Annals of Systems Biology
In vitro cultivation of Leishmania donovani promastigotes: Growth potential of human urine as replacement of fetal calf serum
Abdalla Hassan Sharief1*, Eltahir Awad GAsim Khalil1 and Suad M Suliaman2
2Sudanese National Academy of Sciences, Khartoum, Sudan
Cite this as
Sharief AH, GAsim Khalil EA, Suliaman SM (2021) In vitro cultivation of Leishmania donovani promastigotes: Growth potential of human urine as replacement of fetal calf serum. Ann Syst Biol 4(1): 001-004. DOI: 10.17352/asb.000010In vitro cultivation of Leishmania parasites plays an important role in diagnosis and treatment of leishmaniasis and in vaccine and drug development studies. There is no information about the effects of urine within culture on the infectivity of Leishmania parasites. In this study we used two culture media with different overlays to enhance the In vitro growth rates of Leishmania donovani.
In this study we used two culture media with different overlays to enhance the In vitro growth rates of Leishmania donovani. Addition of female human urine to these media enhanced the growth of the promastigotes by at least 40 times when compared to the classical Novy-MacNeal-Nicole medium (NNN). Analysis of the effect of urine have showed that proliferation indexes were significantly increased in culture medium supplemented with human urine to 12.84×106 parasite / ml in day 12. On the other hand, when 5% fresh human urine was overlaid, the promastigotes’ count was increased to 32.8×106 parasite / ml on day 4 and further increase to 58.5 x 106 parasite / ml on day 8 was significantly (p=0.002) noted) to 137.8×106 parasite / ml on day 12.
In conclusion, human urine in parasite cultures differentially affected the infectivity and proliferation of L.donovani species promastigotes. Further identification of components found in urine that play a role in infectivity of parasites may be important for understanding transmission mechanisms of parasites into infective stages.
Introduction
The In vitro cultivation of protozoan parasites involves highly complex procedures, which are subject to many variables. Some of these parasites have a very complex life cycle that may require different culture parameters [1]. The culture medium is used to simulate the biochemical environment found within the sand fly vector [2] and there are different researches to make the optimal ones that used in various purposes e.g. diagnosis, immunologic and molecular testing and vaccination [1]. A variety of culture media have been used to cultivate Leishmania; these can be divided into three main categories: semi-solid, biphasic media and liquid monophasic media [3]. Since biphasic and semisolid culture media need blood, an important factor for the reproduction of parasites, which was later enriched with bacteriologic additives such as brain heart infusion is used with various modifications of the liquid phase added to the solid phase [4]. Most liquid media may require Fetal Calf Serum (FCS) or erythrocyte lysate, but some recent studies suggested serum-free liquid medium [3,5]. The Isolation of Leishmania promastigotes from culture is not always successful and this may be due to several factors related to the interaction of parasites with the ingredients that supplemented to media. These stimulate the attempts of modifying the culture media by various components to enhance the growth of promastigotes that lead to increasing of their numbers [6]. As promastigotes growth need nutrients, stimulatory components, and good environmental conditions i.e. sugar is considered as a source of carbon, proteins as source of amino acid, and lipids as source of energy [7-9]. Moreover other stimulatory components governed by suitable temperature are vital for good growth [10,11].
Several studies have shown the stimulatory effect of human urine on Leishmania promastigotes [11-13]. When supplemented with human urine, Schneider’s Drosophila culture media was found to increase the prolif eration of different Leishmania parasite species. In the same study, it was found that culturing amastigotes that were isolated from Leishmania –infected hamsters in a culture environment containing urine increased promastigote differentiation and proliferation compared with controls [11]. Another study questioned whether urine from a healthy human donor, a patient with nephritis, and several types of animals could be an alternative to Fetal Calf Serum (FCS) for In vitro L. donovani culture. Results have indicated that urine was not a valid alternative to FCS, but can be used to increase the proliferation in L. donovani cultures [12].
Materials and methods
Leishmania donovani promastigotes used in this study were obtained from the Leishmania parasite bank, Department of Clinical Pathology and Immunology, Institute of Endemic Diseases, University of Khartoum, Sudan. Novy-MacNeal-Nicole (NNN) was prepared by mixing 1.92 g of blood base agar (Hi Media Laboratories, India) and 0.3 g NaCl with 90 ml of distilled H2O. The mixture was then dissolved and sterilized by autoclaving at 121 °C for 30 minutes. The mixture was allowed to cool to 55 °C before 10% defibrinated rabbit blood was aseptically added gently mixed with the contents by rolling, then 1% of penicillin (50 μg/ml), and streptomycin (50 μg/ml) solution was added to the blood agar mixture. Slopes of culture medium were then prepared by dispensing 2-3 ml of the blood agar mixture into sterile containers that were then set in a slope position until the agar completely solidified. Full fatty and skimmed cow milk were purchased from the local market. Three grams from each milk form was added separately to 30 ml of distilled H2O and sterilized by autoclaving at 110°C for 10 minutes. Agar was added to milk at the same concentration similar to that used in preparing blood-agar medium and other procedures were followed. Five liquid overlays were freshly prepared before being added to the solid phases of the culture media. These were 5% dextrose, 10% full fatty milk, 10% skim (non fatty) milk, 5% human urine and normal saline. Both NNN and milk agar media were supplemented with 20% heat-inactivated Fetal Bovine Serum (FBS) for control cultures. Urine samples were collected from 10 healthy apparently healthy men who had normal prostate, kidney and bladder, and had tested negative for HIV and urinary schistosmiasis on several occasions. Urine samples were taken in the morning before any food or drink was consumed. All urine samples were sterilized by 0.22µm filtration and stored in sterile falcon tubes at 4°C. An aliquot of 1x107 parasites/ml was inoculated into each culture vessels containing solid medium overlaid with 2 ml of each liquid phase and incubated at 26oC. Cultures were daily seen and the growth and behavior of parasites in the media will be observed daily and the media will be change after 3 days. After day 6 of cultivation the overlays were centrifuged to pellet the parasites, supernatants were discarded and each solid medium was overlaid with free liquid phase. Leishmania count was adjusted to 1x106 parasites/ml and inoculated into each culture container. All cultures were incubated for 12 days at 26°C and the growth was counted at days 4, 8 and 12. The parasites was counted by adding 100 µl from each overlay to 1 ml of 5% formalin (25 ml of 0.9 % NaCl + 4.1 ml fromaldyhide), using counting chamber.
Stationary phase promastigotes of L. donovani promastigotes were washed twice by centrifugation (1,500 × g for 5 minutes) in PBS, pH 7.2, and their concentration was adjusted to 1x106 parasites/ml. Infection of macrophages was carried out in triplicate and the infection index of promastigotes in medium supplemented with 5% urine was based on the mean of these triplicate values. After 12 days of macrophage-Leishmania interaction in culture, char slides containing promastigotes were washed in PBS, fixed in absolute methyl alcohol (5 minutes), and stained with Giemsa for determining the infection index.
The number of infected macrophages and amastigotes per macrophage was determined for 100 cells on a chamber slides for promastigotes examined. The infection index was expressed as the percentage of infected macrophages multiplied by the average number of amastigotes per macrophage. In addition, the final infection index of promastigotes was represented by the mean of infection index values found in the different Leishmania species examined.
All experiments were repeated at least three times. The results were expressed as the mean ± SD. A parametric test (paired Student’s t test, analysis of variance, F test, and Tukey’s post-hoc test) was used to evaluate the significance of the results. All data were analyzed by using SPSS version 16.0 for Windows and P values < 0.05 were considered statistically significant.
Results
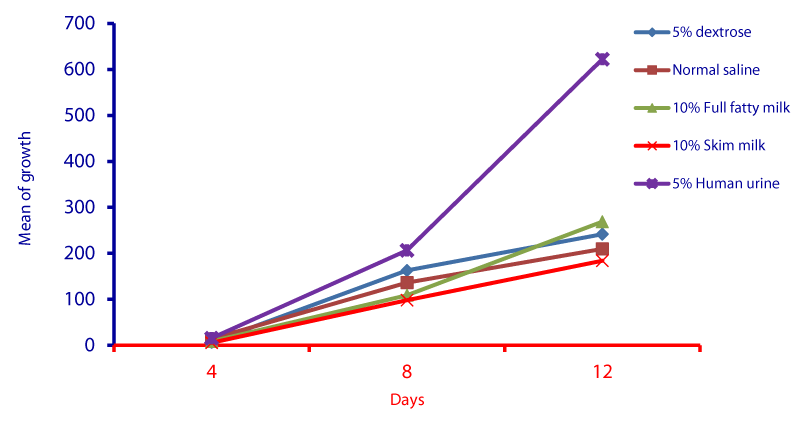
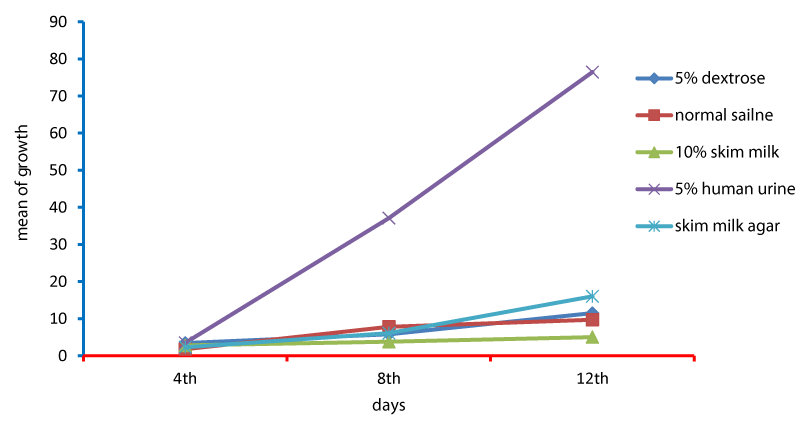
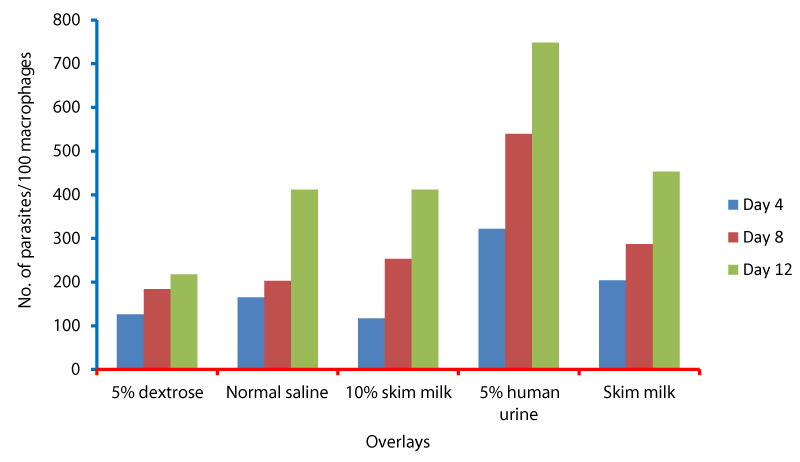
Cell cycle parameters and proliferation index of L. donovani in medium supplemented with 5% urine are shown in Figure 1. After 12 days of incubation, there was a significant increase in the percentage of the parasites in S phases in cultures containing 5% urine in contrast to other overlays (P < 0.05). The NNN medium supported the growth of Leishmania promastigote and the growth rate increased from 7.96 x 106 parasite / ml in day 4 to 56.62 x 106 parasite / ml in day 8 and further increase to 71.2 x 106 parasite / ml in day 12 (Figure 1). When 5% of fresh human urine was overlaid the blood agar medium, the number of Leishmanias was increasing in days 4, 8 (15.53 x 106, 206.4 x 106) and significantly raised (622.4x106 parasite / ml) in day 12 (p=0.0001). Skim milk showed slower elevation (compared to urine) as the growth rates were 5.4 x 106 in day 4, 98.08 x 106 in day 8. This growth was significantly (p=0.001) increased to 183.9 x 106 in day 12 (Figure 1). These findings of a high percentage of cells in the stationary phase and at a high proliferation index indicates a stimulatory effect of 5% urine on cell proliferation Medium composed of full fatty milk as a solid phase (with no overlay) produced 13.4 x 106 parasite / ml in day 4 and this count was elevated to 18.25 x 106 in day 8 and continue increasing up to day 12. Enhancing the medium with 10% full fatty milk showed a very low count of the parasites in both days 4 and 12. The count of the parasites was rapidly elevated to 12.84 x 106 parasite / ml in day 12. On the other hand, when 5% fresh human urine was overlaid, the promastigotes’ count was increased to 32.8 x 106 parasite / ml on day 4 and further increase to 58.5 x 106 parasite / ml on day 8 was significantly (p=0.002) noted) to 137.8 x 106 parasite / ml on day 12 (Figure 2). Skim milk media with no overlay insignificantly (p-value) increased the parasites count from 2.3 x 106 to 16.02 x 106 in the consecutive intervals. Addition of 10% skim milk supported the growth rates of the promastigotes from 2.8 x 106 in day 4 to 5.04 x 106 in day 12. The human urine overlaid this medium showed a significant increase in the number of the parasites in all intervals respectively (3.5 x 106, 37.1 x 106, 76.44 x 106), (Figure 3). Results showed that the infection index for L donovani (P = 0.009; n = 20promastigotes exposed to urine was greater than those cultured in other liquid overlays (by paired Student’s t test). In addition, the infection indexes for L. donovani (P = 0.038; n = 20) promastigotes were also different. The presence of urine also caused significant difference in infection indexes of the parasites that cause visceral leishmaniasis.
L. donovani pomastigotes at stationary phase were washed twice by centrifugation (1,500 × g for 5 minutes) in PBS, pH 7.2, and their concentration was adjusted to 1x107 parasites/ml. In vitro infection of macrophages was carried out in triplicate. Therefore, the infection index of promastigotes in medium supplemented with 5% urine was based on the mean of this triplicate values. Parasites were fixed, stained with Giemsa and counted after all day intervals for determining the infection index (Figure 4). The number of infected macrophages and amastigotes per macrophage was determined for 100 cells on a coverslip for each strain examined. The infection index was expressed as the percentage of infected macrophages multiplied by the average number of amastigotes per macrophage (Figure 4). 18 In addition, the final infection index of promastigotes was represented by the mean of infection index values found in the different Leishmania species examined.
Discussion
Inoculation of Leishmania in NNN medium was considered as our measuring scale for parasite growth in modified media. Although addition of blood may render blood-agar media to become more susceptible to contamination but it is still be as an essential component of vital importance to parasite growth.
In this study, cultivation of Leishmania parasites in NNN medium was considered Addition of female human urine to this medium enhanced the growth of the promastigotes by at least 40 times when compared to the classical NNN medium. Such significant increase in parasite growth has previously been reported by several authors who substituted serum with mammalian urine for enhancement of various Leishmania species [13-16]. Furthermore, Warburg, et al. [12] found that 5% of human urine has contained 10µl xanthine that had significantly enhanced the growth of Leishmania major promastigotes In vitro. However, increasing the concentration of urine up to 4% exhibited no satisfactory effect on the growth of the parasites. This later observation seems to contradict with our results in which the concentration of urine was 5% and in spite of that it did not retard the growth of the parasites. These differences might be attributed to the quality and handling of the urine used e.g. male or female urine. On the other hand, human urine induced amplification in the number of promastigotes when added to full fatty milk agar. However, when the urine was added to skim milk, the growth rate of promastigotes was statistically declined which reflects an inhibitory and/or lethal effect.
This may also indicates an enrichment and metabolic enhancement factor that might be contained in the fatty milk. Furthermore, other milk components were also studied in regards to their influence in the parasites In vitro growth. Tyndalized milk of cow, goat and buffalo were found to possess some components that may replace fetal bovine serum during the cultivation of Leishmania donovani promastigotes [8]. Another observation in our study was that urine concentrations greater than 5% may inhibit proliferation of Leishmania parasites [16-18]. In our study, distinct from the other studies, the reason for increased development of parasites in culture containing 5% urine may be dependent on use of parasite species and types of culture medium.
Conclusion
Human urine in parasite cultures differentially affected the infectivity and proliferation of L.donovani species promastigotes. Further identification of components found in urine that play a role in infectivity of parasites may be important for understanding transmission mechanisms of parasites into infective stages.
- Visvesvara GS, Garcia LS (2002) Culture of protozoan parasites. Clin Microbiol Rev 15: 327-328. Link: https://bit.ly/3qNIq3f
- Raymond RW (2008) Exploration of potential reservoir hosts and vectors of Leishmania in Nicaragua. PhD. Dissertation, Texas A & M University. Link: https://bit.ly/3izcUTq
- Rodrigues Ide A, da Silva BA, dos Santos AL, Vermelho AB, Alviano CS, et al. (2010) A new experimental culture medium for cultivation of Leishmania amazonensis: its efficacy for the continuous in vitro growth and differentiation of infective promastigote forms. Parasitol Res 106: 1249-1252. Link: https://bit.ly/3p8tnka
- Wheeler RJ, Gluenz E, Gull K (2011) The cell cycle of Leishmania : morphogenetic events and their implications for parasite biology. Mol Microbiol 79: 647–662. Link: https://bit.ly/2LNqYNs
- Sharief AH, Khalil EAG, Omer SA, Abdalla HS (2007) Innovative serum-free medium for in vitro cultivation of promastigote forms of Leishmania species. Parasitol Int 57: 138-142. Link: https://bit.ly/3qF8kGa
- Frederick LS, James JS (2002) Cultivation of Clinically Significant Hemoflagellates. Clinical Microbiology Reviews 15: 374-398. Link: https://bit.ly/3sWzFFU
- Naderer T, Heng J, McConville MJ (2010) Evidence That Intracellular Stages of Leishmania major Utilize Amino Sugars as a Major Carbon Source, PLoS Pathog 6: e1001245. Link: https://bit.ly/3p2hD2x
- Muniara JMJ, Lal CS, Kumar S, Sinha PK, Das P (2007) Milk of cow (Bos taurus), buffalo (Bubalus bubalis), and goat (Capra hircus): a better alternative than fetal bovine serum in media for primary isolation, in vitro cultivation, and maintenance of Leishmania donovani promastigotes. J Clin Microbiol 45: 1353-1356. Link: https://bit.ly/396qDyc
- Costa TL, Ribeiro-Dias F, Oliveira MAP, Bezerra JCB, Vinaud MC (2011) Energetic metabolism of axenic promastigotes of Leishmania (Viannia) braziliensis. Experimental Parasitology 128: 438-443. Link: https://bit.ly/2Y5gYkV
- Schuster LF, Sullivan JJ (2002) Cultivation of Clinically Significant Hemoflagellates. Clin Microbiol Rev 15: 347-389. Link: https://bit.ly/3qCgXRG
- Özbilgin A, Zeyrek F, Limoncu ME, Östan P, Tabak T, et al. (2010) Comparison of culture media in the isolation and diagnosis of cutaneous leishmaniases. African Journal of Microbiology Research 4: 1038-1104. Link: https://bit.ly/3iBdlg4
- Iqbal J, Jamshid M, Ahmed B, Bukhari I, Bashir S, et al. (2006) Some studies on human urine as promoter for the growth of Leishmania in vitro. Pak J Pharm Sci 19: 152-155. Link: https://bit.ly/3c2LXqi
- Warburg A, Gelman S, Deutsch J (2008) xanthine in urine stimulates growth of Leishmania promastigotes in vitro. J Med Microbiol 57: 136-138. Link: https://bit.ly/3bVG6Tw
- Armstrong TC, Patterson JL (1994) Cultivation of Leishmania braziliensis in an economical serum-free medium containing human urine. J Parasitol 80: 1030-1032. Link: https://bit.ly/3iDMzUy
- Ali SA, Iqbal J, Ahmad B, Masoom M (1998) A semisynthetic fetal calf serum–free liquid medium for in vitro cultivation of leishmania promastigotes. Am J Trop Med Hyg 59: 163-165. Link: https://bit.ly/363pfKK
- Singh S, Mohapatra DP, Sivakumar R (2000) Successful replacement of fetal calf serum with human urine for in vitro culture of Leishmania donovani. J Commun Dis 32: 289-294. Link: https://bit.ly/2Me09So
- Ferreira KA, Lemos-Junior PE, Lages-Silva E, Ramirez LE, Pedrosa AL (2007) Human urine stimulates in vitro growth of Trypanosoma cruzi and Trypanosoma rangeli. Parasitol Res 101: 1383-1388. Link: https://bit.ly/3iAcj40
- Allahverdiyev AM, Bagirova M, Elcicek S, Cakir Koc R, Oztel ON (2011) Effect of Human Urine on Cell Cycle and Infectivity of Leismania Species Promastigotes In Vitro. Am J Trop Med Hyg 85: 639-643. Link: https://bit.ly/3p9m0sy
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
