Open Journal of Parkinson's Disease and Treatment
How does medicine Parkon® affect the MPTP-induced oxidation stress and MAO systems of the rats brain
N Goldstein1,2*, A Kamensky1, T Arshavskaya2 and R Goldstein2
2Latvian Medical Academy, Toxicological Laboratory, Riga, Latvia
Cite this as
Goldstein N, Kamensky A, Arshavskaya T, Goldstein R (2021) How does medicine Parkon® affect the MPTP-induced oxidation stress and MAO systems of the rats brain. Open J Parkinsons Dis Treatm 4(1): 001-004. DOI: 10.17352/ojpdt.000010Introduction
The review of the literature seems to confirm effects of negative air ions (NAIs) on several brain functions. Indeed, a significant association between NAIs exposure and both wellbeing and high cognitive performances has been described [1-3].
We performed tests in order to study participation of nerve centers of the brain, whose exteroceptor afferent input originates from receptors of the nasal cavity mucosa. The focus was on the role of hypothalamus and basal ganglia structures in physiological reactions stimulated by the new-generation anti-Parkinsonian drug Parkon® [4] (hereafter: Parkon).
The special interest in that regard was the investigation of MAO regulation in the level of neuromediator monoamines and their follow-up product - hydrogen peroxide. This product is created, in particular, in catalyzed by monoamine oxidases in the process of oxidation of serotonin and dopamine and is considered to be the main reason for the increase in the level of lipid peroxidation and destruction of dopaminergic neurons in the basal ganglia in case of Parkinson’s disease [1].
Gradual neuronal loss develops over a long period of time, and interrupting this process is an important goal of disease prevention and treatment. We have previously shown that air contains natural levels of atmospheric reactive oxygen species, primarily O2- and H2O2 [5] and that low levels of these ROS are vital environmental factors [6]. This work is based on our earlier studies of the vital role of nanomolar concentrations of atmospheric O2- ions and hydrogen peroxide as a product of its transformations.
A common biochemical model of Parkinson’s disease in the experiment is MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), which induces oxidative stress and damage to the structures involved. These processes represent the main factor in degeneration of dopaminergic neurons of Substantia nigra pars compacta and formation of dopaminergic deficit in Striatum. Inhibition and interruption of this process is the goal of the search for methods of etiotropic prevention and treatment of oxidative damage and dysfunction of brain cell structures [7-10].
The goal of this prospective study was to explore a new approach to therapeutic strategies related to the control of brain oxidative stress as an etiological basis of Parkinson’s disease. In this work we schow that endonasal application of low concentrated hydrogen peroxide as a part of the new medicine Parkon® normalizes the oxidation stress of the brain tissue connected by decreasing MAO activity in the brain tissues. This was proven in the following experiments:
1. Measurement of MAO-A and MAO-B activity in hypothalamus and basal ganglia;
2. Measurement of the concentration of dopamine and its metabolites, as well as morphological changes in Substantia nigra and Striatum, including the role played by reactive oxygen species under the influence of xenobiotics (the pharmaceutical drug Reserpine and the toxic xenobiotic MPTP (1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine);
3. Indicators of oxidative stress (malondialdehyde (MDA) and conjugated diene) in brain tissue.
The study was conducted on more than 300 healthy laboratory rats.
Antireserpine action and the activation of monoaminergic systems of the brain
After a continuous administration of Parkon, rats demonstrated a suppression of MAO-B activity in the hypothalamus and basal ganglia and MAO-A activity in hypothalamus, accompanied by the decrease in the level of the oxidation stress in these structures (Table 1).
Also, it was determined that Parkon decreases the oxidation stress and normalizes increased MAO-B activity in the hypothalamus and basal ganglia, caused by the de-sympathization of the animals (rats, reserpine intraperitoneal, 5 mg/kg once or 2mg/kg thrice), and restores reserpine-dependent spontaneous motor activity (rats, mice). Similar effects were noted when inhaling gaseous superoxide O2●— or by nasal spraying of extremely low concentrations of hydrogen peroxide [1,2]. These results agree with the hypothesis of the involvement of serotoninergic mechanisms in the reactions caused by negative air ions and leaning towards the central physiological mechanisms of actions of ultralow-concentrated hydrogen peroxide and Parkon [2].
Protection of basal ganglia structures against the destructive actions of MPTP
In experiments on animals, the parkinsonian symptoms may be induced by the neurotoxin MPTP (1-Methyl-4-phenyl- 1,2,3,6-tetrahydropyridine). The role played by the MAO-B-dependent destruction of dopamine and oxidative stress in the nuclei of the basal ganglia of the brain has been previously demonstrated in various pathological conditions), including Parkinson’s disease. Monoamine oxidase B, present in the mitochondrial outer membrane of glial cells, catalyzes the oxidation of MPTP to the toxic 1-methyl-4-phenylpyridinium ion (MPP+), which then targets the dopaminergic neurons causing neuronal death. One of possible ways to slow down the development of this pathology is the pharmaceutical inhibition of MAO-B activity. The free radical activity of MPTP metabolism products causes degenerative changes and death of the neurons in substantia nigra and a decrease of dopamine in the striatum, which is accompanied in rats by the suppression of motor and other behavioral reactions, inclusive the development of depressive signs.
Using the MPTP model (MPTP dose = 25 mg/kg i.p.), we researched the effect of “prophylactic” and “therapeutic” use of intranasal applications of micromolar hydrogen peroxide to rats. We investigated the changes in the dopamine metabolism (dopamine, content of deoxy phenylacetate and homovanillic acid), morphological changes in the basal ganglia structures (striatum, midbrain), and the spontaneous motor activity of the animals. It was shown that hydrogen peroxide, in both prophylactic and therapeutic methods of application, compensated for MPTP dependent decrease in the dopamine production (Table 2).
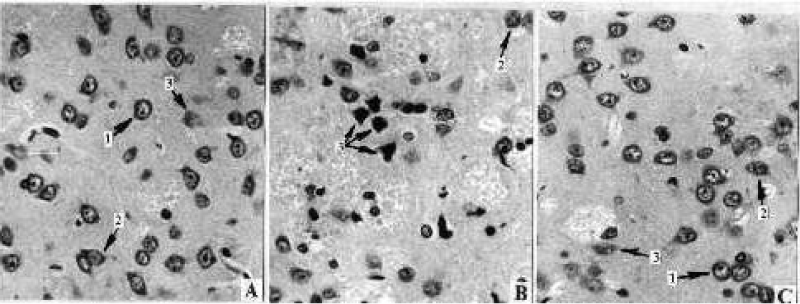
Morphometric analysis of neostriatum tissue in MPTP-treated animals according to the method described in work of Avrushenko [11] showed a 23.5% decrease in total density of neurons and 32.5% increase in quantity of morphologically modified neurons (Figures 1-3). It is significant that free neurons were the most sensitive (25.1% decrease, p<0.005) when the neuron population with the satellite glia was not affected. The “therapeutic” application of H2O2 completely abolished the functional fallout and morphological changes of the neurons and the neuroglia of neostriatum. In the “prophylactic” application, the neuroprotective effect of the exogenous hydrogen peroxide was less significant; however, even in this case, the damage to the structures was much less compared to the group that received only MPTP.
In the substantia nigra we observed MPTP-dependent decrease (35.6%) of various types of neurons and the increase (51.2%) of morphologically modified neurons – effects that are completely prevented by the “therapeutic” application of the hydrogen peroxide. In contrast to the “therapeutic” effect, the prevention of the development of the degenerative processes in the neuron population in the substantia nigra in the “prophylactic” application of hydrogen peroxide was not very significant.
As a result of both “therapeutic” and “prophylactic” application of hydrogen peroxide, the described correction of pathological changes in MPTP-treated animals was quite significant. This can be explained by the time-dependent functional compensation of the dopaminergic system of the basal ganglia and testify to a neuroprotective and/or dopaminoprotective effect. On the whole, the results of the study indicate an important role of intranasal application of low-concentration hydrogen peroxide in the regulation and restoration of impaired functional activity of the studied brain neurotransmitter systems and involvement of sensory neurons of the nasal cavity mucosa in the mechanisms of signaling action of hydrogen peroxide. It is also known that reactive oxygen species are usually considered not only as dangerous exogenous or endogenous products, but also as regulators of important physiological functions, the so-called “redox signaling” [12].
Conclusion
The experimental results confirmed the therapeutic efficacy of the intranasal application of Parkon in case of pathological changes in the structures and neurotransmitter systems of the CNS that are involved in the pathogenesis of Parkinson’s disease [13]. The determined and also supposed mechanisms of the therapeutic action of Parkon for the treatment of the Parkinson’s disease have a lot in common with the pharmacological action of selegiline, the first and, so far, the only widely used in the therapy of the Parkinson’s disease MAO-B inhibitor [14]. The common traits are:
- inhibition of MAO-B activity in CNS and reduction of endogenous oxidative stress;
- reducing the toxic effects of exogenous and endogenous neurotoxins by inhibiting MAO-B and preventing and damaging neurons as a result of selective return of toxins to nerve endings;
- reduction of endogenous oxidative stress in brain tissues by increasing the level of endogenous antioxidant protection.
In addition, a separate observation showed activation of the dopaminergic (DA) brain system by inhibition of the functional dopamine antagonist – the lactotropic hormone (data not shown).
- Parkon (2004) In: Register of Medicinal Products of Russia (RLS) 7: 665.Link:
- Goldstein NI (2000) Biophysical aspects of the physiological action of exogenous O2● ̅on animals. Post.doc. diss Link:.
- Della Vecchia A, Mucci F¸ Pozza A, Marazziti D (2020) Negative air ions in neuropsychiatric disorders. Curr Med Chem 40: S167-S168. Link: https://bit.ly/3bOGlio
- Meredith GE, Halliday GM, Totterdell S (2004) A critical review of the development and importance of proteinaceous aggregates in animal models of Parkinson's disease: New insights into Lewy body formation. Parkinsonism Relat Disord 10: 191–202. Link: https://bit.ly/3wwhYOu
- Avruschenko M (1994) Changes in heterogeneous neural populations in the postresuscitation period after cardiac arrest in rats. Anesteziol Reanimatol 5: 41-44. Link: https://bit.ly/34dLU5z
- Magyar K (1997) Effect of selegiline against selective neurotoxins. Vopr Med Khim 6: 504-514. Link: https://bit.ly/3fePNNU
- Rhee SG (1999) Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med 31: 53-59. Link: https://bit.ly/3vgYoFC
- Coughlan MP, Rajagopalan KV (1980) The kinetic mechanism of xantine dehydrogenase and related enzymes. Eur J Biochem 105: 81-84. Link: https://bit.ly/3fKPDx7
- Cohen G (1984) Oxy-radical toxicity in catecholamine neurons. Neurotoxicology 5: 77-82. Link: https://bit.ly/3vfE8UP
- Cohen G, Pasik P, Cohen B, Leist A, Mytilineou C, et al. (1985) Pargiline and deprenyl prevent the neurotoxicity of 1-methyl-4-phenyl- 1,2,5,6-tetrahydropyridine (MPTP) in monkeys. Eur J Biochem 106: 209-214. Link: https://bit.ly/34aXuhV
- Goldstein NI, Goldstein RN, Merzlyak MN (1992) Negative air ions as a source of superoxide. Int J Biometeorol 36: 118–122. Link: https://bit.ly/3vt0Glf
- Goldstein N, Arshavskaya T (1997) Is atmospheric superoxide vitally necessary? Accelerated death of the animals in a quasi-neutral electric atmosphere. Z Naturforsch C J Biosci 52: 396-404. Link: https://bit.ly/3visDf8
- Goldstein N (2002) Reactive oxygen species as essential components of ambient air. Biochemistry 67: 161–170. Link: https://bit.ly/34eRjJP
- Bajpai P, Sangar MC, Singh S, Tang W, Bansal S, et al. (2013) Metabolism of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine by Mitochondrion-targeted Cytochrome P450 2D6. J Biol Chem 288: 4436–4451. Link: https://bit.ly/3fePylY
- Golubev VL, Sadekov PA, Pilipovich AA and Goldstein NI (2019) Parkon as a treatment of the Parkinson’s disease. Open J Parkinsons Dis Treatm 2: 001-005. Link: https://bit.ly/2QLw52Z
- Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344: 721-724. Link: https://bit.ly/2RGP6nJ
- Fowler JS, Logan J, Volkow ND, Shumay E, McCall-Perez F et al. (2015) Evidence that Formulations of the Selective MAO-B Inhibitor, Selegiline, which Bypass First-Pass Metabolism, also Inhibit MAO-A in the Human Brain. Neuropsychopharmacology 40: 650–657. Link: https://bit.ly/2TfFqB6
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
