Annals of Marine Science
Four consecutive coral bleaching events in the Northern Persian Gulf: 2014–2017
Javid Kavousi1*, Parviz Tavakoli-Kolour2, Sanaz Hazraty-Kari2 and Forough Goudarzi3
2Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, Sesoko 3422, Okinawa 905-0227, Japan
3Department of Natural Resources, Isfahan University of Technology, Isfahan, Iran
Cite this as
Kavousi J, Tavakoli-Kolour P, Hazraty-Kari S, Goudarzi F (2021) Four consecutive coral bleaching events in the Northern Persian Gulf: 2014–2017. Ann Mar Sci 5(1): 007-014. DOI: 10.17352/ams.000025Climate change-induced bleaching is a serious threat to coral reefs worldwide. In recent years, the number of repeated extensive bleaching events has increased globally. Here, we present four consecutive bleaching events and post-bleaching mortalities from four sites on Hormuz and Larak Islands, Iran, in the Persian Gulf from 2014 to 2017. The high thermotolerance of the corals and their endosymbiotic algae and the strong water currents and sites’ turbidity could not protect the majority of the corals against bleaching. Back-to-back bleaching events left almost no unbleached coral colony at any site by 2017. Despite that, coral mortality did not increase at the sites of Hormuz Island that may be a sign of the fast recovery of the Persian Gulf corals after each bleaching event. However, the abundance of coral colonies with 81%-100% mortality at the sites of Larak Island that was constantly minimal in the first three years significantly increased in 2017. Considering bleaching and mortality responses, and abundance dynamics of the coral genera at the study sites, it seems that Dipsastraea at the southwest of Larak Island was a short-term winner; despite facing widespread moderate bleaching (i.e., < 50 of a colony was bleached) in 2014–2016, it managed to significantly increase its population in 2015 and 2016. However, in 2017, when almost all colonies were severely bleached (i.e., > 50 of a colony was bleached), there was a non-significant 27% reduction in its abundance. Dipsastraea at the north of Larak Island also survived in the first three years, but > 66% of its colonies showed 81%–100% mortality in 2017. Such findings warn that the aforementioned successes by corals are unlikely to persist under annual severe bleaching as is predicted for coming decades.
Introduction
In the past few decades, global coral reefs have been threatened by a wide range of human activities such as accelerated industrialization, urbanization, agriculture, and natural phenomena including storms and biological stressors [1-4]. However, global warming-induced coral bleaching and mortalities due to fast increases in temperatures that pass coral species’ thermal tolerance have become the most prominent concern [5-7]. Such temperature anomalies have destroyed coral reefs worldwide, particularly during the four-year period from 2014 to 2017 [6-12]. For example, mass bleaching happened in the Great Barrier Reef, Australia, in 2016 and 2017 [7,8] and in the central Indian Ocean in 2015 and 2016 [13] including the Persian Gulf [14-18].
As the warmest coral sea [19], the Persian Gulf has been known for its thermotolerant corals, which exhibit the highest thermal tolerance limits known globally [20] and has been frequently considered one of the coral reef refugia against global warming by the end of the century [21,22]. Despite that, coral reefs in the Persian Gulf have frequently encountered massive bleaching and mortality events, especially in the southern part [15,23,24], where temperatures exceeding 35°C for a few weeks initiate bleaching [25,26]. These high temperatures occur due to the shallow depth (mean < 30 m) of the Persian Gulf, restricted exchange with the Indian Ocean, and the hyper-arid nature of its surrounding environment. Some coral reefs of the southern Persian Gulf have experienced three consecutive coral bleaching and mortality events during 2010–2012 [27]. Severe bleaching has led to mass mortality of the reef-building corals of the Persian Gulf [14,28,29]. In particular, reports from the southern Persian Gulf show post-bleaching mortality events with different intensities in the late 1970s, 1996, 1998, 2002, 2010, 2011, 2012, and 2017 [15,24,27,30,31].
In the northern Persian Gulf, where bleaching threshold temperatures are at least 1.5℃ to 2.5℃ lower than the southern part, bleaching and mortality events were reported in 2012 [29], 2007, and 2017, with severe long-term consequence [14,16,18]. Here, we report four back-to-back bleaching and mortality events at four sites on two Iranian islands, Hormuz and Larak Islands, during the period 2014–2017. This is to our knowledge the first time in the history of modern coral reefs that some reefs have experienced four consecutive bleaching events.
Materials & methods
Study sites
Two major reef sites of Hormuz Island, located in the south (known as the Red Soil, H-RS; 27°01’N, 56°27’E) and in the east (H-E; 27°03’N, 56°30’E) and two sites on Larak Island, at the north of the island (L-N; 26°88’N, 56°35’E) and at the southwest (L-SW; 26°49’N, 56°18’E) were chosen. The 2012 mass bleaching was previously studied and recorded at both the Hormuz Island and L-SW site of Larak Island [29]. The composition of coral taxa was different among sites and faced various stressors in the past [28,29,32,33]. Turbidity is a natural characteristic of all sites, particularly at the sites of Hormuz Island. The most turbid site is H-RS, where horizontal visibility declines to < 2 meters. In addition, water currents at the H-E site are very strong so that it even made it difficult for us to conduct this research. The depth of corals at all sites was less than 10 m.
Survey method
At each site in each year, eight belt transects of 20m*1 m were randomly selected and photographed/recorded. The coral colonies inside each transect were identified to the genus level. Each colony was categorized based on its bleaching status as unbleached or Healthy (H) if there was no sign of bleaching, moderately bleached (M) if it was bleached < 50%, or severely bleached (S) if it was bleached for > 50%. Each coral colony was identified based on its mortality status as one of the following categories: 1) 0%–20%; 2) 21%–40%; 3) 41%–60%; 4) 61%–80%, and 5) 81%–100%. Mean abundance of coral colonies at each site was calculated based on the average number of coral colonies per transect (n= 8).
Statistical analysis
All statistical analysis were performed in RStudio software (RStudio, Boston, United States). Bleaching rate, mortality rate, and abundance of corals at the study sites in 2014–2017 were assessed using a three-way ANOVA. For all the aforementioned parameters, data were averaged per site (n = 8 transects). The normality of the residuals was verified with a Shapiro–Wilk’s test and the homogeneity of variances was tested using Levene’s test. A Tukey-adjusted pairwise comparison between years or between bleaching/mortality categories at each year at each site was applied as a post hoc test when the ANOVA analysis showed a significant effect.
Results
There were significant differences among the three bleaching categories in each year and for each bleaching category among years for each site (Table 1). There were four distinguishable bleaching patterns observed at the sites (Figure 1, Table 2). At site H-E, about 69% of the coral colonies were unbleached in 2014 while in 2017 the percent of unbleached coral colonies dropped to < 20%. In 2015, the percent of moderately bleached corals rose from < 20% in 2014 to about 78% and remained almost constant in 2016 and 2017. However, the percent of severely bleached colonies, which was < 15% in the first there years, significantly increased in 2017. At site H-RS, in all four years, the percent of severely bleached colonies was significantly higher than the other two categories and the percent of moderately bleached coral colonies was significantly higher than that of healthy (unbleached) corals in 2015 and 2017, and numerically higher in 2014 and 2016. None of the bleaching categories showed a difference between years. At site L-N, the percent of unbleached coral colonies was about 5% in 2014 and reached 0 in the following years. In 2014, there was no significant difference between the percent of moderately and severely bleached corals (Table 1). However, in the following two years, moderately bleached corals reached a significantly higher proportion and in 2017 the percent of severely bleached corals increased to about 78%, which was significantly higher than the percent of moderately bleached corals. The bleaching pattern at L-SW was similar to that of L-N with one major difference: the differences between the proportion of moderately and severely bleached corals in 2014, 2015, and 2016 were non-significant.
Bleaching intensity for the majority of coral genera under consecutive bleaching events significantly increased in 2017 compared to previous years so that coral genera that were mainly moderately bleached in the first three years were severely bleached in 2017 (Figure S1). Dipsastraea at the site H-RS, whose whole population was severely bleached in the previous three years, showed a significant reduction in the percent of severely bleached corals without any increase in the percent unbleached corals (Figure S1).
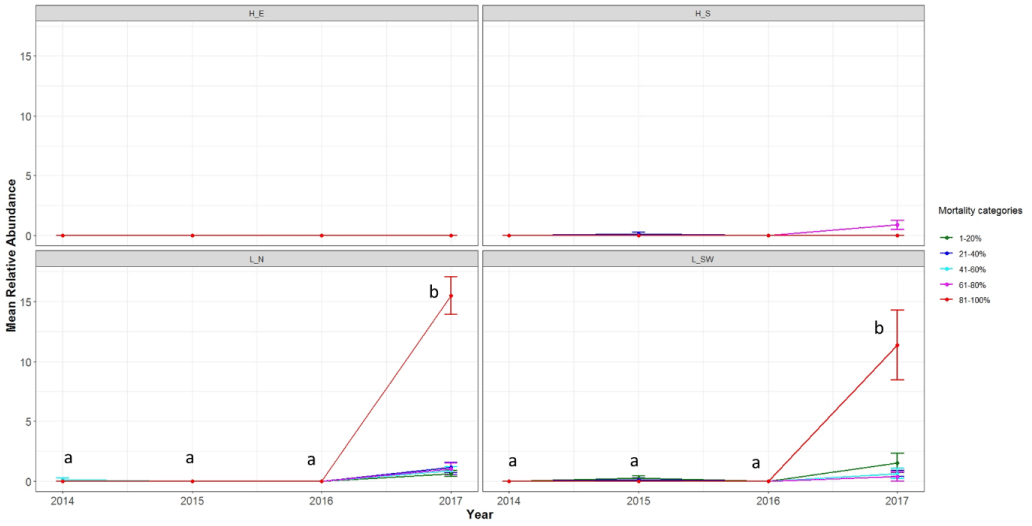
There were significant differences among the mortality categories in each year and for each bleaching category among years for each site (Table 3). Mortality remained low at H-E and H-RS with no significant difference between mortality categories in each year or for each mortality category between years (Figure 2, Table 4). Similarly, mortality rates at L-N and L-SW were low with no significant difference except in 2017, when abundance of colonies with 81–100% mortality was significantly higher than the other categories (Table 4).
The coral genera mainly showed limited mortality and many of them showed signs of mortality in just one year (Figure S2). For example, almost all Acropora colonies at the site L-N that were recorded with minimal mortality in the first three years showed 81%-100% mortality in 2017. Acropora at the site L-SW and Porites at the site L-N showed significant increases in percent coral colonies that were severely bleached from 2014 toward 2017 (Figure S2).
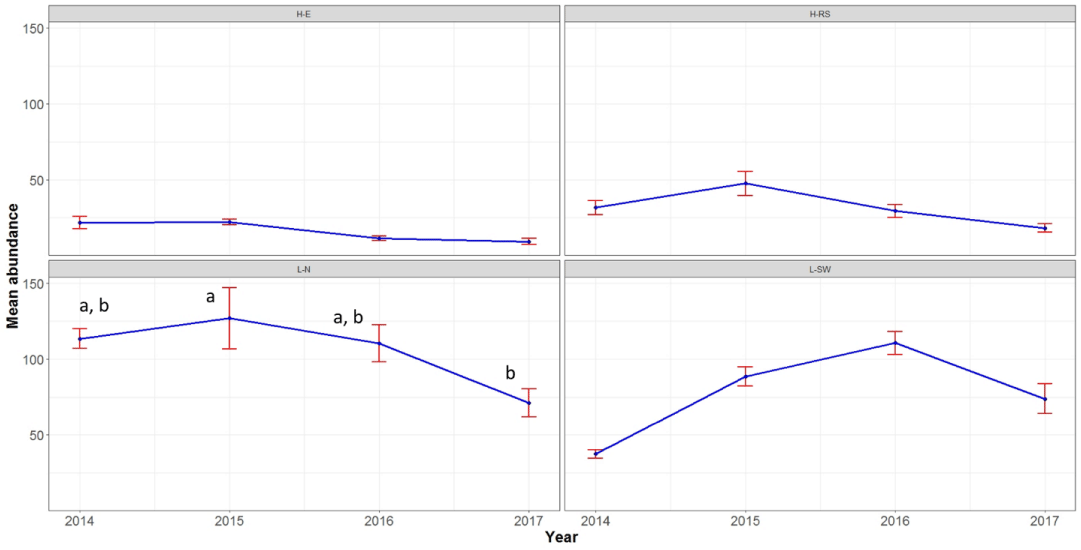
The abundance of coral colonies at none of the sites showed any significant difference between years, except for L-N, where the abundance of corals significantly declined in 2017 compared to 2015 (Figure 3). The majority of coral genera did not show significant changes. Those genera were mainly limited to < 5 colonies per transect per year at all sites (Figure S3). Favites from the site H-RS that comprised 15 colonies per transect in 2014 declined to almost 0 in 2017 (only 3 colonies in 8 transects; Figure S3). Porites at the site L-N declined 66% in 2017 compared to 2014. Acropora at the site L-N and Porites at the site L-SW showed significant increases in 2015 and 2016 compared to the previous years, respectively; however, they both declined by approximately 50% in the following year (significantly for Acropora and non-significantly for Porites; Figure S3). The abundance of Dipsastraea at the site L-SW showed significant increases in 2015 and 2016 compared to previous years, but a non-significant decline in 2017 (Figure S3).
Discussion
To the best of our knowledge, this is the first time in the history of modern coral reefs that four consecutive bleaching events have been reported. It must be taken as a serious warning message for all the world’s coral reefs because first, the Persian Gulf has been considered a coral reef refugium, where corals could survive climate change by 2100 [21,22]. Secondly, these corals are known to encompass some of the most thermotolerant reef-building corals [19,34] and associated endosymbiotic algae [35-38] and face the highest SST records in the world [14,39]. Third, seawater turbidity, which may reduce the severity of bleaching [40-45] is an intrinsic characteristic of the Persian Gulf including our study sites, particularly on Hormuz Island [28,29]. Fourth, strong water currents that were suggested to ameliorate the negative effects of thermal stress on corals [45-47], were present at the site H-E. Conversely, our data question such predictions and show that neither the thermotolerance of the corals and their symbionts nor the natural turbidity and strong water currents could protect coral reefs of the Persian Gulf. It was suggested that the Persian Gulf will be the last place where coral reefs will face annual severe bleaching [21]. However, our study warns that the coral reefs of the Persian Gulf may be among the first reefs in the world to disappear. Mass coral bleaching events have been frequently reported from the Persian Gulf in the past three decades [15,24,27,30,31]. Three back-to-back bleaching events have happened before this in the southern Persian Gulf in 2010, 2011, and 2012 [27]. However, it seems that the northern Persian Gulf is becoming a major bleaching hotspot.
The four consecutive bleaching events in the northern Persian Gulf happened just one year after the 2012 massive bleaching that resulted in the bleaching of 84% of the corals [29]. For example, 100% of the corals at H-E were to some degree bleached in 2012. On the other hand, in 2014, 69% of the corals at H-E did not show any sign of bleaching unlike the other three sites where at best <15% of their coral communities were unbleached. This suggests that reef-building corals at H-E (and likely other sites) managed to recover fast from the 2012 bleaching. Fast recovery may be a characteristic of the Persian Gulf corals, particularly at Hormuz Island because despite four consecutive bleaching events, there was no increase in coral mortality intensity and decline in coral abundances at H-E and H-RS. Another reason may be that the intensities of the bleaching events were not much higher than the maximum monthly mean temperatures of the sites for a long enough time to kill the corals. This needs to be further studied. However, the back-to-back bleaching events from 2014 to 2017 left almost no unbleached coral colony at none of the study sites (except a few massive Porites colonies at H-E) by the end of the 2017 bleaching. In fact, at all sites, except H-E, 73%-86% of the coral colonies were severely bleached. Even the percent of severely bleached corals significantly increased at H-E in 2017. The corals at the L-N and L-SW sites, which did not show mortality in the first three years, showed significant increases in percent of coral colonies with 81–100% mortality in 2017. This confirms previous studies that suggested consecutive bleaching events can lead to coral reef degradation because they do not give the bleached corals enough time to recover and produce larvae in order to return the reefs to their original, pre-bleached state [27,48]. Bleaching events may have long-term dramatic impacts on coral reef ecosystems and services that can be observed years later [14,49-51].
The bleaching pattern observed at H-E was different from the other study sites. The corals at H-E survived the bleaching in 2014 so that only 31% of the corals were bleached and by the end of the 2017, only 34% of the corals were severely bleached (against 73%-86% at other sites). One reason could be the fact that the dominant coral genus at H-E is the massive Porites, which accounted for > 85% of the coral cover [28]. In other sites, massive Porites was not the dominant genus. Massive Porites species are among the most tolerant reef-building coral species against elevated temperatures [52,53]. Some studies suggested that bleaching events may increase the thermotolerance capacity of the corals via acclimation/adaptation [54-56]. However, that seems to be very unlikely here (Figure 1) perhaps due to the limited capacity of tolerant corals to extreme heat stress [57]. In 2012, an elevated temperature-related White Mat Disease infected 96% all the Porites colonies and killed 58% of all Porites tissues at H-E [28]. By 2017, bleaching severity had increased; the most likely reason behind less severe bleaching at H-E compared to other sites is that the majority of the coral colonies that were vulnerable to global warming-induced bleaching died and the most tolerant colonies survived. Such huge intercolonial differences in given coral populations facing thermal stress are abundant [58,59].
No coral genus was a winner against bleaching. However, Dipsastraea at the site H-RS showed significant decline in percent severely bleached colonies by 2017. This did not lead to an increase of unbleached corals, but as there was no significant reduction in the population of Dipsastraea, it may be a sign of acclimation/adaptation induced by previous bleaching events. However, changes in coral populations can happen years later after bleaching. Interestingly, Dipsastraea at the site L-SW showed significant increases in its population in 2015 and 2016 compared to previous years. In both years, about 75% of Dipsastraea colonies were moderately bleached. Increases in abundance despite being bleached may suggest that the bleached colonies managed to reproduce larvae as did other genera around the world [60-62]. However, > 90% of fully bleached colonies showed a non-significant 27% reduction in the abundance of Dipsastraea in 2017, which may suggest limitations in the physiological capabilities of strong coral taxa under repeated bleaching events [57,62-64]; this was observed for the same species at the site L-N with 65% of colonies showing 81–100% mortality in 2017, whereas they were mortality-free in previous years. Similarly, significant increases in the abundance of Acropora at the site L-N and Porites at the site L-SW in 2015 and 2016 compared to the previous years, respectively, suggest either tolerance to thermal stress and successful reproduction, or fragmentation. However, their following decline shows their limitations as well. Such limitation under consecutive bleaching led to a 66% decline in Porites population at the site L-N and almost 100% decline of Favites at the site H-RS. Experimental studies showed that under two consecutive bleaching events, some species may manage to recover and survive while others may not [57,65]. Even short-term winners may become losers over time [52]. Therefore, although some tolerant colonies may survive even four back-to-back bleaching events, by increasing the number of severe bleaching events all coral taxa may be losers.
Conclusion
In conclusion, our study shows that even the most thermotolerant coral reefs of the world can face back-to-back bleaching events that lead to increased coral mortality and reduction in coral abundance at some sites. The significant increases in the frequency of Acropora coral colonies with 81%-100% mortality at the sites of Larak Island in 2017 despite being constantly minimal in the first three years highlights the fact that we cannot rely on the physiological acclimation/adaptation of corals or natural phenomena such seawater turbidity to save coral reefs. The repeated bleaching events did not seem to lead to increased tolerance of the corals of the study sites, except for Dipsastraea at the southwest of Larak Island that managed to significantly increase its population in 2015 and 2016. However, the non-significant 27% reduction in its abundance in 2017, when almost all colonies were severely bleached suggest that these results must be interpreted with caution. In particular that, for Dipsastraea at the north of Larak Island that managed to survive in the first three years, > 66% of its colonies showed 81%–100% mortality in 2017. Unfortunately, there is no effective management strategy to protect global coral reefs from climate change-induced thermal stress [7]. Therefore, as previously suggested, it seems the only way to prevent reef-building corals from reaching extinction is to substantially reduce greenhouse gas emissions, including carbon dioxide.
Author contributions
JK designed the experiment, collected the data, wrote the paper, PTK collected the data, SHK collected the data, and FG analyzed the data.
- Januchowski-Hartley FA, Bauman AG, Morgan KM, Seah JC, Huang D, et al. (2020) Accreting coral reefs in a highly urbanized environment. Coral Reefs 39: 717-731 . Link: https://bit.ly/3xUMcdX
- Heery EC, Hoeksema BW, Browne NK, Reimer JD, Ang PO, et al. (2018) Urban coral reefs: Degradation and resilience of hard coral assemblages in coastal cities of East and Southeast Asia. Mar Pollut Bull 135: 654-681 . Link: https://bit.ly/3smiY6X
- Cheal AJ, MacNeil MA, Emslie MJ, Sweatman H (2017) The threat to coral reefs from more intense cyclones under climate change. Global Change Biology 23: 1511-1524 . Link: https://bit.ly/3g8YqtI
- Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute . Link: https://bit.ly/3yQrRYx
- Frieler K, Meinshausen M, Golly A, Mengel M, Lebek K, et al. (2013) Limiting global warming to 2℃ is unlikely to save most coral reefs. Nature Climate Change 3: 165-170 . Link: https://bit.ly/3xPSLyD
- Eakin CM, Sweatman HP, Brainard RE (2019) The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38: 539-545 . Link: https://bit.ly/3m4f7Kn
- Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, et al. (2017) Global warming and recurrent mass bleaching of corals. Nature 543: 373-377. Link: https://go.nature.com/2VZ5l1n
- Hughes TP, Kerry JT, Baird AH, Connolly SR, Chase TJ, et al. (2019) Global warming impairs stock–recruitment dynamics of corals. Nature 568: 387-390. Link: https://go.nature.com/3z3d1hq
- Bahr KD, Jokiel PL, Rodgers KS (2015) The 2014 coral bleaching and freshwater flood events in Kāneʻohe Bay, Hawaiʻi. PeerJ 3: e1136. Link: https://bit.ly/3g69k3j
- Monroe AA, Ziegler M, Roik A, Röthig T, Hardenstine RS, et al. (2018) In situ observations of coral bleaching in the central Saudi Arabian Red Sea during the 2015/2016 global coral bleaching event. PLoS One 13: e0195814. Link: https://bit.ly/3iMj5Wa
- Raymundo LJ, Burdick D, Hoot WC, Miller RM, Brown V, et al. (2019) Successive bleaching events cause mass coral mortality in Guam, Micronesia. Coral Reefs 38: 677-700 . Link: https://bit.ly/3jVgR6d
- Smith KM, Payton TG, Sims RJ, Stroud CS, Jeanes RC, et al. (2019) Impacts of consecutive bleaching events and local algal abundance on transplanted coral colonies in the Florida Keys. Coral Reefs 38: 851-861 . Link: https://bit.ly/3xPKxXa
- Head CE, Bayley DT, Rowlands G, Roche RC, Tickler DM, et al. (2019) Coral bleaching impacts from back-to-back 2015–2016 thermal anomalies in the remote central Indian Ocean. Coral Reefs 38: 605-618. Link: https://bit.ly/37NiqNW
- Bargahi HR, Shokri MR, Kaymaram F, Fatemi MR (2020) Changes in reef fish assemblages following multiple bleaching events in the world’s warmest sea (Kish Island, the Persian Gulf). Coral Reefs 39: 603-624. Link: https://bit.ly/3g9maOb
- Burt JA, Paparella F, Al-Mansoori N, Al-Mansoori A, Al-Jailani H (2019) Causes and consequences of the 2017 coral bleaching event in the southern Persian/Arabian Gulf. Coral Reefs 38: 567-589 . Link: https://bit.ly/3xPy45X
- Oladi M, Rouzbehani S, Ahmadzadeh F, Ghazilou A (2021) Dynamics of Dipsastraea pallida-symbiont association following bleaching events across the northern Persian Gulf and Gulf of Oman. Symbiosis 84: 141-149. Link: https://bit.ly/37Sv8L3
- Paparella F, Xu C, Vaughan GO, Burt JA (2019) Coral bleaching in the Persian/Arabian Gulf is modulated by summer winds. Frontiers in Marine Science 6: 205 . Link: https://bit.ly/2VXKkUu
- Ranjbar MS, Jahromi MS, Javid P (2018) The status of coral reefs in the Larak Island, Persian Gulf, from 2012 to 2018. International Journal of Veterinary and Animal Research (IJVAR) 1: 49-53 . Link: https://bit.ly/2VZh5k9
- Riegl BM, Purkis SJ, Al-Cibahy AS, Abdel-Moati MA, Hoegh-Guldberg O (2011) Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS One 6: e24802. Link: https://bit.ly/3CTp4Aw
- Riegl BM, Purkis SJ, Al-Cibahy AS, Al-Harthi S, Grandcourt E, et al. (2012) Coral bleaching and mortality thresholds in the SE Gulf: highest in the world. Coral Reefs of the Gulf: Adaptation to Climatic Extremes, eds B. M. Riegl and S. J. Purkis (Berlin: Springer), 95–105.
- Van Hooidonk R, Maynard JA, Planes S (2013) Temporary refugia for coral reefs in a warming world. Nature Climate Change 3: 508-511. Link: https://go.nature.com/3sjnqDc
- Cacciapaglia C, van Woesik R (2016) Climate‐change refugia: Shading reef corals by turbidity. Global Change Biology 22: 1145-1154 . Link: https://bit.ly/2XxkYhe
- Coles SL, Riegl BM (2013) Thermal tolerances of reef corals in the Gulf: A review of the potential for increasing coral survival and adaptation to climate change through assisted translocation. Marine Pollution Bulletin 72: 323-332. Link: https://bit.ly/3xQi8QI
- Riegl B, Johnston M, Purkis S, Howells E, Burt J, et al. (2018) Population collapse dynamics in Acropora downingi, an Arabian/Persian Gulf ecosystem-engineering coral, linked to rising temperature. Glob Chang Biol 24: 2447–2462. Link: https://bit.ly/2XvxIoC
- Sheppard C, Price A, Roberts C (1992) Marine Ecology of the Arabian Region: Patterns and Processes in Extreme Tropical Environments. Toronto: Academic Press. Link: https://bit.ly/3ySCtpR
- Coles S (2003) Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: a comparison to the Indo-Pacific region. Atoll Res Bull 507: 1–19. Link: https://s.si.edu/3AKdZjd
- Riegl B, Purkis S (2015) Coral population dynamics across consecutive mass mortality events. Global Change Biology 21 : 3995–4005. Link: https://bit.ly/3iNEOgb
- Kavousi J, Tavakoli-Kolour P, Barkhordari A (2013) Mass mortality of Porites corals on northern Persian Gulf reefs due to sediment-microbial interactions. International Journal of Marine Science 3: 306-310 .
- Kavousi J, Tavakoli-Kolour P, Mohammadizadeh M, Bahrami A, Barkhordari A (2014) Mass coral bleaching in the northern Persian Gulf, 2012. Scientia Marina 78: 397-404 .
- Sheppard C, Loughland R (2002) Coral mortality and recovery in response to increasing temperature in the southern Arabian Gulf. Aquatic Ecosystem Health & Management 5: 395–402. Link: https://bit.ly/3iQRuTE
- Shuail D, Wiedenmann J, D’angelo C, Baird AH, Pratchett MS, et al. (2016) Local bleaching thresholds established by remote sensing techniques vary among reefs with deviating bleaching patterns during the 2012 event in the Arabian/Persian Gulf. Mar Pollut Bull 105: 654–659. Link: https://bit.ly/3AKe7zd
- Rezai H, Kamrani E, Samimi-Namin K, et al. (2010) Coral degradation, distribution and abundance around Larak, Hengam and Kish Islands, Persian Gulf. Iranian National Centre for Oceanography, Tehran.
- Mohammadizadeh M, Tavakoli-kolour P, Rezai H (2013) Coral reefs and community around Larak Island (Persian Gulf). Caspian Journal of Applied Sciences Research 2: 52-60 . Link: https://bit.ly/3iPp907
- Sheppard C (2003) Predicted recurrences of mass coral mortality in the Indian Ocean. Nature 425: 294-297 . Link: https://go.nature.com/2VXKETc
- Mostafavi PG, Fatemi SMR, Shahhosseiny MH, Hoegh-Guldberg O, Loh WKW (2007) Predominance of clade D Symbiodinium in shallow-water reef-building corals off Kish and Larak Islands (Persian Gulf, Iran). Marine Biology 153: 25-34 . Link: https://bit.ly/3xTtJ1s
- Varasteh T, Shokri MR, Rajabi-Maham H, Behzadi S, Hume BC (2018) Symbiodinium thermophilum symbionts in Porites harrisoni and Cyphastrea microphthalma in the northern Persian Gulf, Iran. Journal of the Marine Biological Association of the United Kingdom 98: 2067-2073 . Link: https://bit.ly/3m3zozS
- Rahmani S, Ghavam Mostafavi P, Shahhosseiny MH, Vosoughi G, Faraji A (2011) Genetic Identification of Symbiodinium in Genus Acropora off Farur Island, Persian Gulf. International Journal of Marine Science and Engineering 1: 43-50 . Link: https://bit.ly/3m7ut0I
- Hume BC, D'Angelo C, Smith EG, Stevens JR, Burt J, et al. (2015) Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world's hottest sea, the Persian/Arabian Gulf. Scientific Reports 5: 8562 . Link: https://go.nature.com/2VQs1kN
- Brandl SJ, Johansen JL, Casey JM, Tornabene L, Morais RA, et al. (2020) Extreme environmental conditions reduce coral reef fish biodiversity and productivity. Nature Communications 11 : 3832 Link: https://go.nature.com/2UkzgjY
- Goreau T, McClanahan T, Hayes R, Strong A (2000) Conservation of coral reefs after the 1998 global bleaching event. Conservation Biology 1: 5-15 . Link: https://bit.ly/3yXpvHK
- Golbuu Y, Victor S, Penland L, Idip D, Emaurois C, et al. (2007) Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs 26: 319-332 . 3
- Takahashi S, Nakamura T, Sakamizu M, van Woesik R, Yamasaki H (2004) Repair Machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol 45: 251-255 . Link: https://bit.ly/3CRTsuX
- Sully S, Van Woesik R (2020) Turbid reefs moderate coral bleaching under climate‐related temperature stress. Glob Chang Biol 26: 1367-1373 . Link: https://bit.ly/37IKC4x
- Wagner DE, Kramer P, van Woesik R (2010) Species composition, habitat, and water quality influence coral bleaching in south-eastern Florida. Marine Ecology Progress Series 408: 65-78 . Link: https://bit.ly/3xPTIXJ
- Bayraktarov E, Pizarro V, Eidens C, Wilke T, Wild C (2013) Bleaching susceptibility and recovery of Colombian Caribbean corals in response to water current exposure and seasonal upwelling. PloS One 8: e80536 . Link: https://bit.ly/2VXbLhL
- Nakamura T, Van Woesik R (2001) Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Marine Ecology Progress Series 212: 301-304 . Link: https://bit.ly/3xQUMuz
- Nakamura T, Yamasaki H (2005) Requirement of water-flow for sustainable growth of Pocilloporid corals during high temperature periods. Mar Pollut Bull 50: 1115-1120 . Link: https://bit.ly/3siNBdg
- Hughes TP, Tanner JE (2000) Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81: 2250-2261 . Link: https://bit.ly/3CUhMfC
- Burt J, Al-Harthi S, Al-Cibahy A (2011) Long-term impacts of coral bleaching events on the world’s warmest reefs. Mar Environ Res 72: 225-229. Link: https://bit.ly/3z3dQa0
- Garpe KC, Yahya SA, Lindahl U, Öhman MC (2006) Long-term effects of the 1998 coral bleaching event on reef fish assemblages. Marine Ecology Progress Series 315: 237-247. Link: https://bit.ly/3xQ269E
- McClanahan TR, Ateweberhan M, Omukoto J (2008) Long-term changes in coral colony size distributions on Kenyan reefs under different management regimes and across the 1998 bleaching event. Marine Biology 153: 755-768. Link: https://bit.ly/3k4iSx4
- van Woesik R, Sakai K, Ganase A, Loya YJMEPS (2011) Revisiting the winners and the losers a decade after coral bleaching. Marine Ecology Progress Series 434: 67-76 . Link: https://bit.ly/3snrqm5
- Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, et al. (2001) Coral bleaching: the winners and the losers. Ecology letters 4: 122-131 Link: . https://bit.ly/3COlSWJ
- Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, et al. (2016) Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352: 338-342 . Link: https://bit.ly/3AM4BM1
- Brown BE, Dunne RP, Goodson MS, Douglas AE (2000) Bleaching patterns in reef corals. Nature 404: 142-143 . Link: https://bit.ly/3m60eaw
- Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, et al. (2012) Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One 7: e33353 . Link: https://bit.ly/3CS5NiD
- Schoepf V, Grottoli AG, Levas SJ, Aschaffenburg MD, Baumann JH, et al. (2015) Annual coral bleaching and the long-term recovery capacity of coral. Proceedings of the Royal Society B: Biological Sciences 282: 20151887 . Link: https://bit.ly/3xRgzCi
- Kavousi J, Denis V, Sharp V, Reimer JD, Nakamura T, et al. (2020) Unique combinations of coral host and algal symbiont genotypes reflect intraspecific variation in heat stress responses among colonies of the reef-building coral, Montipora digitata. Marine Biology 167: 23 . Link: https://bit.ly/2VX6wya
- Kavousi J, Reimer JD, Tanaka Y, Nakamura T (2015) Colony-specific investigations reveal highly variable responses among individual corals to ocean acidification and warming. Mar Environ Res 109: 9-20 . Link: https://bit.ly/3iNUVdK
- Cox EF (2007) Continuation of sexual reproduction in Montipora capitata following bleaching. Coral Reefs 26: 721-724 . Link: https://bit.ly/3skyeRy
- Godoy L, Mies M, Zilberberg C, Pastrana Y, Amaral A, et al. (2021) Southwestern Atlantic reef-building corals Mussismilia spp. are able to spawn while fully bleached. Marine Biology 168: 15. Link: https://bit.ly/3m4xHCj
- Ward S, Harrison P, Hoegh-Guldberg O (2002) Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. In Proceedings of the Ninth International Coral Reef Symposium Bali 2: 1123-1128. Link: https://bit.ly/3jXdg7y
- Howells EJ, Berkelmans R, van Oppen MJ, Willis BL, Bay LK (2013) Historical thermal regimes define limits to coral acclimatization. Ecology 94: 1078-1088 . Link: https://bit.ly/3AI0gtl
- Coles SL, Brown BE (2003) Coral bleaching—capacity for acclimatization and adaptation . Adv Mar Biol 46: 183-223. Link: https://bit.ly/3m8l7BN
- Grottoli AG, Warner M, Levas SJ, Aschaffenburg M, Schoepf V, McGinley M, et al. (2014) The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Global Change Biology 20: 3823– 3833 . Link: https://bit.ly/3xST9MN

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
