cycling procedures consisted of the following steps: initial temperature 950C for 2 min, then the 40 cycles including 950C for 15 sec, annealing for 30 sec at 55-600C (FAS, ACC 60.5 0C, LPL 55.6 0C), and finally extension at 72 0C for 40 sec.
Data analysis
The relative gene expressions were calculated using the equation RQ= (fold) on the basis of the ΔΔCT-formula [22]. The variance of relative lipid metabolizing enzymes content and relative gene expression level was analyzed using ANOVA from the StatView 5.0 software (SAS Institute, Gary, NC, USA).
Results
The levels of FAS, ACC, LPL in liver tissue
Compared with blank control group, the concentration of FAS and ACC were significantly increased in the untreated high fat model group (P < 0.05), whereas liver LPL level was not significantly different (P >0.05). After treatment with the natural bee bread, the FAS concentrations in liver tissue of rats in three bee bread dosage groups were significantly decreased (P < 0.05), and the ACC contents in liver tissue in middle and high bee bread dosage groups were significantly decreased (P < 0.05) compared with the high fat model group. However, there was no significant differences (P>0.05) between the three bee bread dose groups and high fat model group with the levels of LPL in liver tissue (Table 2).
Effects of bee bread on the expression levels of FAS genes, ACC genes, LPL genes in the liver tissue
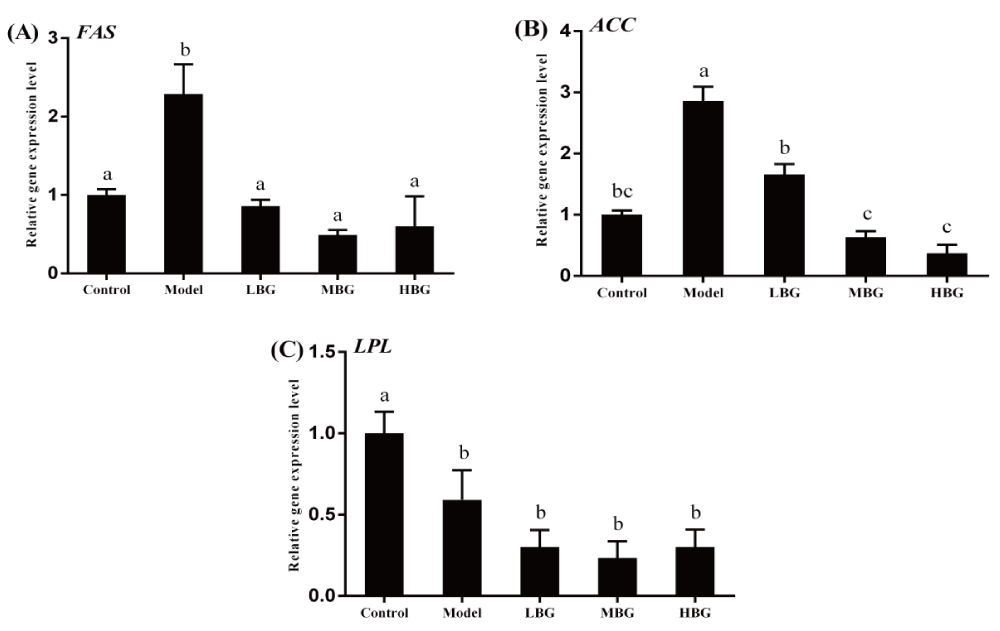
The trend of expression levels of FAS, ACC and LPL was consistent with the changes in the levels of the lipid metabolic enzymes encoded by each gene respectively. Compared with the blank control group, the expression of FAS gene and ACC gene in the liver tissue of model rats were significantly increased (P < 0.05) by high fat diet. The high fat diet significantly decreased the expression of LPL gene (P < 0.05). By contrast, three treatments with different doses of bee bread significantly decreased the FAS gene and ACC gene expression levels (P< 0.05), but had no significant effect on the LPL mRNA expression levels (P>0.05) compared with the high fat model group (Figure 1).
Discussion
Natural bee bread is a fermentation product of a mixture of plant pollen particles, nectar and bee secretions [23]. It is superior to bee pollen with better nutritional value, higher digestibility and richer natural active ingredients [24]. After fermentation by lactic acid bacteria, the cell wall of plant pollen was broken and a large number of nutrients were released which are better absorbed by the human body than pollen [2,25].
The lipogenic capacities of both the liver and adipose tissue are component amounts which are essentially controlled by FAS [26]. FAS in the liver has long been classified as housekeeping protein, which produces fat to store energy during periods of overnutrition. The primary product of the FAS reaction is palmitate (C16:0). Stearate (C18:0) and shorter fatty acids may also be produced [27]. Ob/ob mice have increased hepatic FAS gene expression as well as increased hepatic FAS activity and fatty liver compared to non-obese mice [28]. It has been reported that overexpression of FAS gene in human adipose tissue can increase the risk of obesity and prostate cancer [29,30]. FAS, a nearly-universal druggable target in many human carcinomas and their precursor lesions, offers new therapeutic opportunities for metabolically treating and preventing obesity, hyperlipidemia, diabetes and cancer [29,31]. In addition, inactivating FAS in the hypothalamus prevents diet-induced obesity and systemic inflammation [32]. In this study, FAS content and FAS gene expression in rat liver tissue were significantly increased in high fat model group compared to blank control group, this is in accordance with previous studies [17]. Meanwhile, we also provided experimental evidence that bee bread has suppressive effects on adipogenesis by decreasing the production of FAS in the liver. Natural bee bread is enriched in flavonoids, polyphenols and unsaturated fatty acids, which can restrain fat growth and prevent hyperlipidemia [33,34].
ACC plays a unique role in lipid synthesis as the rate-limiting enzyme by catalyzing the first step of fatty acid biosynthesis to produce malonyl coenzyme A (MA), then to further form long-chain fatty acids, and finally to synthesize triacylglycerol and phospholipids. It can also regulate the conversion of carbohydrates to fat and store carbohydrate energy in the form of fat energy in the body [35,36]. There are two forms of Acetyl CoA Carboxylase 1 (ACC1) and Acetyl Coa Carboxylase 2 (ACC2) genes in animal tissues, which catalyze two opposite metabolic reactions. ACC1 is widely distributed in the cytoplasm, mainly responsible for regulating the synthesis of long-chain fatty acids. On the opposite side, ACC2 is associated to the mitochondria and it play major roles in regulating the rate of fatty acid oxidation. Increasing evidence suggests that the ACC2 has attracted more and more attention due to its ability to block active sites in the treatment of obesity, diabetes and other metabolic diseases [37]. Therefore, these two carboxylases relate to energy homeostasis. Furthermore, ACC2 mutant mice have a normal lifespan and higher fatty acid oxidation rate and accumulate less fat, which means it also can protect against obesity and diabetes induced by high-fat/high-carbohydrate diets. Kim and coauthors [38] reported that ACC inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans. In this study, we also found that lower ACC content (except for the low dose of bee bread treatment group) and ACC gene expression in bee bread treatment groups than in the high fat model group. Additionally, the serum triglycerides in the treatment groups were also significantly decreased compared with the high fat model group in our previous research. The results reveal that the active ingredient in natural bee beard may be an excellent ACC inhibitor. The advantages of ACC gene and protein levels decreased were reduce obesity and prevent the onset of type 2 diabetes [39].
LPL involved in the conversion of dietary lipids into energy sources of peripheral tissues. Free fatty acids and TG were separated from chyle particles and very low density lipoprotein. Lipoprotein hydrolysis rich in triglycerides also releases apolipoproteins and phospholipids, which are precursors of High Density Lipoproteins (HDLs) [40-42]. HDLs are key component of reverse cholesterol transport, which carries cholesterol from peripheral cells to the liver where it is excreted via the bile. Therefore, an effective LPL is associated with lower triglyceride and LDL levels but higher HDL levels and is potential non-atherosclerotic protective effect [9]. The findings suggest that high fat diet could reduce LPL activity and LPL gene expression in rat livers compared with the control group. There was no significant difference between the treatment groups and the high fat model group.
Overall, natural bee bread showed a positive regulatory effect on lipid metabolism in hyperlipidemic rats, which may inhibit lipid synthesis, indicating that it has a good prospect for development for treatment of hyperlipidemia.
This work was supported by China Agriculture Research System of Ministry of Finance, Ministry of Agriculture and Rural Affairs of the People’s Republic of China (CARS-44- KX J15).
- Singh MB, Bhalla PL (2010) Control of male germ‐cell development in flowering plants. BioEssays 29: 1124-1132. Link: https://bit.ly/2YsNNvJ
- Degrandi-Hoffman G, Chen Y, Rivera R, Carroll M, Chambers M, et al. (2016) Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie 47: 186-196. Link: https://bit.ly/3n3o8UK
- Wang J, Wang D, Luo Q, Xiao H, Han S (2017) Lipid oxidation and color degradation kinetics under different storage conditions of pollen. Trans Chin Soc Agric Eng 33: 367-373. Link: https://bit.ly/3mWLBH4
- Kieliszek M, Piwowarek K, Kot AM, BAEjak S, Chlebowska-Migiel A, et al. (2018) Pollen and bee bread as new health-oriented products: a review. Trans Chin Soc Agric Eng 71: 170-180. Link: https://bit.ly/3BFgq79
- Krell R (1996) Value-added products from beekeeping. Fao Agricultural Services Bulletin 36: 229–234. Link: https://bit.ly/3yKnk9x
- Loper GM, Standifer LN, Thompson MJ, Gilliam M (1980) Biochemistry and microbiology of bee-collected almond (prunus dulcis) pollen and bee bread. i-fatty acids sterols vitamins and minerals. Apidologie 11: 63-73.
- Filipa S, Ricardo C, Lillian B, Montserrat D, Andreia T, et al. (2017) Flavonoid composition and antitumor activity of bee bread collected in northeast portugal. Molecules 22: 248-251. Link: https://bit.ly/2WLYets
- Ivanišová E, Kačániová K, Frančáková H, Petrová J, Musilová J (2017) Bee bread-perspective source of bioactive compounds for future. Potravinarstvo 9: 592-598.
- Ruili YMS, Le G, Anlin LMS, Jianliang ZMS, Yonghui SMS (2006) Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 22: 1185-1191. Link: https://bit.ly/3BAWrXc
- Mathur M, Devi VK (2016) Potential of novel drug delivery strategies for the treatment of hyperlipidemia. J Drug Targeting 24: 916-926. Link: https://bit.ly/3jIrABQ
- Mogensen SS, Schmiegelow K, Grell K, Albertsen BK, Wehner PS, et al. (2017) Hyperlipidemia is a risk factor for osteonecrosis in children and young adults with acute lymphoblastic leukemia. Haematologica 102: e175-e178. Link: https://bit.ly/2WW5IdR
- Larsson SC, Markus HS (2018) Does treating vascular risk factors prevent dementia and alzheimer's disease? a systematic review and meta-analysis. J Alzheimers Dis 64: 657-668. Link: https://bit.ly/3tcT5H5
- Yin R, Chen Y, Pan S, He F, Liu T, et al. (2006) Comparison of lipid levels hyperlipidemia prevalence and its risk factors between guangxi hei yi zhuang and han populations. Arch Med Res 37: 787-793. Link: https://bit.ly/3yE3KM5
- Bulur H, Gülzdemirler B, Gülin T, Muzaffer Z, Uysal M (1995) High cholesterol diet supplemented with sunflower seed oil but not olive oil stimulates lipid peroxidation in plasma liver and aorta of rats. J Nutr Biochem 6: 547–550. Link: https://bit.ly/3h4r7bs
- Blazovics A, Lugasi A, Kemeny T, Hagymási K, Kéry Á (2001) Membrane stabilising effects of natural polyphenols and flavonoids from Sempervivum tectorum on hepatic microsomal mixed-function oxidase system in hyperlipidemic rats. J Ethnopharmacol 73: 479-485. Link: https://bit.ly/3BD4CCo
- Huang L, Wen K, Gao X, Liu Y (2010) Hypolipidemic effect of fucoidan from laminaria japonica in hyperlipidemic rats. Pharm Biol 48: 422-426. Link: https://bit.ly/3DJaax4
- Nan L, Ouyang K, Cai J, Yang W, Wang W (2014) Research on anti-oxidant activity and hypolipemic mechanism of aloes flavonoids in mice. Journal of Food and Nutrition Research 2: 601-607. Link: https://bit.ly/3BCZUEP
- Semenkovich CF (1997) Regulation of fatty acid synthase (FAS). Prog Lipid Res 36: 43-53. https://bit.ly/3h4uEqj
- Marini PE, Carlos AP, de Mendoza D (2001) Growth-rate regulation of the Bacillus subtilis accBC operon encoding subunits of acetyl-CoA carboxylase the first enzyme of fatty acid synthesis. Arch Microbiol 175: 234-237. Link: https://bit.ly/3n15lt3
- Zhao T, Guo J, Li H, Huang W, Xian X, et al. (2006) Hemorheological abnormalities in lipoprotein lipase deficient mice with severe hypertriglyceridemia. Biochem Biophys Res Commun 341: 1066-1071. Link: https://bit.ly/3mYg47D
- Lourenço AP, Mackert A, Cristino ADS, Simões ZLP (2008) Apidologie 39: 372-385. Link: https://bit.ly/2YsC1By
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-[delta][delta] CT-method. Methods 25: 402-408. Link: https://bit.ly/3DPYvwG
- Barene I, Daberte I, Siksna S (2014) Investigation of bee bread and development of its dosage forms. Medicinos teorija ir praktika 21: 16-22. Link: https://bit.ly/3yL6Vl9
- Habryka C, Kruczek M, Drygas B (2016) Bee products used in apitherapy. World Sci 48: 254-258. Link: https://bit.ly/3yIUnux
- Deveza MV, Keller KM, Lorenzon MCA, Nunes LM (2015) Mycotoxicological and palynological profiles of commercial brands of dried bee pollen. Braz J. Microbiol 46: 1171-1176. Link: https://bit.ly/3n2GbKw
- Huang HC, Lin J (2012) Pu-erh tea green tea and black tea suppresses hyperlipidemia hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct 3: 170-177. Link: https://bit.ly/2WRr4Ja
- Anne PL, Jensen-Urstad, Semenkovich CF (2012) Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta 1821: 747-753. Link: https://bit.ly/3jL0g6i
- Perfield JW, Ortinau LC, Taylor PR, Ruebel ML, Meers GM, et al. (2013) Altered hepatic lipid metabolism contributes to nonalcoholic fatty liver disease in leptin-deficient ob/ob mice. Int J Obesity 2013: 296537. Link: https://bit.ly/2WW5Nyb
- Nguyen PL, Ma J, Chavarro JE, Freedman ML, Loda M (2010) Fatty acid synthase polymorphisms tumor expression body mass index prostate cancer risk and survival. J Clin Oncol 28: 3958-3964. Link: https://bit.ly/3h10hBk
- Matthias B, Nora K, Stephan W, Schoenle EJ, Schn MR, et al. (2014) Fas and fasl expression in human adipose tissue is related to obesity insulin resistance and type 2 diabetes. J Clin Endocrinol Metab 99: E36-44. Link: https://bit.ly/3jIFjsB
- Wu M, Singh SB, Wang J, Chung C (2011) Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc Natl Acad Sci U S A 108: 5378-5383. Link: https://bit.ly/2WSZY4w
- Chakravarthy MV, Zhu Y, Yin L, Coleman T, Pappan KL, et al. (2009) Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation. J Lipid Res 50: 630-640. Link: https://bit.ly/3td02HY
- Feng L, Yu C, Ying K, Jian H, Dai X (2011) Hypolipidemic and antioxidant effects of total flavonoids of perilla frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res Int 44: 404-409. Link: https://bit.ly/2WNwpAY
- Zeynep T, Cemal O, Nurhan S, , Kazim S (2017) Cinnamon Polyphenol Extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxid Med Cell Longev 20171-10. Link: https://bit.ly/3yL773R
- Manco M, Calvani M, Mingrone G (2004) Effects of dietary fatty acid on inslin sensitivity and secretion. Diabetes Obes Metab 6: 402-413. Link: https://bit.ly/3hl980T
- Lane MD, Cha S (2009) Effect of glucose and fructose on food intake via malonyl-CoA signaling in the brain. Biochem Biophys Res Commun 382: 1-5. Link: https://bit.ly/38E2eyP
- Abu-Elheiga L, Oh W, Kordari P, Wakil SJ (2003) Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A 100: 10207-10212. Link: https://bit.ly/38FbjaH
- Kim CW, Addy C, Kusunoki J, Norma N, Anderson S, et al. (2017) Acetyl coa carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab 26: 394-406. Link: https://bit.ly/3jL0rhY
- Minokoshi Y, Kim YB, Peroni OD, Fryer LGD, Müller C, et al. (2012) Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature (London) 415339-343.
- Merkel M, Eckel RH, Goldberg IJ (2002) Lipoprotein lipase: genetics lipid uptake and regulation. J Lipid Res 43: 1997-2006. Link: https://bit.ly/3BIi7ka
- Li Z, Liu Z, Jiang W, He X, Yan W, et al. (2019) Effects of natural bee bread on blood lipid antioxidant and immunity in rats with hyperlipidemia. Sci Agric Sin 52: 2912-2920. Link: https://bit.ly/3zFS5gW
- Sinpoo C, Williams GR, Chantawannakul P (2017) Dynamics of fungal communities in corbicular pollen and bee bread. Chiang Mai J Sci 44: 1244-1256. Link: https://bit.ly/38HFy0O
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
