Archives of Clinical Nephrology
Impact of Diabetes Mellitus on Renal transplant outcome
Muhammad Tanzeel Abbasi*, Mariam Arif and Nayyar Saleem
Cite this as
Abbasi MT, Arif M, Saleem N (2021) Impact of Diabetes Mellitus on Renal transplant outcome. Arch Clin Nephrol 7(1): 057-064. DOI: 10.17352/acn.000057Copyright
© 2021 Abbasi MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Diabetes Mellitus (DM) is a potent risk factor for post-transplant cardiovascular complications and infections. Management of diabetes and its complications in renal transplant recipients is a challenging task. This is a frequently encountered predicament in transplant setups. An erratic glycemic control during dialysis is a predictor of poor graft and patient outcomes after kidney transplantation.
Literature review reveals majority of studies explaining post-transplant diabetes and its role in graft and patient survival. However, a wide range of opinions exists about the impact of pre-transplant DM on transplant outcomes. Measurement of HbA1c levels is a significant tool for assessment of glycemic control. A target HbA1c level of <7% is recommended for diabetic patients irrespective of presence or absence of Chronic Kidney Disease (CKD). However, diabetic patients with CKD are at risk of hypoglycemia owing to decreased insulin metabolism so it is safe to keep HbA1c levels between 7-8% in this population.
Immunosuppressive medications have a strong contributory role in deterioration of glycemic control. So, it is imperative to achieve strict pre-transplant diabetes control in order to avoid post-transplant complications. Post-transplant diabetes mellitus (PTDM) has been a subject of a large number of trials and is not only considered a serious metabolic complication but also a predisposing factor of diabetic nephropathy in transplanted kidney.
Abbreviations
DM: Diabetes mellitus; CKD: Chronic Kidney Disease; PTDM: Post-Transplant Diabetes Mellitus; MODY: Maturity Onset Diabetes of Young; LADA: Latent Autoimmune Diabetes of Adults; ECD: Expanded Criteria Donor; AGE`s: Advanced Glycation End Products; mTORi: mammalian Target of Rapamycin Inhibitors; VADT: Veterans Affairs Diabetes Trial; KDIGO: Kidney Disease: Improving Global Outcomes; GFR: Glomerular Filtration Rate; DPP4: Dipeptidyl Peptidase 4 inhibitors; SGLT2: Sodium Glucose Co-Transporter Type 2; CNI: Calcineurin Inhibitors; SPK: Simultaneous Pancreas and Kidney; PAK: Pancreas After Kidney; KTA: Kidney Transplantation Alone; CAN: Chronic Allograft Nephropathy; MMF: Mycophenolate Mofetil; CT: Computed Tomography; HIV: Human Immunodeficiency Virus; CMV: Cytomegalovirus; EBV: Epstein Bar Virus; CHD: Coronory Heart Disease; ECG: Electrocardiography; ABD: Adynamic Bone Disease; iPTH: Intact PTH; TMV: Turnover, Mineralization, Osteoid Volume; DEXA: Dual-Energy X-ray Absorptiometry
Introduction
Diabetic nephropathy is the commonest cause of end stage kidney disease worldwide. Developed countries have been facing an enormous impact of Chronic Kidney Disease (CKD) due to diabetic nephropathy for the past few decades. Among US population, prevalence of type 1 diabetes is 5.2% and type 2 diabetes is 91.2% [1]. Diabetic nephropathy can develop not only during the course of type 1 and type 2 diabetes but also as a consequence of Maturity Onset Diabetes of Young (MODY) Latent Autoimmune Diabetes of Adults (LADA) and gestational diabetes. Equal risk of diabetic kidney disease has been found in type 1 and type 2 diabetic patients. Overall prevalence of CKD in diabetics is 40% [2]. According to Pérez-Sáez MJ, et al. 95% of patients with type 2 diabetes and only 5% of type 1 diabetes in US population represent total burden of diabetic kidney disease [3].This difference can be attributed to a comparatively less number of type 1 diabetic patients. It has been estimated that renal failure accounts for approximately 10% deaths in diabetic patients [4].
Kidney transplantation is the preferred treatment option for all suitable diabetic patients with End Stage Renal Disease (ESRD). It offers better quality of life and better survival. The preferred approach of transplantation in diabetic end stage kidney disease is:
1. Pre-emptive renal transplantation is better option than starting dialysis followed by transplantation
2. Living donor kidney is preferred to a deceased donor.
3. All diabetic CKD patients should be enlisted in standard donor waitlist as well as Expanded Criteria Donor (ECD) waitlist. Although the outcome of ECD kidney does not meet the standard criteria kidney but compared with dialysis, these patients do well after transplantation in terms of graft and patient survival.
There are certain challenges in pre-transplant evaluation and post-transplant period attributed to control of diabetes. These challenges are related to cardiovascular complications and infections. In addition to these complications, glycemic control becomes difficult after transplantation because immunosuppressive medications have a deleterious effect on islet cells of pancreas and insulin sensitivity. It makes difficult to achieve a target blood glucose level and to prevent recurrence of diabetes related histopathological changes in renal allograft [5].
C-peptide is found as a part of pro-insulin and is an important measure of pancreatic islets cell function. Insulin and C-peptide are secreted in equal amounts but the rate of C-peptide secretion is more constant and prolonged. C-peptide is helpful in differentiating types of diabetes mellitus. A low level of C-peptide (usually < 0.2ng/ml) favors type 1 diabetes [6]. Although early age at diagnosis of diabetes, frequent episodes of diabetic ketoacidosis and insulin dependence favor type-I diabetes but in certain clinical situations, there remains a diagnostic uncertainty especially if previous records are not available. Measurement of C-peptide levels is of great importance in such scenarios.
Renal transplantation in diabetics sometimes becomes a challenging task owing to control of diabetes, selection of patient, presence of co-morbidities and role of immunosuppression especially steroids. Therefore, it is imperative to discuss pre-transplant evaluation, post-transplant complications, donor selection and impact of diabetes and its control on graft and patient survival.
Pre-transplant control of diabetes
Kidneys have an important role in metabolism of insulin and therefore renal functions have a particular influence on glycemic control. A persistently raised HbA1c of >8% during dialysis period is a predictor of high all-cause and cardiovascular mortality. Several mechanisms have been proposed in literature, explaining association of glycemic control with post-transplant complications. The cardinal pathological mechanisms responsible for high mortality include:
a. Generation of Advanced Glycation End products (AGE`s) can shorten patient survival because they induce macrovascular complications
b. Chronic inflammation as a result of high HbA1c levels is a predictor of increased mortality and allograft dysfunction
c. Increased infection rate further predisposes to risk of graft failure.
Renal allograft restores insulin metabolism and hyperglycemia is commonly seen in previously diabetic patients after transplantation. Immunosuppressive medications especially tacrolimus, mammalian target of rapamycin inhibitors (mTORi) and steroids have a contributing role in diabetes after transplantation. Diabetic patients with CKD are at high risk of hypoglycemia and dose of hypoglycemic drugs needs to be adjusted cautiously. Various anti-diabetic drugs have renal excretion, and their accumulation in CKD patients can cause adverse effects. In case of uncertainty about glycemic control, history and medical record suggestive of uncontrolled diabetes include:
a. Frequent hospital admissions due to DKA that shows either non-compliance to treatment or inadequate dose of insulin.
b. Heavy proteinuria and significant hypoalbuminemia.
c. Evidence of vascular calcifications on pelvic X-ray suggesting diffuse atherosclerosis.
d. Presence of micovascular and macrovascular complications of diabetes. These complications usually take more than five years to develop after diagnosis if diabetes remains uncontrolled.
Pre-transplant target levels of HbA1c
The role of strict HbA1c targets in CKD population has been controversial. The ADVANCE trial and Veterans Affairs Diabetes Trial (VADT) showed no benefit of strict diabetes control on cardiovascular outcome among renal transplant recipients. According to one study, HbA1c level of >9% or <6.5% was associated with increased mortality in non-dialysis CKD patients [7]. An HbA1c level of >8.0% is also responsible for increased all cause and cardiovascular mortality among transplant recipients and dialysis patients [8]. Lower HbA1c levels also failed to show a beneficial effect. It is important to individualize glycemic targets based on risks and benefits in advanced diabetic CKD patients. Previously, Kidney disease: Improving Global Outcomes (KDIGO) 2007, recommended HbA1c target level of <7% with or without CKD [9].Usually, in CKD-V patients, an HbA1c target of 7-8% is considered safe to avoid risk of hypoglycemia [Table 1].
Options for diabetes control
A. Medical management
i. Insulin therapy: All types of insulin including short, intermediate and long acting are safe in diabetic CKD-V and transplant patients [Table 2]. However due to risk of hypoglycemia, insulin dosing needs an individualized approach.
ii. Oral hypoglycemic drugs: a. Biguanides (Metformin): Metformin is not recommended below glomerular filtration rate (GFR) of 30ml/min/1.73m2 due to risk of lactic acidosis. It is euglycemic and does not cause weight gain. However, it is not a preferred agent for diabetes control after transplantation due to lack of clinical trials about its risk and benefit ratio [10].
b. Sulfonylureas: They are frequently prescribed in transplant recipients. The risk of hypoglycemia is high with sulfonylureas except glipizide and gliclazide. They should preferably be avoided below GFR 30. Some studies have also shown their role in apoptosis and exhaustion of pancreatic beta cells [11].
c. Dipeptidyle peptidase 4 (DPP4) inhibitors: DPP4 inhibitors (sitagliptin) require dose adjustment in CKD stage 4 and 5 patients. In contrast to sulfonylureas, they are found to preserve pancreatic islet cells in animal models. They can cause prolonged QT interval especially when used with cyclosporine. Limited studies have shown their safety in post-transplant diabetes mellitus (PTDM) [12].
d. Thiazoledinediaones: Thiazoledinediones (rosiglitazone) do not require dose adjustment in CKD patients and are associated with a lower risk of progression to ESRD [13]. However, there is no sufficient data supporting their use in pre-transplant CKD patients and transplant recipients.
e. Sodium-glucose co-transporter type 2 (SGLT2) inhibitors: SGLT2 inhibitors (empagliflozin) increase renal glucose excretion causing a reduction in HbA1c of 0.9 to 1% [14]. Increased incidence of genitourinary infections and lack of sufficient data limits their use in transplant and CKD population.
iii. Modification of immunosuppressive regimens: Glucocorticoids, calcineurin inhibitors (CNI`s) and mTOR-i contribute to impaired glycemic control after transplantation. Different strategies regarding modification of immunosuppression are in practice including early and late steroid withdrawal, low dose maintenance steroids, CNI withdrawal or CNI to mTORi switch. The purpose of these strategies is to reduce immunosuppression related complications including PTDM. However, it is of immense importance to keep a balance between management of diabetes and avoidance of rejection.
B. Transplant options
Strategies of transplantation in diabetic patients include:
a. Simultaneous Pancreas and Kidney (SPK) transplantation
b. Pancreas After Kidney (PAK) transplantation
c. or Kidney Transplantation Alone (KTA)
There is still some controversy about using these options in diabetic patients and their impact on transplant outcome. In type-I diabetes, SPK is usually preferred at least from a deceased donor. Kidney transplantation from a living donor followed by pancreatic transplant is also preferred at some setups with comparable results. However it is less clear whether SPK from a deceased donor has additional benefits over KTA from a living donor.
Based on literature and our local experience, SPK will be the most suitable option in type-1 diabetic CKD patients.
Impact of Pre-transplant glycemic control on transplant outcome
Insulin and oral hypoglycemic drugs play a vital role in achieving target HbA1c levels before and after transplantation. Pre-transplant diabetes has a variable impact on patient and graft survival and post-transplant complications. A definite association has not yet been studied in detail. Previously, it was hypothesized that higher pre-transplant HbA1c levels were associated with worse patient and graft outcome. In a study by Kuo HT et al, pre-transplant diabetes alone was found to have a significant role in cardiovascular mortality but had no effect on graft outcome [15]. However, pre-transplant diabetes plus acute rejection during first post-transplant year were responsible for increased mortality and graft failure.
In another trial by Molnar MZ, et al. 2872 patients with poorly controlled pre-transplant diabetes were studied. Based on his findings, an HbA1c level of >8% was a predictor of increased mortality. Deaths due to cardiovascular complications were high in patients with poor glycemic control [16]. However, pre-transplant glycemic control was not found to have significant association with graft failure or delayed graft function (DGF). Mild hyperglycemia during CKD stage V or during dialysis may not be a risk factor for a poor short term or long term post-transplant outcome. However, the risk of post-operative complications including delayed wound healing and infections is high. Further studies are required to elaborate its role in detail.
In order to prevent negative outcomes after transplantation, a strict pre-transplant glycemic control by achieving an HbA1c target of 7-8% is of utmost importance.
In addition to poor pre-transplant glycemic control, use of corticosteroids after transplantation increases incidence of PTDM. Compared with low maintenance steroids, PTDM is less prevalent in early steroid withdrawal groups [17] but the risk of Chronic Allograft Nephropathy (CAN) is increased [18, 19].
The risk of PTDM with tacrolimus is 10% and cyclosporine is 3% [20]. CNI avoidance regimens including Mycophenolate Mofetil (MMF)/steroids, sirolimus/MMF/steroids and belatacept showed increased long term risk of graft and patient death [21,22].
Sirolimus does not improve insulin sensitivity and is diabetogenic. Sirolimus and CNI`s have comparable risk of PTDM and patient and graft survival. However, compared with CNI`s, it is less diabetogenic [23]. Its widespread use is still hindered due to its side effect profile.
Impact of surgical options on transplant outcome
A comparison of data regarding patient survival between SPK and deceased donor KTA has been discussed in literature. Ten years mortality was high in deceased donor KTA compared to SPK [24]. Another study by Rahill et al also found significantly high mortality in deceased donor KTA compared to living donor KTA and SPK transplants [25].
Overall allograft survival with SPK and living donor KTA transplant is better than deceased donor KTA transplant [26]. Outcome of SPK transplant recipients with functioning pancreas during first post-transplant year is better than deceased and living donor KTA transplant [27]. Those with failed pancreatic graft have high renal allograft loss and patient mortality.
In addition to improved survival, pancreatic transplantation also reduces morbidity. Complications related to glucose metabolism, hyperlipidemias, risk of fractures, fertility, retinopathy and nephropathy after transplantation are reduced.
PTDM and transplant outcome
It was previously believed that post-transplant glycemic control had no impact on patient or graft survival [28]. A report about the impact of PTDM suggested one year survival of 98% in non-diabetics and 83% in diabetic transplant recipients [29]. A later study by Kuo HT, et al. also failed to show any impact of PTDM on transplant outcome [15]. In a study by Sheu A et al, no significant difference of graft and patient survival was found in transplant recipients with or without diabetes [30]. However, the risk of cardiovascular complications was significantly high.
The awareness about the impact of impaired glycemic control was increased by Casio et al who described a two fold increase in mortality in transplant recipients [31]. So, according to evidence till date, it is mabdatory to manage all modifiable factors causing poor glycemic control before and after transplant to avoid its impact not only on patient and graft survival and but also on post-operative complications.
Pre-transplant assessment
A. General workup: The application of transplant suitability is not simple and straightforward in patients with multiple co-morbid conditions. Routine pre-transplant evaluation of all diabetic patients is similar to non-diabetic transplant candidates [Table 3].
B. Targeted workup of diabetic recipients:
i. Cardiovascular evaluation: Most common cause of death in diabetic transplant recipients is related to cardiovascular diseases. These complications are usually observed in early post-transplant period. Pre-transplant as well as post-transplant patients should be screened for modifiable cardiac risk factors. Coronory Heart Disease (CHD) is quite prevalent (30-50%) in asymptomatic diabetic patients [32] and diabetic patients are at a high risk of CHD.
A suggested approach of evaluation in potential diabetic recipients is shown in Figure 1.
a. All diabetic CKD patients need evaluation based on history, clinical examination, Electrocardiographic (ECG) changes and chest radiograph.
b. Patients with CHD or previous myocardial infarction should undergo cardiac catheterization and revascularization (if needed) before transplantation.
c. It is reasonable to perform non-invasive cardiac screening in all diabetics irrespective of age. Cardiac catheterization is not recommended if there is no evidence of angina, peripheral heart disease, smoking or significant Electrocardiographic (ECG) changes.
d. Dobutamine stress echocardiography is done in all symptomatic diabetics who cannot undergo initial screening coronary angiography and if positive, the decision to proceed for angioplasty or cardiac bypass should be done after consultation with a cardiologist.
Among non-invasive tests, dobutamine stress echocardiography has better sensitivity in transplant candidates. Its positive predictive value is 75% [33].
ii. Peripheral vascular disease: Asymptomatic diabetic patients with palpable femoral and peripheral pulses usually require no further investigations. Approach to evaluate peripheral vascular disease is:
a. All diabetic patients with poor peripheral pulses, evidence of vascular calcifications and those above 45 years of age should undergo initial evaluation with Doppler ultrasound.
b. In case of inconclusive Doppler scan, non-contrast abdominopelvic CT scan is performed.
c. All patients with evidence of peripheral vascular disease should be evaluated by vascular disease specialist.
d. Significant vascular calcifications make transplant difficult and sometimes impossible. Although it is not an absolute contraindication but is associated with increased morbidity and mortality as well as graft loss [34].
e. Due to non-compressible vessels, standard ankle brachial index or toe brachial index remains inconclusive for diagnosis.
Any evidence of significant vascular disease precludes renal transplantation.
iii. Bone mineral profile: Adynamic Bone Disease (ABD) affects > 40% of CKD-V patients and has strong association with vascular calcifications, increased incidence of fractures and cardiovascular mortality [35,36]. So, a co-existence of ABD and DM adds on the risk of cardiac complications. Screening of bone mineral profile includes:
a. Biochemical parameters: Serum calcium and phosphate, serum vitamin D and intact Parathyroid Hormone (iPTH) levels need to be done. Low iPTH levels (<100-150pg/ml) are indicative of ABD. However, histologically evident ABD can occur with iPTH levels of up to 300pg/ml [37].
b. Bone biopsy: Bone biopsy is the gold standard for evaluation of mineral bone disorders and is based on turnover, mineralization and osteoid volume (TMV) [38]. Symptomatic patients with persistent hypercalcemia, progressive extra osseous calcification, fractures and bone pains should undergo bone biopsy.
c. Dual Energy X-ray Absorptiometry (DEXA) scan: DEXA scan gives an idea about normal, low or high bone density but it does not give idea of bone turn over. Although it is a less significant tool in evaluating mineral bone density but due to its non-invasive nature, it is still in frequent use. Its findings are based on T and Z scoring [Figure 2].
Restoration of normal serum calcium, phosphate and target iPTH levels, reduction or withdrawal of active vitamin D products are the key steps in the management of ABD. Co-existing osteoporosis needs to be excluded and managed accordingly before transplantation.
iv. Pulmonary assessment: Smoking is an independent risk factor for poor graft and patient survival and this risk is further increased in diabetic transplant recipients. Physical examination and chest radiograph are part of initial pulmonary assessment. If there is history of smoking, pulmonary function tests and CT scan of chest may be required. Patients with uncontrolled asthma, cor-pulmonale and severe obstructive lung disease (FEV1 < 25% or PO2 < 60mmHg) should be excluded from transplantation [39]. Cigarette smoking is associated with increased mortality and graft loss so it is preferable to cease smoking before transplantation.
v. Psychiatric evaluation: Non-conformity towards treatment culminating in repeated hospital admissions due to diabetic complications, history of smoking and alcohol addiction in potential transplant recipients necessitate psychological evaluation. Non-compliant attitude towards anti-diabetic medications and immunosuppression due to psychiatric disorders is a contraindication to transplantation.
Effect of smoking on transplantation in diabetic recipient
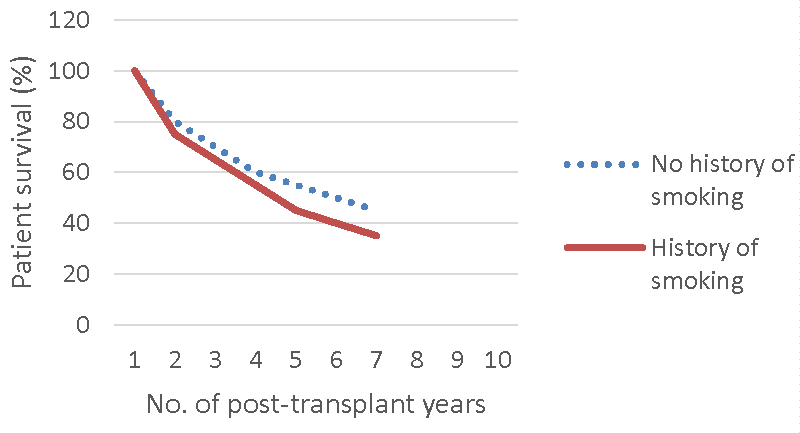
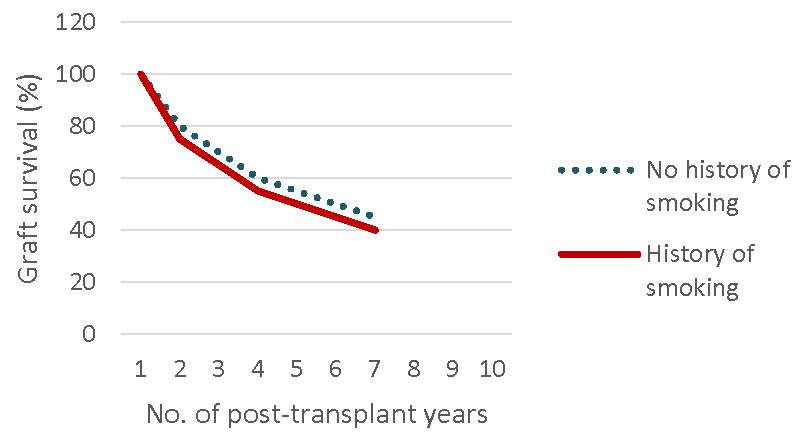
The association of smoking with cardiovascular, pulmonary and malignant diseases and death has been documented in many studies. The data about impact of smoking on renal allograft is surprisingly low. The risk of these complications in diabetic patients with history of smoking is greater than those with no history of smoking. Smoking is one of the modifiable causes of death. Smoking increases the risk of recipient death as well as graft loss. This effect is mediated by immunocompromised status and metabolic effects of medications [40].
Smoking is associated with 30% increased risk of graft failure and most of its effects on allograft are also related to mortality due to smoking [41]. [Figure 3a,3b] show high risk of mortality and graft loss in smoker transplant recipients.
In addition to immunosuppression, smoking has a strong association with post-transplant malignancy which is another leading cause of death and graft loss in transplant recipients [42,43].
Studies have documented a role of smoking in histopathological findings of chronic sclerosing nephropathy and arteriolar hyalinosis [44]. Smoking also has a strong correlation with cardiovascular diseases and due to the combined effect, mortality is high in transplant recipients [42].
The risk of mortality and graft failure is surprisingly high in recipients who received renal allograft from a smoker [40].
Effect of alcohol consumption on transplant outcome in diabetic recipient
Chronic inflammatory process is one of the cardinal mechanisms of renal damage in diabetic patients. Although the role of alcohol consumption on renal functions has got less attention but some studies have mentioned a favorable outcome of alcohol on kidney functions in general population [45]. Anti-oxidant and anti-inflammatory properties of alcohol are thought to counteract the chronic inflammation caused by DM and therefore, have a reno-protective effect [46]. Some other studies have documented an increased risk of CKD and its progression due to alcohol consumption [47].
Among transplant recipients, it has been described that a combined effect of alcohol and diabetes can increase the risk of cardiovascular and liver diseases with increased risk of death. However the direct effect of alcohol on patient and graft survival needs further studies.
According to available data, it has been shown that moderate alcohol consumption can reduce the risk of PTDM and mortality [48]. Some studies have also documented that 1-3 drinks/day reduce the incidence of CHD and diabetes not only in general population but also in transplant recipients [49].
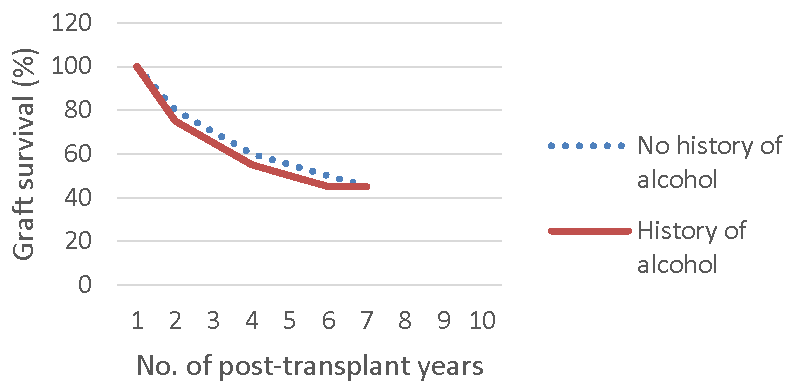
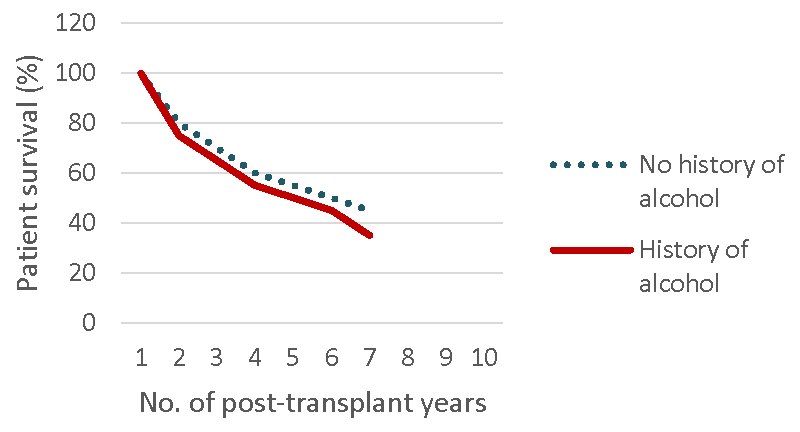
Heavy alcohol drinking has an opposite effect. In a study by Abdou et al, about 425 patients with habit of heavy alcohol consumption before transplantation were evaluated for patient and graft survival. The results of study showed a strong association with reduced patient as well as graft survival [50]. Among these recipients, no renal allograft survived beyond six years [Figure 4a] and very few recipients survived at six post-transplant years [Figure 4b].
The available data in favor of or against alcohol consumption is not sufficient and needs further studies to document its definite role on transplant outcomes. Due to deleterious multi-system effects of alcohol and diabetes, all transplant recipients should be advised to cease or at least reduce alcohol consumption.
Diabetes and kidney donation
Available data has no conclusive agreement in accepting pre- diabetic donors for kidney donation. There is a small percentage of transplanted kidneys donated from diabetic patients ranging from 3.5-6% [51]. Although the risk of diabetic nephropathy and CHD in patients with diet control diabetes or with impaired fasting glucose is not increased after kidney donation but the scenario is not same for young and elderly donors. The risk of these complications in young diabetic patients is high because the risk has longer time to unfold in this population [52]. There is high likely chance of turning pre-diabetes into diabetes and since diabetic nephropathy is a leading cause of renal failure so this raises a concern for kidney donation. However, patients with uncontrolled diabetes or having end organ involvement are not considered for donation so far.
Conclusion
In a comprehensive way, target HbA1c level in CKD is still controversial and needs individualized approach. Modifiable risk factors including smoking and alcohol abuse are not only associated with reduced graft and patient survival but also increase all-cause mortality especially due to cardiac dysfunction. A positive approach towards management of these factors includes cessation of smoking and alcohol abuse to reduce post-transplant complications. Selection of a donor with pre-diabetes is still based on individualized approach and needs further studies about its impact in pre-diabetic young donors.
- Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, et al. (2018) Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ 362: k1497. Link: https://bit.ly/3uVU27A
- Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12: 2032-2045. Link: https://bit.ly/3li2JFT
- Pérez-Sáez MJ, Pascual J (2015) Kidney Transplantation in the Diabetic Patient. J Clin Med 4: 1269-1280. Link: https://bit.ly/2YsUtdN
- vanDieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B (2010) The global burden of diabetes and its complications: an emerging pandemic. Eur J CardiovascPrev Rehabil 17: S3- S8. Link: https://bit.ly/3DpsZVg
- Gaston RS, Basadonna G, Cosio FG, Davis CL, Kasiske BL, et al. (2004) National Kidney Foundation Task Force on Diabetes and Transplantation. Transplantation in the diabetic patient with advanced chronic kidney disease: a task force report. Am J Kidney Dis 44: 529-542. Link: https://bit.ly/2YwHkQx
- Leighton E, Sainsbury CA, Jones GC (2017) A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther 8: 475-487. Link: https://bit.ly/3v5O2t3
- Hahr AJ, Molitch ME (2015) Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol 1. Link: https://bit.ly/3my9MKa
- Ricks J, Molnar MZ, Kovesdy CP, Shah A, Nissenson AR, et al. (2012) Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes 61: 708-715. Link: https://bit.ly/3Aper5M
- KDOQI (2007) KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 49: S12-154. Link: https://bit.ly/3oKfuey
- Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, et al. (2014) Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant 14: 1992-2000. Link: https://bit.ly/3AmQKLB
- Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, et al. (2005) Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 90: 501-506. Link: https://bit.ly/3oFVERJ
- Haidinger M, Werzowa J, Hecking M, Antlanger M, Stemer G, et al. (2014) Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation--a randomized, double-blind, placebo-controlled trial. Am J Transplant 14: 115-123. Link: https://bit.ly/30bPyhT
- Chen YH, Chiang MH, Liu JS, Chang YK, Kuo KL, et al. (2015) Thiazolidinediones and Risk of Long-Term Dialysis in Diabetic Patients with Advanced Chronic Kidney Disease: A Nationwide Cohort Study. PLoS One 10: e0129922. Link: https://bit.ly/2WT52G9
- Kalra S (2014) Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther 5: 355-366. Link: https://bit.ly/3lkLDqN
- Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S (2010) Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis 56: 1127-1139. Link: https://bit.ly/3mzQlAz
- Molnar MZ, Huang E, Hoshino J, Krishnan M, Nissenson AR, et al. (2011) Association of pretransplant glycemic control with posttransplant outcomes in diabetic kidney transplant recipients. Diabetes Care 34: 2536-2541. Link: https://bit.ly/3iIBzq6
- Thomusch O, Wiesener M, Opgenoorth M, Pascher A, Woitas RP, et al. (2016) Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open-label, multicentre, randomised controlled trial. Lancet 388: 3006-3016. Link: https://bit.ly/3lmnf8t
- Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, et al. (2008) A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564-577. Link: https://bit.ly/3iHgA73
- Sinclair NR (1992) Low-dose steroid therapy in cyclosporine-treated renal transplant recipients with well-functioning grafts. Can Med Assoc J 147: 645–657. Link: https://bit.ly/3iDSlGM
- Heisel O, Heisel R, Balshaw R, Keown P (2004) New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant 4: 583-595. Link: https://bit.ly/3AhAThi
- Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, et al. (2006) Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant 6: 514-522. Link: https://bit.ly/3oIZUja
- Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, et al. (2016) Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med 374: 333-343. Link: https://bit.ly/3afOCe5
- Sawinski D, Trofe-Clark J, Leas B, Uhl S, Tuteja S, et al. (2016) Calcineurin Inhibitor Minimization, Conversion, Withdrawal, and Avoidance Strategies in Renal Transplantation: A Systematic Review and Meta-Analysis. Am J Transplant 16: 2117-2138. Link: https://bit.ly/3Dpu2oa
- Tydén G, Bolinder J, Solders G, Brattström C, Tibell A, et al. (1999) Improved survival in patients with insulin-dependent diabetes mellitus and end-stage diabetic nephropathy 10 years after combined pancreas and kidney transplantation. Transplantation 67: 645-648. Link: https://bit.ly/3oNUvHX
- Rayhill SC, D'Alessandro AM, Odorico JS, Knechtle SJ, Pirsch JD, et al. (2000) Simultaneous pancreas-kidney transplantation and living related donor renal transplantation in patients with diabetes: is there a difference in survival? Ann Surg 231: 417-423. Link: https://bit.ly/3DlrPd9
- Gaston RS, Basadonna G, Cosio FG, Davis CL, Kasiske BL, et al. (2004) National Kidney Foundation Task Force on Diabetes and Transplantation. Transplantation in the diabetic patient with advanced chronic kidney disease: a task force report. Am J Kidney Dis 44: 529-542. Link: https://bit.ly/2YwHkQx
- Weiss AS, Smits G, Wiseman AC (2009) Twelve-month pancreas graft function significantly influences survival following simultaneous pancreas-kidney transplantation. Clin J Am Soc Nephrol 4: 988-995. Link: https://bit.ly/3oIlTaf
- Cole EH, Johnston O, Rose CL, Gill JS (2008) Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 3: 814-821. Link: https://bit.ly/3oHqVUg
- Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, et al. (1987) The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 44: 376-381. Link: https://bit.ly/3BCsxm5
- Sheu A, Depczynski B, O'Sullivan AJ, Luxton G, Mangos G (2016) The Effect of Different Glycaemic States on Renal Transplant Outcomes. J Diabetes Res 2016: 8735782. Link: https://bit.ly/3DpCu6v
- Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, et al. (2002) Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 62: 1440-1446. Link: https://bit.ly/3ApgczU
- Ramanathan V, Goral S, Tanriover B, Feurer ID, Kazancioglu R, et al. (2005) Screening asymptomatic diabetic patients for coronary artery disease prior to renal transplantation. Transplantation 79: 1453-1458. Link: https://bit.ly/3mDtR1F
- Sharma R, Pellerin D, Gaze DC, Gregson H, Streather CP, et al. (2005) Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol Dial Transplant 20: 2207-2214. Link: https://bit.ly/3AnMo6P
- Schiffrin EL, Lipman ML, Mann JF (2007) Chronic kidney disease: effects on the cardiovascular system. Circulation 116: 85-97. Link: https://bit.ly/2WQx7h8
- London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, et al. (2004) Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15: 1943-1951. Link: https://bit.ly/3Bn9JqA
- Malluche HH, Mawad H, Monier-Faugere MC (2004) The importance of bone health in end-stage renal disease: out of the frying pan, into the fire? Nephrol Dial Transplant 19: i9-i13. Link: https://bit.ly/3FqtCzB
- Barreto FC, Barreto DV, Moysés RM, Neves KR, Canziani ME, et al. (2008) K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int 73: 771-777. Link: https://bit.ly/3DnNdyo
- Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, et al. (2006) Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945-1953. Link: https://bit.ly/3iDb19J
- Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, et al. (2005) Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation [published correction appears in CMAJ 173: 1181-1184. Link: https://bit.ly/306Zr0a
- Aref A, Sharma A, Halawa A (2017) Smoking in Renal Transplantation; Facts Beyond Myth. World J Transplant 7: 129-133. Link: https://bit.ly/2YrPeKP
- Kasiske BL, Klinger D (2000) Cigarette Smoking in Renal Transplant Recipients. JASN 11: 753-759. Link: https://bit.ly/3ajT5MK
- Ponticelli C, Villa M, Cesana B, Montagnino G, Tarantino A (2002) Risk factors for late kidney allograft failure. Kidney Int 62: 1848-1854. Link: https://bit.ly/3FrAMDw
- Nourbala MH, Nemati E, Rostami Z, Einollahi B (2011) Impact of cigarette smoking on kidney transplant recipients: a systematic review. Iran J Kidney Dis 5: 141-148. Link: https://bit.ly/3FvvJSw
- Zitt N, Kollerits B, Neyer U, Mark W, Heininger D, et al. (2007) Cigarette smoking and chronic allograft nephropathy. Nephrol Dial Transplant 22: 3034-3039. Link: https://bit.ly/3Bno721
- Lee YJ, Cho S, Kim SR (2021) Effect of alcohol consumption on kidney function: population-based cohort study. Sci Rep 11: 2381. Link: https://go.nature.com/3agBk0K
- Yahfoufi N, Alsadi N, Jambi M, Matar C (2018) The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 10: 1618. Link: https://bit.ly/3iEiBRd
- Shankar A, Klein R, Klein BE (2006) The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 164: 263-271. Link: https://bit.ly/3iIqWmZ
- Zelle DM, Agarwal PK, Ramirez JL, van der Heide JJ, Corpeleijn E, et al. (2011) Alcohol consumption, new onset of diabetes after transplantation, and all-cause mortality in renal transplant recipients. Transplantation 92: 203-209. Link: https://bit.ly/3BuOVha
- Ajani UA, Hennekens CH, Spelsberg A, Manson JE (2000) Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Arch Intern Med 160: 1025-1030. Link: https://bit.ly/3oJ9ToI
- Gueye AS, Chelamcharla M, Baird BC, Nguyen C, Tang H, et al. (2007) The association between recipient alcohol dependency and long-term graft and recipient survival. Nephrol Dial Transplant22: 891-898. Link: https://bit.ly/3uU6jcH
- Truong LD, Suki WN, Gaber LW, Gaber OA, Khan F (2019) Kidney Donors With Diabetes: Renal Biopsy Findings at Time of Transplantation and Their Significance. Transplant Direct 5: e465. Link: https://bit.ly/3iK1TjH
- Vigneault CB, Asch WS, Dahl NK, Bia MJ (2011) Should living kidney donor candidates with impaired fasting glucose donate? Clin J Am Soc Nephrol 6: 2054-2059. Link: https://bit.ly/3mDv3Cb
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
