International Journal of Clinical Endocrinology and Metabolism
Metformin use and the Risk of Gastrointestinal Malignancies in Diabetic Populations: A Meta-Analysis
Gil Hevroni, Samara Skwiersky, Angelina Zhyvotovska and Samy I McFarlane*
Cite this as
Hevroni G, Skwiersky S, Zhyvotovska A, McFarlane SI (2020) Metformin use and the Risk of Gastrointestinal Malignancies in Diabetic Populations: A Meta-Analysis. Int J Clin Endocrinol Metab 6(1): 035-041. DOI: 10.17352/ijcem.000052Background: Metformin use has been associated with a decreased risk of cancer and improvement in overall cancer survival rates. However, scant data available regarding metformin’s role in the risk of Gastrointestinal (GI) malignancies in patients with type II diabetes. Our study aimed to conduct a meta-analysis to evaluate the association of metformin use and GI cancer risk.
Methods: We conducted electronic search by two independent investigators using the PubMed and Cochrane library databases. Studies were assessed for design and quality, and a meta-analysis was conducted to quantify the effect of metformin on the odds of developing specific GI malignancies. The final papers that met our prespecified inclusion criteria included 4 case-control studies that address metformin’s effect on pancreatic cancer, and 4 case-control studies that address metformin’s effect on colorectal cancer in patients with type II DM.
Results: Of 2258 articles screened, 8 eligible studies were identified comprising 483,561 participants diagnosed with DM. Our analysis showed that metformin use was not associated with a significant effect on the odds of developing pancreatic cancer (OR .98; 95% CI 0.82-1.17, P=0. 83). Metformin use was associated with significantly lower odds of having Colorectal Cancer (CRC); (OR 0.84; 95% CI 0.81-0.87, p < 0.01). Sufficient data were not available to conduct analyses on the impact of metformin dose and duration.
Conclusion: Our findings suggest that metformin could be a useful neoadjuvant agent for CRC cancer and as a possible preventive therapy for other inflammatory conditions related to colorectal pathologies such as adenomatous polyps and inflammatory bowel disease. Further research is warranted to elucidate the role of metformin on the risk of developing pancreatic cancer, given the complex nature of the organ’s regulation on insulin production.
Introduction
Diabetes Mellitus (DM) is a chronic disease which affects approximately 10.5% of the United States population to date, and has been recognized as a risk factor for the development of a variety of malignancies [1,2]. A recent review of observational studies on cancer incidence and Type 2 Diabetes (T2DM) found a significant association between T2DM and cancers of the breast, bile duct, colon, and endometrium, but revealed the strongest associations between T2DM and hepatocellular and pancreatic cancer risk [2]. Various mechanisms have been hypothesized to explain the association between diabetes and these malignancies, most of which involve hyperglycemia, insulin resistance and the resulting hyperinsulinemia, and increased inflammation [3].
Moreover, the use of certain anti-diabetic medications has been hypothesized to have an effect on the risk of cancer among diabetics as well. One such medication is metformin, a dimethylbiguanide, an oral insulin sensitizer, which has become the first-line agent for the treatment of type 2 diabetes, as well as an off-label treatment for Polycystic Ovarian Syndrome (PCOS) and metabolic syndrome [4]. It is widely recognized that metformin improves glycemic control with a good safety profile, weight neutrality or weight loss, lack of associated hypoglycemia, reduced cardiovascular mortality and low cost [5]. Metformin normalizes serum glucose levels by reducing gluconeogenesis and improving insulin mediated glucose uptake by liver and muscle cells thus increasing insulin sensitivity and decreasing serum insulin levels [6]. It accomplishes this through the Adenosine Monophosphate-Activated Kinase (AMPK) signaling pathway [6]. Generally, metformin inhibits mammalian Target of Rapamycin (mTOR) activity by activating ATM (ataxia telangiectasia mutated), Liver Kinase B1 (LKB1) , and then AMPK, which ultimately prevents protein synthesis and cell growth [6]. Aside from metformin’s role in glucose control, studies have shown that it might also reduce the risk and mortality of certain malignancies, and improve diabetics response to certain cancer treatments [4].
On the contrary, exogenous insulin and insulin secretagogues such as sulfonylureas and meglitinides have been thought to increase the risk of malignancy in diabetic patients [7]. This association, however, has not been well-established with evidence from meta-analysis conducted on randomized controlled trials showing no evidence of association of sulfonylurea and the risk of malignancy at any site [7]. Although their exact mechanism is not fully understood, the effects of high serum insulin levels are predicted to contribute to increased malignancy in diabetes. This hypothesis may also explain why metformin, an insulin sensitizer, thereby reducing serum insulin levels, has been found to be associated with a decreased cancer risk [2].
Based on current literature, metformin has been shown to be associated with a decreased risk of pancreatic, breast cancer and malignancies overall [2]. Additionally, a retrospective study conducted by our group, from 2002-2014, by Zhu, et al. (2017) revealed that while the diagnosis of diabetes had no impact on CRC survival or disease, diabetic patients on metformin had significantly increased survival when compared to diabetic without metformin use and non-diabetic populations [8]. However, the effect of metformin on colorectal cancer risk has been inconsistent in the literature to date [9]. In this meta-analysis, we aim to assess the effect of metformin on the risk of gastrointestinal cancers, specifically pancreatic and colorectal cancers among individuals with T2DM.
Methods
Literature search and data sources
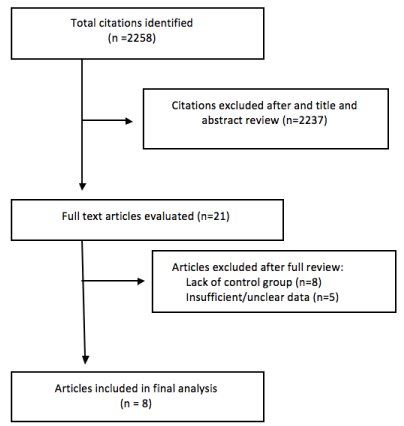
An electronic search was performed by two investigators using the PUBMED and Cochrane library databases at the library of the University Hospital of Brooklyn. We used a time (from 2000 to 2018) and language restricted to English. Utilizing this strategy, we were able to identify 2,258 articles (Figure 1). Three sets of search terms were used to ensure an adequate and comprehensive literature review. Search terms utilized included “Metformin and Cancer risk,” “Metformin and pancreatic cancer,” “Metformin and colorectal cancer.” After title and abstract review, the bibliographies of studies that met our inclusion criteria were examined for suitable additional literature. We excluded studies in which sufficient information to allow adequate estimation of hazard ratio, relative risk or odds ratios, and 95% confidence interval was not available. We excluded studies that were not published to maintain a high-quality meta-analysis.
Study selection and quality assessment
The inclusion was limited to studies that were (1) case-control in design, (2) performed after 2000, (3) compared cancer odds in diabetic patients on metformin versus those not treated with metformin, (4) cancer sites limited to the colon and pancreas. No study was excluded based on the size of the study population, but duplicate articles were excluded. For each study, we applied the Newcastle-Ottawa Scale to assess its quality. The quality scale included three components: selection of the study groups with points ranging from 0 to 4; compatibility of the study groups, with points from 0 to 2; and ascertainment of study groups exposure, points from 0 to 3. The maximum for each study is 9, studies with less than 5 points carry a high risk of bias and were excluded (N=2).
Data extraction
A total of 2,250 studies were excluded for not meeting the inclusion criteria. Eight (8) studies met the inclusion criteria and were reviewed in full; five (5) case control studies were removed due to unclear findings, and an additional eight cohort studies were removed because the results for their control groups were unclear. The Newcastle-Ottawa Scale for case-control studies was used to assess the quality of the selected articles.
Statistical analysis
The meta-analysis was performed using Review Manager, version 5.3 (Cochrane Collaboration). Mean differences were evaluated along with 95% confidence intervals (95% CIs). Each included cancer was summarized using the random-effects model. Heterogeneity, Cochran’s Q, tau-squared test, and I2 index were assessed for each study.
Results
A total of 8 studies with 487,591 subjects were included in our meta-analysis; 484,368 subjects were included in the analysis of colorectal carcinoma (CRC) incidence, and 3,223 in the analysis of pancreatic carcinoma incidence. All subjects included were previously diagnosed with T2DM and were age and sex matched. All studies included were case-control in study design. Four studies were from the United States, two from the United Kingdom, one from Korea and Denmark respectively. Some of the following subject qualities were excluded from the studies: prior diagnosis of PCOS, Inflammatory Bowel Disease (IBD), type 1 diabetes, prior cancer, and pancreatic disease. The key points of the individual studies are represented in (Table 1).
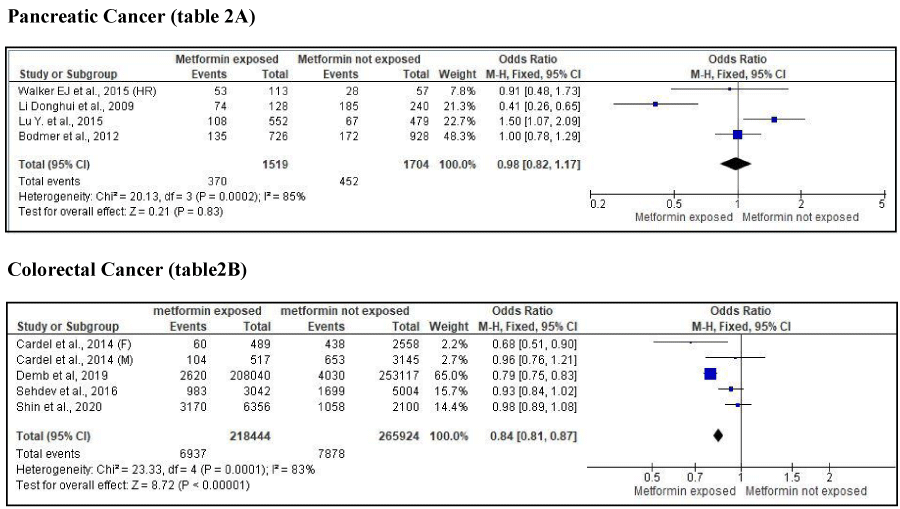
Our meta-analysis showed that metformin use did not have any effect on the risk of pancreatic cancer (OR .98; 95% CI 0.82-1.17, p=. 83) in the diabetic population (Table 2A). Metformin was associated with a decreased risk of colorectal cancer; (OR .84; 95% CI 0.81-0.87, p < 0.01) in patients with diabetes (Table 2B).
Table 2A shows association of metformin exposure with pancreatic cancer risk. Table 2B shows association of metformin exposure with colorectal cancer risk. Solid square represents the odds ratio for the risk of cancer on diabetic patients who were exposed to metformin. Solid diamond represents point estimate and confidence interval after analyzing and averaging all combined individual studies.
Discussion
Metformin and pancreatic cancer
Pancreatic cancer is the fourth most common cause of death from malignancy in the United States [10]. T2DM has been implicated as a predisposing factor for Pancreatic Cancer (PC) along with cigarette smoking and obesity [11]. In fact, those with T2DM are at 1.5-2.0 times increased risk of developing PC [11]. Although an association between T2DM and PC has been established, the causality of the relationship is complex because while T2DM has been identified as a risk factor for PC, it can also be seen as an early manifestation of PC. Individuals who are diagnosed with DM within two years of PC diagnosis are often considered to have ‘secondary diabetes’ or pancreatic cancer-associated diabetes in which the diabetes developed as a result of PC [11]. It has been hypothesized that precancerous pancreatic cells are incapable of producing insulin, which might contribute to this phenomenon [12]. The prevalence of diabetes and impaired glucose tolerance in individuals with PC has been reported to be as high as 50-80% [11]. However, given that there are no clinical or laboratory methods to accurately determine exact timing of the onset of diabetes or distinguish T2DM from diabetes caused by PC, many of these cases are likely to be misclassified.
Despite the fact that DM can result from PC itself, there is still evidence indicating that long term DM is an independent risk factor for PC [11]. This is likely due to the insulin resistance and resulting hyperinsulinemia and elevated levels of insulin-like growth factors (IGFs) characteristic of individuals with T2DM. Insulin, a growth promoting hormone, works to promote cellular proliferation and increase glucose consumption. Both of these actions are needed for cancer cell growth and proliferation. Additionally, islet cell turnover, characteristic of individuals with T2DM and insulin resistance promotes pancreatic carcinogenesis [11]. IFG-1, has a tumor promoting effect and is overexpressed in pancreatic cancer cells [13].
As previously mentioned, the use of antidiabetic medications may play a role in the association between T2DM and PC. Treatments such as exogenous insulin and sulfonylureas, which induce high levels of systemic insulin, have been thought to increase risk of PC, but this has not been confirmed, with evidence providing inconclusive results [10] . On the other hand, Metformin, has been shown to have both a protective effect on PC risk and survival among diabetics with pancreatic tumors [7, 14]. Various mechanisms have been proposed to explain metformin’s protective effect on PC, the majority of which center around its ability to increase insulin sensitivity thereby reducing plasma insulin and IFG-1 levels and normalizing the rate of islet cell turnover [10]. Metformin also stimulates AMP- activated protein kinase (AMPK), which inhibits mTOR, a signaling molecule that regulates cell growth and cycling, including pancreatic cancer cells [15].
Despite this evidence, some studies examining the role of metformin on PC risk in diabetic patients have revealed a protective effect, while others have shown no effect at all [7,14,16]. Results from our meta-analysis showed that metformin use is not associated with PC risk. Of the four case-control studies included in our analysis, two studies revealed statistically insignificant results, one revealed metformin to have a protective effect on PC, and the other a harmful effect on PC (Table 2A).
Our results are inconsistent with the meta-analysis performed by Wang, et al. (2014), which included 11 studies, and revealed a significantly lower risk of pancreatic cancer among diabetics taking metformin [RR 0.63, 95% CI 0.46-0.86, p=0.003] [14]. Their analysis included both case control and cohort studies, which may have led to heterogeneity, thereby rendering their pooled data less consistent. Moreover, most of the studies in their analysis included the effects of multiple anti-diabetic agents and/or incidences of all cancers, rather than the effect of metformin on PC risk alone. In contrast, our analysis included only case-control studies, which increases outcome consistency but may result in diminished validity.
Adjusting for duration and dose of metformin exposure as well as duration and severity of T2DM prior to PC diagnosis are important factors to account for when attempting to elucidate the effect of metformin use on pancreatic cancer risk. The duration of T2DM prior to PC diagnosis is important to consider given that it might predispose to protopathic bias [17]. Protopathic bias arises when a medication is initiated as a result of a complication of the disease of interest, which is not yet diagnosed [18]. As discussed previously, secondary diabetes can occur as a result of PC, and unfortunately there are no current clinical or laboratory methods available to accurately differentiate T2DM from pancreatic-cancer associated diabetes [11]. To minimize bias, the studies conducted by Walker, et al. and Li, et al. imposed a lag-period of six months to two years between diabetes diagnosis or metformin initiation and cancer incidence [10,13]. Individuals with a new PC diagnosis within that time period were excluded in order to control for reverse causality due to DM cases, which resulted from PC [10,13]. In contrast, the study conducted by Lu and colleagues in 2015, did not impose a lag-period, and revealed a positive association of anti-diabetic medication use with a risk of pancreatic cancer [19]. This association cannot be differentiated from the positive association between diabetes and pancreatic cancer which might have introduced both time related bias and misclassification bias into their study [19].
Duration of DM diagnosis is important to consider because longer duration of DM often infers greater disease severity, which has been hypothesized to increase one’s risk for PC [11]. In fact, the case-control study conducted by Lu and colleagues revealed that individuals with elevated HbA1c levels were at three times greater risk of developing PC [19]. However, despite this significance, the other studies in our analysis did not account for DM severity or HbA1c levels. Therefore, in order to maintain homogeneity among studies included, we did not account for this factor, which is one of the major limitations of our analysis.
The dose and duration of metformin exposure could be an independent factor for PC risk, but it could also correlate with disease severity. The case-control study conducted by Walker, et al. adjusted for diabetes duration, and stratified by metformin duration in months [13]. However, stratification of participants by never-ever metformin use or duration of metformin exposure did not reveal a statistically significant relationship between metformin and PC risk [13]. On the other hand, the study conducted by Li and colleagues revealed a statistically significant reduction risk of PC in metformin users, which was greater with increased duration, specifically those with >5 years of use [10]. While some of the studies included in our meta-analysis did in fact calculate ORs stratified by metformin exposure duration or dosages, we decided to limit our study to ratios calculated on ever or never use in order to maintain homogeneity among studies. This might have caused a source of error in our analysis as well.
Our analysis has strengths and limitations, which are important to note. One limitation is the possibility that the studies included in our analysis are too heterogenous to provide reliable pooled data. This heterogeneity in part can be explained by differences in each of the studies analytic approach and study design, which will be discussed later on. Additionally, it is important to note that when compared to the analysis of the effect of metformin use on CRC risk, that of PC risk included a notably smaller sample size.
Another limitation of our meta-analysis is that we used unadjusted ORs for our analysis, and thus did not adjust for potential confounding variables, specifically those known or suspected to predispose to PC, despite the fact that the majority of the studies included did adjust for a number of these potential confounding variables in their analysis in order to maintain heterogeneity among studies. These variables include but are not limited to: age, lifestyle factors (obesity/BMI, alcohol use, smoking status, and physical activity), and exposure to other medications that might affect the risk of pancreatic cancer. Additionally, our analysis focused on diabetic patients alone, thus eliminating the diagnoses of diabetes itself as a confounding variable. Obesity is an important variable to adjust for as it is a significant risk factor for both DM and PC and is a frequent indication for metformin prescription in diabetics due to its modest weight-reducing effect. Each of the case-control studies included in our meta-analysis controlled for obesity. Walker, et al. Li, et al. Bodmer, et al. and Lu, et al. utilized propensity score weighting and/or multivariate logistical regression for other potential confounding variables such as alcohol and smoking status [9,10,13,19].
Metformin and colorectal carcinoma
Colorectal Cancer (CRC) is the third major cause of cancer-related deaths worldwide, with a large geographical variation in incidence and mortality across the world. According to recent literature, individuals with type II diabetes are at an increased risk of developing colorectal cancer when compared to non-diabetics [20]. Hyperglycemia, insulin resistance, and the resulting hyperinsulinemia and advanced glycation end-products are thought to lead to CRC through the direct stimulation of colorectal tumor cell growth, DNA synthesis and enhanced resistance to apoptosis [20,21]. Additionally, decreased peristalsis and constipation characteristic of patients with diabetes mellitus increases exposure to bowel toxins including potential carcinogens which might also contribute to carcinogenesis [12].
As discussed previously, metformin improves glucose control by increasing insulin sensitivity and decreasing serum insulin levels, thus counteracting the effects of hyperinsulinemia. This might also help to explain its ability to reduce CRC risk in individuals with T2DM. Evidence suggests that metformin may interfere with colorectal carcinogenesis through both indirect (insulin-dependent) and direct (insulin-independent) mechanisms. Moreover, there is a growing body of evidence indicating that metformin exerts its anticancer activity not only through its systemic effects but also via its cellular effects. Similar to its effects on PC cells, metformin may produce an anticancer effect by inhibiting mTOR, a critical mediator of cancer development, either through direct activation of the liver kinase B1 (LKB1)/Adenosine Monophosphate Kinase (AMPK) pathway or through an AMPK-independent pathway [22].
Various mechanisms have been proposed with regards to how metformin specifically protects colonic tissues from malignant events vivo and in vitro. A pilot study conducted by Paleari, et al. found that metformin absorption in the colon is 150-fold higher than in plasma, and the levels found in colonic tissue are in the range of direct antitumor effect shown in preclinical models [23]. In addition, metformin plasma and tissue concentrations were closely correlated, suggesting that the exchange between blood and tissue occurs rapidly [23].
Our meta-analysis suggests that metformin therapy is associated with 16% decreased risk of colorectal cancer among individuals with T2DM. Three of the four studies included in our analysis found that metformin has a protective effect on CRC rate among diabetic patients. Of those three, two studies showed that metformin has a protective effect on CRC, with one of these studies statistically significant among females alone. Our results are consistent with previous findings, which suggest a protective role for metformin therapy on CRC risk. For example, Zhang, et al. conducted a cohort study which found that metformin therapy was associated with a significantly lower risk of colorectal cancer (OR=0.63, 95% CI: 0.47-0.84, p=0.002) [24]. A systematic review conducted by Franciosi, et al. reported that the odds of colorectal cancer decreased by 17% (OR=0.83, 95% CI: 0.74–0.92, p=0.0009) in individuals with metformin use [25]. The largest and most recent study conducted by He, et al. found that metformin is associated with a pooled 10% relative reduction in CRC risk in persons with diabetes, compared to persons without diabetes (OR: 0.90, 95% CI: 0.85–0.96) [26]. This rate is lower than the 16% risk reduction demonstrated in our current meta-analysis. However, it is important to note that in He, et al analysis, out of the studies included in his analysis, five found a significant protective effect of metformin on CRC risk, whereas the other ten showed no significant effect [26]. These conflicting results may have been caused by methodical problems within studies.
It must be noted that as in the review conducted by He, et al., there is a degree of heterogeneity among the studies included in our meta-analysis as well [26]. These can be attributed to variations in study design, study population, and adjustments for potential confounders. For example, Demb, et al. performed a case-control study of US veterans using data from the Department of Veteran Affairs (VA) electronic health records, in which the majority of the populations were male subjects [27]. This study included few women, consistent with the VA demographic, limiting the ability to examine sex- specific effects on CRC risk. On the other hand, Shin, et al. and Cardel, et al. limited their study population to those of Korean and Danish nationality respectively [28,29]. Moreover, Shin, et al. examined the effect not only of metformin but also of different anti-diabetic medications on CRC odds [28]. In the matching process, the study did not adjust for exposure to anti-diabetic drugs other than the drug of interest [28]. In fact, only patients who had a history of taking at least one anti-diabetic medication were included in this study, thus excluding diabetics treated with diet and exercise alone. Consequently, a comparison of CRC risk between diabetic patients with and without therapy was lacking.
Specifically, for CRC, analyses using claim data made it challenging to distinguish rectal cancers from colon cancers, to identify the stage of CRC, and to distinguish between in situ and colorectal cancers. The studies conducted by Shin, Cardel, and Sehdev, et al. looked at colorectal cancer as a single entity, failing to distinguish amongst the aforementioned anatomical sites [28-30]. In contrast, Demb, et al. addressed specific anatomic sites and found that risk reduction associated with metformin exposure was mainly limited to rectal cancer [31]. Of note, there is little to no published literature that is currently capable of separating out site-specific effects and no mechanistic studies, which could explain a site-specific effect of metformin on risk of developing rectal cancer.
Similar to the point made in the discussion of pancreatic cancer, another important limitation to consider is the duration and dosage of metformin use. For instance, a major limitation of the study conducted by Demb, et al. is that they examined metformin as a binary single time exposure such that duration and accumulation were not considered; all of this may underestimate the true protective effect of metformin on CRC [31]. The study conducted by Shin, et al. did not account for dosage of metformin, but did include duration of metformin use and distributed the results as 90-180, 180-360,360-730, and 730> days. Sehdev, et al. accounted for both duration and dosage of metformin use but no significant dose response relationship was found between metformin dose, duration or total exposure (dose × duration) and odds of developing CRC [28]. Finally, the study conducted by Cardel et al. showed a statistical significant dose response association between the use of metformin and a reduced risk of CRC; but the protective effect of metformin ceased after nine years of treatment [29].
Conclusion
Our analysis revealed that metformin use in patients with T2DM significantly decreases the odds of developing CRC, but did not demonstrates an association between metformin use and PC odds. The proposed role of metformin in its protection against CRC is multifactorial and includes both insulin-dependent and insulin-independent mechanisms. This supports the use of metformin in diabetic patients, particularly those with risk factors for CRC such as those with hereditary CRC syndromes, personal or family history of sporadic CRC or adenomatous polyps, inflammatory bowel disease, and obesity. Further investigations, especially large-scale clinical studies and experiments at the cellular level are needed to confirm the relationship between colorectal cancers and metformin treatment. Given that the pancreas is the center of insulin production, the effect of metformin on pancreatic cancer odds/risk in people with type II diabetes is more complex, as pancreatic cancer has been shown to be a risk factor for developing diabetes and vice versa. Although the mechanisms by which metformin influences tumor growth have recently been reviewed, it is uncertain whether the use of metformin can reduce the odds and/or risk of developing pancreatic cancer. Future research should focus on exploring the effect of metformin on non-diabetics and those with pre-diabetes (impaired fasting glucose or impaired glucose tolerance) as well. Moreover, it would be helpful to conduct future research on particular tumor biomarkers in order to elucidate which patients and types of cancers would be most responsive to metformin therapy for the prevention of gastrointestinal cancers.
This work is supported, in part, by the efforts of Dr. Moro O. Salifu M.D., M.P.H., M.B.A., M.A.C.P., Professor and Chairman of Medicine through NIH Grant number S21MD012474.
- Prevention CfDCP National Diabetes Statistics Report, 2020, U.S.D.o.H.a.H.S. Centers for Disease Control and Prevention, Editor. 2020: Atlanta, GA. Link: https://bit.ly/38vDrfW
- Valent F (2015) Diabetes mellitus and cancer of the digestive organs: An Italian population-based cohort study. J Diabetes Complications 29: 1056-1061. Link: https://bit.ly/3hkBZAR
- Tsilidis KK, Kasimis JC, Lopez DS, Ntzani E, Ioannidis J (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ: 350: g7607. Link: https://bit.ly/3aVcxRF
- Chen K, Li Y, Guo Z, Zeng Y, Zhang W, et al. (2020) Metformin: current clinical applications in nondiabetic patients with cancer. Aging 12: 3993-4009. Link: https://bit.ly/3hhkkKu
- McCreight LJ, Bailey CJ, Pearson ER (2016) Metformin and the gastrointestinal tract. Diabetologia 59: 426-435. Link: https://bit.ly/3mQbrsk
- Zaharenko L (2017) Pharmacogenetics of efficiency and tolerance of the peroral antidiabetic drug metformin. Molecular Biology. 2015 University of Latvia: Riga.
- De Souza A, Khawaja KI, Masud F, Saif MW (2016) Metformin and pancreatic cancer: Is there a role? Cancer Chemother Pharmacol 77: 235-242. Link: https://bit.ly/3nRUaAn
- Zhu RC, Rattanakorn K, Pham S, Mallam D, McIntyre T (2017) Survival benefits in colorectal adenocarcinoma with the use of metformin among a black diabetic inner city population. Colorectal Cancer 6: 33-41. Link: https://bit.ly/37OJJZ5
- Bodmer M Becker C, Meier C, Jick SS, Meier CR (2012) Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 107: 620-626. Link: https://bit.ly/34Mn65r
- Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL (2009) Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137: 482-488. Link: https://bit.ly/2WKvA8H
- Li D (2012) Diabetes and pancreatic cancer. Mol Carcinog 51: 64-74. Link: https://bit.ly/3mXRLTP
- Ashamalla M, Youssef I, Yacoub M, Jayarangaiah A, Gupta N, et al. (2018) Obesity, Diabetes and Gastrointestinal Malignancy: The role of Metformin and other Anti-diabetic Therapy. Glob J Obes Diabetes Metab Syndr 5: 008-14. Link: https://bit.ly/3rsqHPO
- Walker EJ Ko AH Holly EA Bracci PM (2015) Metformin use among type 2 diabetics and risk of pancreatic cancer in a clinic-based case-control study. Int J Cancer 136: E646- E653. Link: https://bit.ly/3ppQR3O
- Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, et al. (2014) Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 106: 19-26. Link: https://bit.ly/2KQdG1H
- Rozengurt E (2014) Mechanistic target of rapamycin (mTOR): a point of convergence in the action of insulin/IGF-1 and G protein-coupled receptor agonists in pancreatic cancer cells. Front Physiol 5: 357. Link: https://bit.ly/37MLi9C
- Jang WI, Kim MS, Kang SH, Jo AJ, Kim YJ, et al. (2017) Association between metformin use and mortality in patients with type 2 diabetes mellitus and localized resectable pancreatic cancer: a nationwide population-based study in korea. Oncotarget 8: 9587-9596. Link: https://bit.ly/3hv2dB7
- de Jong RG, Burden AM, de Kort S, van Herk-Sukel MP, Vissers PA, et al. (2017) No Decreased Risk of Gastrointestinal Cancers in Users of Metformin in The Netherlands; A Time-Varying Analysis of Metformin Exposure. Cancer Prev Res 10: 290-297. Link: https://bit.ly/3rs3SvJ
- Sharma A, Chari ST (2018) Pancreatic Cancer and Diabetes Mellitus. Curr Treat Options Gastroenterol 16: 466-478.
- Lu Y, García Rodríguez LA, Malgerud L, González-Pérez A, Martín-Pérez M, et al. (2015) New-onset type 2 diabetes, elevated HbA1c, anti-diabetic medications, and risk of pancreatic cancer. Br J Cancer 113: 1607-1614. Link: https://bit.ly/2WJzLl4
- Gonzalez N, Prieto I, Del Puerto-Nevado L, Portal-Nuñez S, Ardura JA, et al. (2017) 2017 update on the relationship between diabetes and colorectal cancer: epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget 8: 18456-18485. Link: https://bit.ly/3rwMaXN
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, et al. (2010) Diabetes and cancer: a consensus report. Diabetes Care 33: 1674-1685. Link: https://bit.ly/3ptGufz
- Tseng CH (2017) Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: A retrospective cohort analysis. Diabetes Metab 43: 438-445. Link: https://bit.ly/3psUSo3
- Paleari L, Burhenne J, Weiss J, Foersch S, Roth W, et al. (2018) High Accumulation of Metformin in Colonic Tissue of Subjects With Diabetes or the Metabolic Syndrome. Gastroenterology 154: 1543-1545. Link: https://bit.ly/3mRJtN3
- Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, et al. (2011) Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care 34: 2323-2328. Link: https://bit.ly/37QquOs
- Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, et al. (2013) Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One 8: e71583. Link: https://bit.ly/3hk31bG
- He XK, Su TT, Si JM, Sun LM (2016) Metformin Is Associated With Slightly Reduced Risk of Colorectal Cancer and Moderate Survival Benefits in Diabetes Mellitus: A Meta-Analysis. Medicine (Baltimore) 95: e2749. Link: https://bit.ly/3mQ9EDC
- Demb J, Yaseyyedi A, Liu L, Bustamante R, Earles A et al. (2019) Metformin Is Associated With Reduced Odds for Colorectal Cancer Among Persons With Diabetes. Clin Transl Gastroenterol 10: e00092. Link: https://bit.ly/3hmmmJw
- Shin CM, Kim N, Han K, Kim B, Jung JH, et al. (2020) Anti-diabetic medications and the risk for colorectal cancer: A population-based nested case-control study. Cancer Epidemiol 64: 101658. Link: https://bit.ly/3hhhYLE
- Cardel M, Jensen SM, Pottegård A, Jørgensen TL, Hallas J, et al. (2014) Long-term use of metformin and colorectal cancer risk in type II diabetics: a population-based case-control study. Cancer Med 3: 1458-1466. Link: https://bit.ly/38yK1lO
- Sehdev A, Shih YC, Vekhter B, Bissonnette MB, Olopade OI, et al. (2015) Metformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US population. Cancer 121: 1071-1078. Link: https://bit.ly/3aJU8H1
- Demb J, Yaseyyedi A, Liu L, Bustamante R, Earles A et al. (2019) Metformin Is Associated With Reduced Odds for Colorectal Cancer Among Persons With Diabetes. Clin Transl Gastroenterol 10: e00092. Link: https://bit.ly/3hmmmJw
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
