Global Journal of Medical and Clinical Case Reports
Long term outcomes of Hospital-Identified Clostridium Difficile Infection (HICDI): A retrospective cohort analysis of adult patients in a teaching hospital
Samarasimha R Pandhem1*, David Michael2 and Manoj Y Singh3
2Statistician, New castle university, NSW, Australia
3Consultant Senior Intensivist, Australian Capital Territory Health and Australian National University, Canberra, Australia
Cite this as
Pandhem SR, Michael D, Singh MY (2021) Long term outcomes of Hospital-Identified Clostridium Difficile Infection (HICDI): A retrospective cohort analysis of adult patients in a teaching hospital. Glob J Medical Clin Case Rep 8(1): 008-015. DOI: 10.17352/2455-5282.000118Objectives: To study long term outcomes of HICDI.
Design, settings and participants: Retrospective cohort study of adult patients diagnosed with HICDI during their admission to a tertiary teaching hospital between January 1st 2012 and December 31st 2016.
Main outcomes: Primary aim was to study two-year mortality outcome and it’s predictors in HICDI patients. Secondary outcomes were to identify characteristics of HICDI and predictors of time to resolution of infection.
Results: A total of 819 adult HICDI episodes were identified. 544 episodes occurring in 466 patients were included in final analysis. Single CDI episodes occurred in 409 patients, 45 patients had 2 episodes and 12 patients had greater than 2 episodes.
Two-year all-cause mortality was 33% (152/409) in single CDI episodes and 61.4% (35/57) for those with greater than one CDI episode. Of the 466 patients, the in-hospital all-cause mortality directly attributed to CDI was 14 patients (3%).
Risk factors predicting long-term mortality were, chemotherapy (AHR(adjusted hazard ratio)2.7; 95% CI 1.90-3.81;p=0.01), low albumin(AHR 2.44; 95% CI 1.83-3.47; p=0.01), ICU admission(AHR 2.09, 95% CI 1.44-3.03; p=0.01) high WBC count (AHR 1.78,CI 1.28-2.30; p=0.01), multiple CDIs (AHR 1.24,95% CI 1.09-1.39; p=0.01) and age (AHR:1.04;95%CI:1.03–1.05;p=0.01).
Most common type of HICDI was Hospital –Acquired CDI (HA-CDI) (55.8 %; n=260). Antibiotic usage before developing CDI significantly delayed the time to resolution of infection (AIRR: 1.35; 95% CI 1.06-1.71; p=0.01). In 1/3rd (n=180) of HICDI episodes, patients were discharged before resolution of diarrhoea. Majority of HICDI episodes (n=371) were treated with metronidazole.
Conclusion: HICDI was associated with significant long term mortality and morbidity. Mortality increased with more than one CDI infection. Antibiotic usage before developing CDI significantly delayed the time to resolution of infection.
Background
Clostridium Difficile (currently clostridioides) Infection (CDI) is a common cause of diarrhoea, particularly in patients who seek hospital treatment [1]. It is the commonest cause of nosocomial diarrhoea accounting for 15-25% of cases [2]. In the USA, it results in 500 000 infections and 29000 deaths annually [2,3]. It is also recognised as a major and increasing cause of diarrhoea in community settings [1,4].
Following a 2008 recommendation by Australian Commission on Safety and Quality in Health care (ACSQHC), public hospitals in Australia carried out surveillance in 2011 to identify the burden of CDI and help guide infection control measures and treatment [5]. This surveillance initiative reported an incidence of Hospital-Identified CDI (HICDI) in Australia of 3.25 per 10000 patient-days in 2011, which increased to 4.03 per 10000 patient-days in 2012. It was also found that Hospital-Acquired CDI (HA-CDI) was the most common cause of CDI infections contributing to 67% of all CDI diagnoses, with community-acquired CDI (CA- CDI) occurring at a rate of 26% [5]. Similar surveillance findings have been documented in other countries [3,6,7].
There is increased 30-day mortality in patients with CDI compared to patients without CDI [8]. Increased mortality was associated with patients who were elderly, had multiple comorbidities or was admitted to the Intensive Care Unit [1,8,9]. There is also an increase in morbidity and health care costs with CDIs [10,11].
CDI can present acutely as a single episode or as a chronic illness with recurrences and relapses [12]. Short term outcomes have been extensively studied but little is known about long term outcomes [1,8,11-13]. Two international single centre studies reported mortality outcomes of CDI at 6 months as 38% [14] and 34% [15]. Another international single centre study reported 2-year mortality as 32.5% [16]. No long term CDI outcomes have been studied in Australia. The present study primarily aims to review two- year term mortality of HICDI patients and its predictors.
Methods
Design and Study population
The study was conducted at The Canberra Hospital (TCH), a university-affiliated metropolitan tertiary teaching and referral hospital in the Australian Capital Territory (ACT). The study was approved by the ACT Health Human Research Ethics committee (ETHLR.16.175). The department of Infectious disease at TCH prospectively collects data on patients who are confirmed to have CDI using various methods including detection of toxin or glutamate dehydrogenase enzyme and PCR or culture or both.
We included all patients who were diagnosed with HICDI during their hospital stay between 1st January 2012 and 31st December 2016. To assess long-term mortality, we looked up the ACT Patient Administration system (ACTPAS) health record system and noted whether patients were dead or alive as at 31st December 2018. The total number of HICDI patients and the total number of patients admitted per year were also checked on ACTPAS.
Following HICDI episodes were excluded from the study:
• Age <17years
• HICDI episodes that occurred in 198 non-ACT resident patients (mainly from New South Wales) due to difficulties in accessing interstate mortality registries.
• Recurrences of HICDI episode/s defined as CDI occurring within 8 weeks of the previous CDI episode and are not considered as new infections [17] (Appendix).
• HICDI episodes with inadequate information (i.e. more than four missing variables for each episode).
• HICDI episodes detected in an outpatient or emergency department or short stay (not stayed overnight) in the hospital.
Data collection
Reviewed data sources included the TCH CDI infectious surveillance data, laboratory (pathology and microbiology) and radiology results, medical documentation, medication charts, stool charts, and ACTPAS. Data relating to hospitalised patients without CDI was not collected because their baseline characteristics were very different to those who had CDI.
Data collected included
• Patient demographics,
• Date of CDI confirmation
• Type of CDI (HA-CDI or CA-CDI or indeterminate/unknown) [17] (Appendix).
• Time of resolution of infection (defined as 48 hours post-cessation of diarrhoea or time when contact precautions were removed) [17].
• Length of hospital stay
• Risk factors for CDI: Antibiotics (to a maximum of three) received before developing CDI, proton pump inhibitors, H2-receptor antagonists, admission to intensive care in the past six months, presence of diabetes mellitus, active cancer of any type, gastrointestinal disorder (any previous gastrointestinal disease), immunosuppression and chemotherapy [18,19].
• CDI severity factors: temperature (>38.5C), white cell count (>15000 per cu mm), hypoalbuminaemia (<25g/L), lactate (>2.0 mmol/L), creatinine increase by 50% from baseline, Toxic megacolon (based on CT scan or clinical peritonism), colectomy and admission to ICU [9,17,19].
• Antibiotics received during CDI and
• Medications (including duration) used for treating CDI: metronidazole, vancomycin, concurrent metronidazole and vancomycin or sequential metronidazole and vancomycin.
The type of CDI was identified by the hospital surveillance data. Patient stool charts and accompanying notes were evaluated to identify the time to resolution of infection. Patients with end-stage COPD, end-stage renal failure, receiving dialysis, decompensated heart failure, chronic liver disease, metastatic cancer, had received chemotherapy in last 6 months or were on long term steroids (>10mg prednisolone or its equivalent) were considered as immunosuppressed. If HICDI patients died in the hospital, their death certificates were reviewed to identify whether CDI was the primary cause of death.
Study outcomes
• Primary outcome: Two-year mortality and predictors of mortality. Two-year mortality was defined as death taking place within two years of the date of original diagnosis for patients with a single episode of CDI and within two years following the last CDI diagnosis in patients with more than one episode.
• Secondary outcomes: Incidence of types of HICDI, time to CDI diagnosis from admission, length of hospital stay, time to resolution of infection and its predictors, treatment choices for CDI.
Statistical methods
Data were described as frequencies (%) or means (standard deviation) and medians (interquartile range). For the primary outcome, a Cox proportional hazard regression model with time-varying covariates was used to identify and assess the significant predictors. The effect measures were expressed as Hazard Ratios (HR) with 95% confidence Intervals (CIs). For the time to resolution of infection, a Negative Binomial model was used to identify and assess the significant factors. The effect measures were expressed as Incidence Rate Ratios (IRRs) with 95% CIs. A multivariable model was built for each outcome after univariate analysis to identify significant factors. The Link test was used to test if the model specification for both outcomes was valid or not. Incidence and mortality analyses were performed with STATA/SE 15.0 (Stata Corp LP, College Station, Texas, USA). A p-value < 0.05 was considered statistically significant and all tests were two-sided.
Results
As per ACTPAS, the total incidence of adult HICDI in TCH (includes both ACT and non-ACT residents) per year per 1000 patients was: 3.95(2012), 2.65(2013), 2.99(2014), 2.77 (2015) and 1.61(2016).
A total of 864 HICDI episodes were identified during the 5- year study period. Figure 1 is the study consort chart highlighting the patients included and excluded in the study. Four hundred and sixty-six ACT-resident patients accounting for 544 episodes of CDIs were included in the study. Of these, forty episodes of CDI were diagnosed in ICU/ HDU and the remaining episodes (n=504) were diagnosed in general wards. In total, 409 patients had a single episode of CDI, 45 patients had two episodes of CDI diarrhoea and 12 patients had more than two episodes of CDIs.
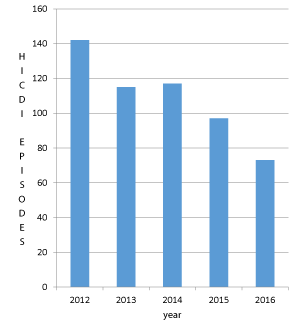
The baseline demographic characteristics of the patients and their HICDI episodes are shown in Table 1. We noted a decrease in the number of HICDI episodes during years 2012-2016 as highlighted in Figure 2.
Table 2 summarises the antecedent risk factors for CDI. Three hundred and sixty-six patients (78.5%) received antibiotics before developing CDI. Approximately 21% of patients (n=100) had no antibiotics before developing CDI. A majority of the patients had multiple risk factors.
Table 3 describes the markers of severity of CDI. One-eighth of HICDI patients were admitted to the ICU and colectomy was rarely performed. In several CDI episodes, serum lactate (n=307) and imaging (n=260) was not performed.
Primary outcome
Of the 466 HICDI patients, the in-hospital mortality directly attributed to CDI (identified on the death certificate) was 14 patients (3%). Patients who died at the end of two years since their last CDI are presented in Table 4. Two-year all-cause mortality for patients who suffered a single episode of CDI was 33% (152/409) and for patients with two or more CDI episodes was 61.4% (35/57). More than half (n= 247/466) of the cohort died due to various causes (including CDI as a primary cause) by the end of the data collection period (i.e. Dec 31st 2018). In patients who suffered single HICDI (n=409), 56.72% (n=232) were HA-CDI and 39.11% (n=160) were CA-CDI. The mortality rate of these single episodes of HA-CDI and CA-CDI by the end of the study period was 54.31% (n=126) and 46.25% (n=74) respectively.
Multivariate long term survival modelling (Table 5) found that the most significant predictors of long term mortality were age (HR 1.04 per year), chemotherapy (HR 2.7), and low albumin (HR 2.44), and ICU admission (HR 2.09).
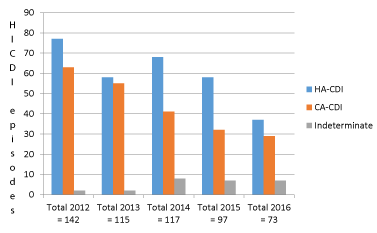
Various secondary outcomes are presented in Table 6 and the incidence of different types of HICDI per year in Figure 3.
Time to resolution: Factors predicting time to resolution of infection are reported in Table 7. Antibiotics usage prior to developing CDI significantly delayed the time to resolution. Compared to patients without antibiotic usage before developing CDI, patient with antibiotic usage took 70% longer time for resolution of infection.
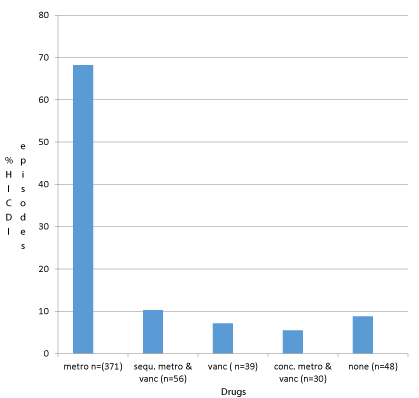
Medications used for treating HICDI are given in Figure 4. Usage of single-agent was common and no antibiotics were given in few HICDI episodes.
Discussion
Our cohort of HICDI patients was elderly, ward-based, mostly female, had a primary medical diagnosis, and was non-indigenous. The incidence of HICDI in TCH (total identified HICDI episodes/1000 patient admissions per year) was 3.95 per 1000 patients in 2012 and 1.61 per 1000 patients in 2016. The potential source/s of this decrease is likely due to improved infection control measures and implementation of antibiotic stewardship programs during this period, but our study was not designed to address this question.
Primary outcome
This descriptive study documented the 2-year all-cause mortality of patients diagnosed with CDI in a tertiary hospital. Similar to previous studies it has found that CDI commonly affects the elderly and immunosuppressed [1-4,14].
The in-hospital mortality of patients directly attributed to CDI was 3%.Unfortunately ,comparison of mortality in hospitalised patients without CDI was difficult given the baseline characteristics of these patients were very different to those who had HICDI. In future, we suggest researchers to focus on subset of patients with similar baseline characteristics for example, haematological patients undergoing chemotherapy and compare patients who had HICDI infections to those who did not. This allows better comparison of long-term mortality outcomes.
Unlike our study, Young, et al. [16] included early CDI recurrences (within 8 weeks of first CDI episode) in their cohort and showed a mortality of 24.6% and 32.5% at 12 months and 24 months respectively. Recurrences are not new CDI infections epidemiologically [18] and our study did not include them. In the literature confusion exists between “recurrences” and “relapses”. Very frequently, these terms are used interchangeably with no clear cut epidemiological time limits or reference to whether it is the same (inadequately treated) old infection or a new C.difficle infection. Our research was based on clear definitions provided by the Australian commission on safety and Quality in Health care.17 There is also no clear demarcation of what constitutes short term and long term mortality (& morbidity). All this commands an attention to formulate uniform world-wide definitions, so that confusion is avoided in interpretation of the results. The current study gives information regarding two year (long term) mortality of HICDI patients in the Australian health system.
In our study, we noted that multiple factors were associated with long term mortality with the most significant being the administration of chemotherapy, the presence of low albumin and admission to ICU. The predictors of mortality we studied have been found to be associated with short term mortality in several CDI studies [9,16,18-20]. However, to validate their influence on long term mortality, large prospective studies are needed to better determine association and causation and to stratify HICDI patients.
The risk factors included in our study were also associated with a higher risk of mortality in several other CDI studies [1,18,21-23]. In our cohort more than half of the patients were immunosuppressed or used PPI and a third had a gastrointestinal disease. Patients in this cohort also had additional recent or concurrent infective diagnoses necessitating antibiotic use (i.e. respiratory infection, neutropenic sepsis). All of these conditions themselves carry an inherent mortality and morbidity risk making the total mortality attributable to CDI difficult to determine in these populations.
Secondary outcomes
Patients developed CDI within a few days of their hospital admission and diarrhoea usually lasted for almost a week. CDI infection was associated with increased length of hospital stay [2,13,14,24].
HICDI was predominantly hospital-acquired (55.8%) in our cohort [1-7]. The type of small proportion of HICDI could not be assessed (indeterminate/unknown).
Time to resolution of infection (diarrhoea) was significantly delayed if patients used antibiotics prior to the development of CDI. This is postulated due to a change in gut microbiota promoting CDI and antibiotic stewardship is recommended [1,24,25]. The most common reasons noted in our study for starting antibiotics were infective exacerbations of COPD, neutropenic sepsis and pneumonia. In some patients (especially haematological and oncological patients) the time when contact precautions were removed for CDI could not be identified due to the presence of other MROs that needed isolation [26]. One-third of the time (in 33% of HICDI episodes) patients were discharged without resolution of diarrhoea. Some of those were discharged to nursing homes as noted in other studies [14,27]. Strict isolation and contact precautions are taken during the care of C. difficle infected patients in the hospital. This may not be possible once patients (with unresolved diarrhoea) are discharged. If there is a susceptible population (viz. children, immunosuppressed or elderly) at the discharged destination they are at high risk of contracting CDI [1,23,25,27]. This calls the researchers and ID specialists to study and plan for safer ways of hospital discharge to minimise community transmission.
Drug choices for treating CDI in our cohort were according to CDI treatment guidelines now considered outdated [28]. The most common drug used was oral metronidazole for 10 days. Vancomycin was chosen if symptoms were severe or diarrhoea did not resolve with metronidazole. However, as per new guidelines, the treatment of choice is oral vancomycin irrespective of disease severity [25] Current Australian therapeutic guidelines still recommend metronidazole as a suitable agent for mild to moderate CDI [29]. Similar to Bond, et al. [24] we noted that no treatment was administered in some HICDI episodes. The reasons for this were unclear but issues such as enterotoxin test being negative, inadequate follow up of investigations, resolution of diarrhoea, and transfer to a different ward are possible explanations raising concern of nosocomial transmission.
Strengths
The major strength of our study was that it allowed investigation of questions relating to surveillance and monitoring of CDI. Our data was collected according to the ACSQHC protocol and recommendations. Diagnosis of CDI was done by using standardised methods. Simple, reliable and easily accessible information in hospital records was collected. Mortality data was collected from a reliable source.
Limitations
There are several limitations in our study. It is a retrospective observational study and limited only to the ACT population. Because the non–ACT resident population that had CDI detected in the hospital was excluded, the results may be an underestimation for the whole hospital. Similarly, details of patients who died outside ACT (interstate or overseas) may not have been entered in the ACTPAS thus potentially leading to underestimation of HICDI long term mortality. The current study did not compare mortality with an age and comorbidity matched control. We were unable to calculate the severity (Horn’s index) [19] of CDI because tests required for calculation were not performed in every patient/episode. There is a possibility of measurement error, estimation bias and inaccurate data affecting our results. Some patients were sent home with symptoms of diarrhoea, so their length of hospital stay and time to resolution may also be subject to error.
Our findings in single tertiary Australian hospital need to be confirmed in a well-conducted prospective study involving multiple sites.
Conclusion
Our study found that patients diagnosed with HICDI had significant long term mortality and morbidity. The two-year mortality increased with multiple CDI episodes. The most common cause of HICDI was HA-CDI. Usage of antibiotics before developing CDI increased the time to resolution. A large percentage of CDI patients were discharged before the resolution of diarrhoea and a small percentage of CDI patients were not treated. These findings raise issues regarding infection control procedures and discharge process for CDI patients in hospital settings.
- Kelly CP, LaMont JT (2008) Clostridium difficile--more difficult than ever. N Engl J Med 359: 1932-194040. Link: https://bit.ly/3jHulBS
- Bhandari S, Pandey RK, Dahal S, Shahreyar M, Dhakal B, et al. (2018) Risk, Outcomes, and Predictors of Clostridium difficile Infection in Lymphoma: A Nationwide Study. South Med J 111: 628-633. Link: https://bit.ly/375S0XQ
- Lessa FC, Winston LG, McDonald LC, Emerging Infections Program C. difficile Surveillance Team (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med 372: 2369-2370. Link: https://bit.ly/3jKqvbo
- Gupta A, Khanna S (2014) Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist 7: 63-72. Link: https://bit.ly/3di3Bab
- Slimings C, Armstrong P, Beckingham WD, Bull AL, Hall L, et al. (2014) Increasing incidence of Clostridium difficile infection, Australia, 2011-2012. Med J Aust 200: 272-276. Link: https://bit.ly/3qiLIeQ
- Khanna S, Pardi DS, Aronson L, Kammer PP, Orenstein R, et al. (2012) The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol 107: 89-95. Link: https://bit.ly/3pd3Up0
- Viseur N, Lambert M, Delmee M, Van Broeck J, Catry B (2011) Nosocomial and non-nosocomial Clostridium difficile infections in hospitalised patients in Belgium: compulsory surveillance data from 2008 to 2010. Euro surveill 16: 20000. Link: https://bit.ly/377hyDF
- Mitchell BG, Gardner A (2012) Mortality and Clostridium difficile infection: a review. Antimicrob Resist Infect Control 1: 20. Link: https://bit.ly/377hFPB
- Kenneally C, Rosini JM, Skrupky LP, Doherty JA, Hollands JM, et al. (2007) Analysis of 30-day mortality for clostridium difficile-associated disease in the ICU setting. Chest 132: 418-424. Link: https://bit.ly/3d5NImQ
- Kwon JH, Olsen MA, Dubberke ER (2015) The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am 29: 123-134. Link: https://bit.ly/37o0mu3
- Kyne L, Hamel MB, Polavaram R, Kelly CP (2002) Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 34: 346-353. Link: https://bit.ly/3qcVRK8
- Maroo S, Lamont JT (2006) Recurrent Clostridium Difficile. Gastroenterology 130: 1311-1316. Link: https://bit.ly/3pdiKvu
- Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, et al. (2012) Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 81: 1-14. https://bit.ly/3rLAWy5
- Dubberke ER, Butler AM, Reske KA, Agniel D, Olsen MA, et al. (2008) Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerg Infect Dis 14: 1031-1038. Link: https://bit.ly/3pm5NzP .
- Musher DM, Nuila F, Logan N (2007) The long-term outcome of treatment of Clostridium difficile colitis. Clin Infect Dis 45: 523-524. Link: https://bit.ly/3d4yVc5
- Doh YS, Kim YS, Jung HJ, Park YI, Mo JW, et al. (2014) Long-Term Clinical Outcome of Clostridium difficile Infection in Hospitalized Patients: A Single Center Study. Intest Res 12: 299-305. Link: https://bit.ly/2Nhq8Zy
- Australian commission on safety and Quality in Health care (2013) Implementation guide for surveillance of clostridium difficile infection. Link: https://bit.ly/3730PkP
- Bloomfield MG, Sherwin JC, Gkrania-Klotsas E (2012) Risk factors for mortality in Clostridium difficile infection in the general hospital population: a systematic review. J Hosp Infect 82: 1-12. Link: https://bit.ly/3b1dGFB
- Arora V, Kachroo S, Ghantoji SS, Dupont HL, Garey KW (2011) High Horn's index score predicts poor outcomes in patients with Clostridium difficile infection. J Hosp Infect 79: 23-26. Link: https://bit.ly/2MQbU28
- Wynell-Mayow W, Cash D, Muniz-Tererra G, Gkrania-Klotsas E, Khanduja V (2017) Factors Affecting Mortality and Length of Stay Following Clostridium Difficile Associated Diarrhoea: Validating A Consistent Scoring System. Arch Clin Microbiol 8: 3. Link: https://bit.ly/2NhqBuM
- Eze P, Balsells E, Kyaw MH, Nair H (2017) Risk factors for Clostridium difficile infections - an overview of the evidence base and challenges in data synthesis. J Glob Health 7: 010417. Link: https://bit.ly/2Z9oaNv
- Kim JW, Lee KL, Jeong JB, Kim BG, Shin S, et al. (2010) Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol 16: 3573-3577. Link: https://bit.ly/3aS6rQc
- Raines DL, Lopez FA (2011) Clostridium difficile infection in non-HIV-immunocompromised patients and in HIV-infected patients. Curr Gastroenterol Rep 13: 344-350. Link: https://bit.ly/3jGZvcE
- Bond SE, Boutlis CS, Yeo WW, Pratt W, Orr ME, et al. (2017) The burden of healthcare-associated Clostridium difficile infection in a non-metropolitan setting. J Hospital Infect 95: 387–393. Link: https://bit.ly/3ad30V1
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll, KC, et al. (2018) Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 66: e1-e48. Link: https://bit.ly/2Zavg4s
- Australian Guidelines for the Prevention and Control of Infection in Healthcare, Canberra (2019) National Health and Medical Research Council. Link: https://bit.ly/3jDTBJm
- Reveles KR, Dotson KM, Gonzales-Luna A, Surati D, Endres BT, et al. (2019) Clostridioides (Formerly Clostridium) difficile Infection During Hospitalization Increases the Likelihood of Nonhome Patient Discharge. Clin Infect Dis 68: 1887-1893. Link: https://bit.ly/2LJN979
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, et al. (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 31: 431-455. Link: https://bit.ly/2NmFdZH
- eTG complete [Internet]. Melbourne (VIC) : Therapeutic Guidelines Ltd. Published April 2019 (eTG December 2019 edition) Clostridium difficle infection.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
