Annals of Marine Science
Feeding Ecology with Prey Electivity and Growth Performance of Indigenous Asian Striped Dwarf Catfish, Mystus Vittatus (Bloch, 1794) in Low Saline Earthen Ponds of Indian Sundarbans
Asish Mondal*, Deepta Chakravartty and Sufia Zaman
Cite this as
Mondal A, Chakravartty D, Zaman S (2017) Feeding Ecology with Prey Electivity and Growth Performance of Indigenous Asian Striped Dwarf Catfish, Mystus Vittatus (Bloch, 1794) in Low Saline Earthen Ponds of Indian Sundarbans. Ann Mar Sci 1(1): 032-038. DOI: 10.17352/ams.000006Feeding ecology with prey preferences and fish growth of Mystus Vittatus (Bloch, 1794) reared in low saline earthen polyculture ponds in Sundarbans were studied for 11th months during July, 2015 to June, 2016. Locally available wild collected mixed bagrid juveniles (4.3±0.14 cm, 1.20±0.99 g) including M. vittatus were stocked @ 1000 juveniles ha-1. No commercial feed or fertilizer was applied following common practice. According to the order of dominance the existing food items in water recorded were Chlorophyceae, Myxophyceae, Bacillariophyceae, Insect parts and larvae, Fish parts & larvae, Rotifers and Cladocera, Copepods and Crustacean larvae. Feeding intensity in terms of stomach fullness upgraded with increasing body weight of fish. The major food constituents in stomach were Copepods followed by Bacillariophyceae, Fish parts and larvae, Insect parts and larvae, unidentified organic matter, Chlorophyceae, Myxophyceae, Rotifers & Cladocera and Crustacean larvae. Prey electivity analysis indicated significantly active selection of Copepods (+0.53±0.05) and Bacillariophyceae (+0.31±0.06). Fish parts and larvae (+0.17±0.11), Insect parts (+0.06±0.07) and larvae and Crustacean larvae (0.02±0.17) were positively selected but not significant. M. vittatus is an omnivorous fish and almost prefer zooplanktons. M. vittatus was attained average body weight 26.50±1.20g (13.6±0.19cm) with mean specific growth rate of 0.96±0.19 % day-1at harvest. Bagrid catfish performed isometric growth (W=0.012TL2.98) and good condition factor (1.14±0.046) indicating sufficient of natural food materials in water. Health status of M. vittatus in low saline polyculture ponds is good.
Background
Asian striped dwarf catfish, Mystus Vittatus (Bloch, 1794), locally known as “Bujuri Tangra or Mitha Tangra”, is an economic important target small bagrid for small-scale fishermanoccurring in the eastern and northeastern regions of India. Due to its high nutritive value and great consumer preference, it has good market demand [1-3]. This bagrid is very common in ponds and estuary all over India as well as widely distributed throughout the Indian subcontinent including Pakistan, Nepal, Malaysia, Sri Lankaand Bangladesh [4,5]. Recently it has also got its entry in ornamental fish markets of India [6] and has been reported to have moderate export price too [7].
Food is one of the important factors promoting growth and enriching the biochemical composition of fishes and helps to find the distribution of a fish population which is highly essential for successful management of any fishery [8]. Understanding of food and feeding habits of the different species is necessary for the proper management of their culture. The success of good scientific planning and management of various fish species largely depends on the knowledge of their biological aspects in which food and feeding habits include a valuable portion [9]. Study of food and feeding habit of fishes have manifold importance in fishery biology and in fisheries management programme [10]. The direct observation of fish feeding behavior or the stomach content analysis of fish can serve as good tool for fish feeding analysis [11]. Quantitative and qualitative changes in fish food during the life span are useful tools to define the diet of a particular fish species [12]. Fish feeding is selective, but it can vary according to availability of food in environment, which means that fish feeding habits are extremely adaptable where fish can use food item readily available in the environment [13]. The study of food selectivity determines the most frequently consumed prey and relative importance of different food types to fish nutrition and quantify the consumption rate of the individual prey types [14].
Previous reports from India [15,16] and Bangladesh [17] reported food and feeding habit of M. vittatus occurring in freshwater and coastal water. There is no information on growth performances and feeding ecology of the species from low saline ponds of Sundarbans in spite of being a highly potential and ecologically important area where M. vittatus is abundantly available and forms a commercially important species. This study aimed to assess feeding ecology along with prey selectivity and growth of M. vittatus in low saline earthen ponds of Indian Sundarbans.
Methodology
The study was conducted at Madhabnagar village (lat. 21.787003–21.7917850N, long. 88.353365–88.3569380E) of Patharpratima block in South 24 Parganas district of West Bengal, India during July, 2015 to June, 2016 in three low saline earthen ponds covering area of 350-450 m2 each. This area exits within the Hooghly-Matla estuarine complex popularly known as ‘Sundarbans’. One house hold farmers allowed to authors to collect water and fish samples. All fishes of previous year were harvested by the drag net. Ponds were prepared with lime @ 200 kg ha-1 and farm yard manure @ 500 kg ha-1 as per traditional practice. With the onset of monsoon during June end, ponds became filled with rain water up to depth of 130 cm within two weeks.
Locally available wild collected mixed bagrid catfish juveniles (4.3±0.14 cm, 1.20±0.99 g) including M. vittatus were stocked @ 1000 juveniles ha-1. Commercial fish feed and fertilizer not applied; farm yard manure @ 150 kg ha-1 was applied monthly. Both fish and water samples were collected monthly during morning in between 08.00 A.M to 09.00 A.M hours and carried to laboratory in ice boxes for subsequent analysis.
Water quality parameters were measured following standard methods [18]. Water temperature and pH were determined using a digital multi-meter (model deluxe 191E). Salinity was recorded using a refractometer (ATAGO, Japan). Dissolved oxygen (DO) was analyzed using modified Winkler’s method and total alkalinity was determined through titration. Nitrogenous metabolites like nitrite-nitrogen (NO2-N) and ammonia-nitrogen (NH3-N) and nutrients like nitrate-nitrogen (NO3-N) and phosphate-phosphorus (PO4-P)concentrations were determined using a digital double-beam spectrophotometer(model UV2310; Tech comp).Plankton samples were collected monthly during midday by filtering 50 L of water through bolting silk plankton net (mesh size 64 μm). Plankton concentrates were immediately preserved in 5% buffered formalin and one ml aliquot were then placed into Sedgwick-Rafter counting cell. Plankton constituents were identified and counted [19,20]. Other suspended constituents were also estimated.
Before gravimetric measurements, the stomach of fish were removed intact by cutting above the cardiac and below the pyloric sphincters and preserved in a vial with 4% formalin. The stomach fullness degree was assessed by visual estimation and classified as gorged, full, 3/4 full, 1/2 full, 1/4 full little and empty [21]. The data have then been used to calculate the monthly fullness index (FI) by determining the percentage of feeding intensity using the following formula:
The stomach contents were transferred into fixed volume of 4% formalin solution. From every vial, one ml aliquot was placed into Sedgwick-Rafter counting cell and constituents were identified and counted [19,20].
Stomach content analysis has been performed following percentage occurrence methods [22]. The dominant food items of water and stomachs were categorized as Bacillariophyceae, Myxophyceae, Chlorophyceae, Copepods, Insect parts and larvae, Rotifers and cladocera, Crustacean larvae, Fish parts and larvae, Unidentified materials.
The percentage compositions of food types in the stomach falling under different groups were then compared with that of fish ponds to evaluate prey preferences. Prey preferences were determined by Ivlev Electivity Index using the formula [23]:
Where, r=percentage of dietary item in ingested food, p= percentageof prey in the environment.
Ten fishes from each three ponds were collected during 2nd week of each month i.e. 30 fish in a month and total 330 fish were collected and analyzed throughout the study period. Gravimetric data such as total length (TL, cm) was recorded using a slide caliper and body weight (W, g) was measured using a digital electronic balance. Daily weight gain (DWG) was calculated using the formula:
Where and are the average final and initial weight in time t.
Specific growth rate (SGR) was calculated using the following equation given by Brown [24]:
Where and are the average final and initial weight in time t.
The mathematical relationship between length and weight was calculated using the conventional formula [25]:
Where W is fish weight (g), TL is total length (cm), ‘a’ is the proportionality constant and ‘b’ is the isometric exponent. The parameters ‘a’ and ‘b’ were estimated by non-linear regression analysis.
Fulton’s condition equation was used to find out the condition factor as [26]:
Where K is the condition factor, is the average weight (g) and is the average total length (cm)
Differences in growth parameters of fish and water quality parameters among ponds were determined by analysis of variance with the General Linear Model procedure using SPSS for Windows v.17.0 programme (SPSS Inc Chicago IL USA). Duncan’s Multiple Range Test [27] was used for comparison of ponds. All data are expressed as mean ± standard error (SE).
Results
The water quality parameters of three ponds are presented in table 1. Average water quality parameters between the ponds during the rearing period were 20.5 to 31.5°C for temperature; 7.50 to 8.85 for pH; 1.50 to 5.05 ppt for salinity; 6.04 to7.39 mg L-1for dissolved oxygen; 120 to 165 for alkalinity; 12.45 to 18.45µg L-l for NO2-N; 28.21 to 38.44µg L-l for NH4-N; 89 to 104 µg L-l for NO3-N and 27.23 to 38.55 µg L-l for PO4-P. Phytoplankton density (16.22±1.94/L-1 ×103) was significantly higher in pond 2 than others. Significantly greater density (3.05±0.24/L-1 ×103) of zooplanktons was observed in pond 1 than others.
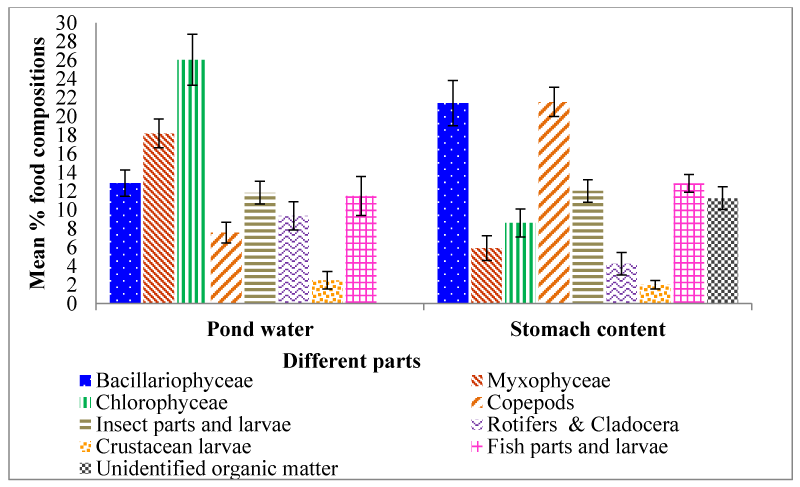
Mean percentage occurrences of planktonic and other suspended food materials in pond water and stomach are presented in figure 1. Monthly percentage occurrences of planktonic food materials in pond water are presented in figure 2. According to the order of dominance the prey groups recorded were Chlorophyceae (26.06±2.71%), Myxophyceae (18.19±1.53%), Bacillariophyceae (12.89±1.40%), Insect parts and larvae (11.84±1.22%), Fish parts and larvae (11.492.10%), Rotifers and Cladocera (9.37±1.52%), Copepods (7.59±1.11%) and Crustacean larvae (2.49±0.93%).
a. Chlorophyceae
Volvox, Enteromorpha, Cladomorpha,Caetomorpha, Ulothrix, Spirogyra, Pediastrum, Chlorella and Tetraedron were the most abundant genera.
b. Myxophyceae
Anabaena, Spirulina, Nostoc, Oscillatoria, Chroococcus, Gleocapsa and Merismopedia, were the main encountered genera.
c. Bacillariophyceae
Coscinodiscus, Gyrosigma, Navicula, Nitzschia, Cyclotella, Melosira, Basilaria, Cymbella, Synedra and Pleurosigma were important existed genera.
d. Insect parts and larvae
Orthopteran and culicid larvae and parts of various insects were present.
e. Fish parts and larvae
Different fish larvae and small fish parts were existed.
f. Rotifers and Cladocera
Brachionus of rotifers and Daphnia andMoina of Cladocera were most abundant genera.
g. Copepods
Cyclops and Calanus were the main identifiable food items.
h. Crustacean larvae
Nauplius larvae and Cypris larvae were common part.
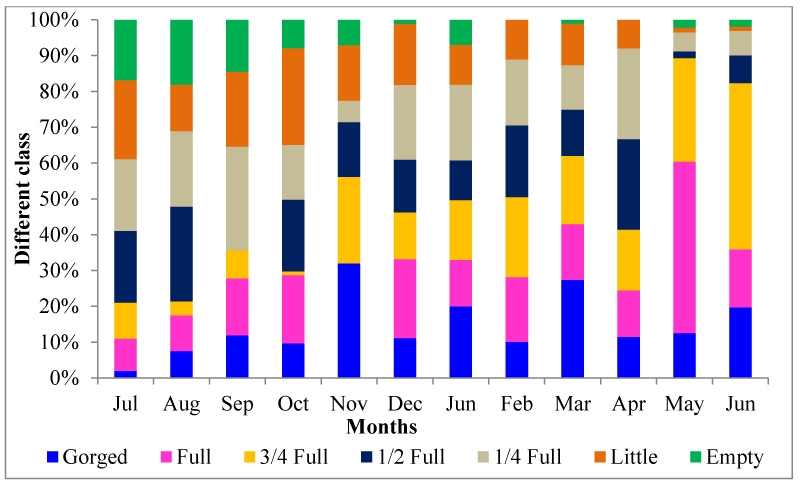
Stomach fullness has been categorized into seven classes namely: as gorged, full, 3/4 full, 1/2 full, 1/4 full little and empty results of monthly analysis of feeding intensity have been represented in figure 3. The stomach fullness indices which have been determined 14.63±3.43, 16.65±3.74, 17.56±3.74, 14.63±2.54, 16.80±2.34, 13.32±2.35 and 6.47±2.02 respectively.
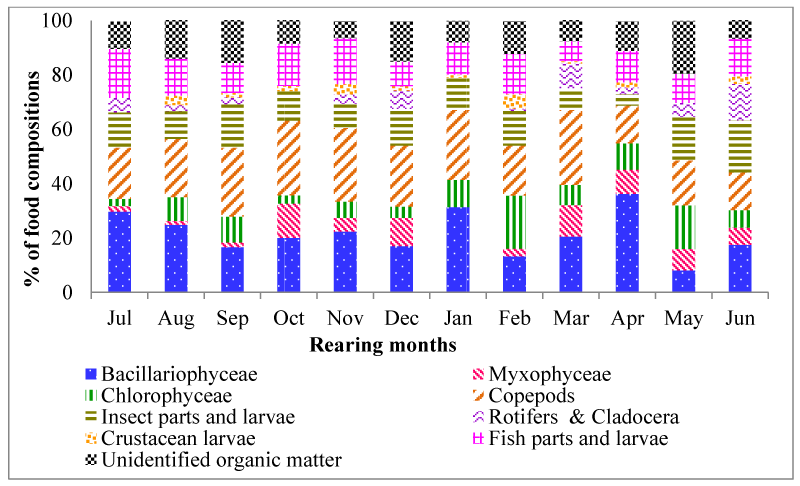
The mean percentage composition of different food in fish stomach is displayed in figure 1. Monthly percentage composition of food items in fish stomach is presented in figure 4. The major constituent of food in the stomach was Copepods (21.55±1.56) followed by Bacillariophyceae (21.43±2.41), Fish parts and larvae (12.85±0.96), Insect parts and larvae (12.03±1.01), Unidentified organic matter (11.27±1.12%), Chlorophyceae (8.61±1.51), Myxophyceae (5.92±1.31), Rotifers & Cladocera (4.26±1.20) and Crustacean larvae (2.01±0.47).
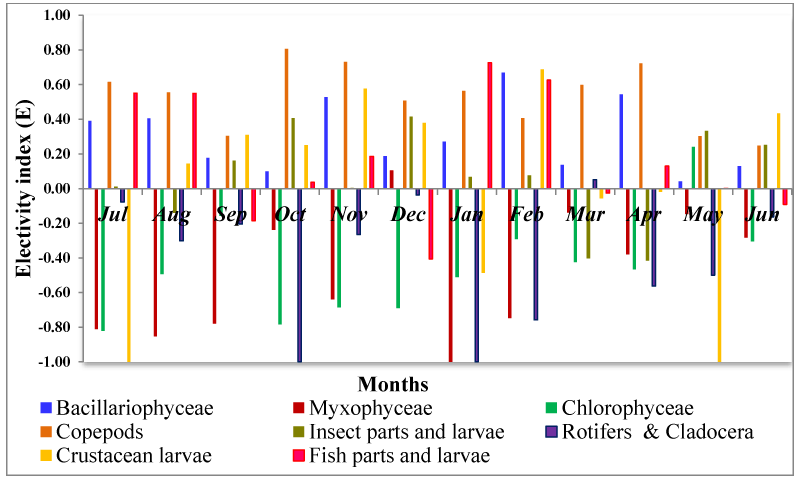
According to prey electivity index (E) of different food items is showed in figure 5. M. vittatus positively preferred Copepods (+0.53±0.05), Bacillariophyceae (+0.31±0.06), Fish parts and larvae (+0.17±0.11), Insect parts (+0.06±0.07) and larvae and Crustacean larvae (0.02±0.17). Rotifers & Cladocera (-0.40±0.11), Chlorophyceae (-0.45±0.09) and Myxophyceae (-0.49±0.11) were the negatively preferred prey items to the fish.
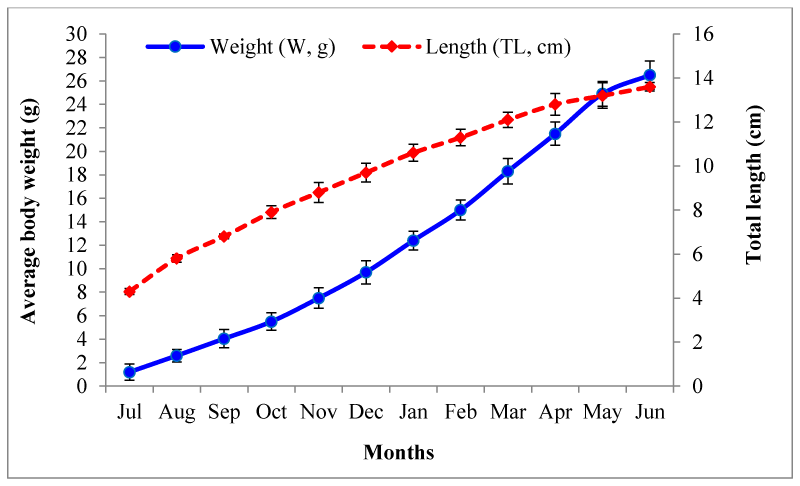
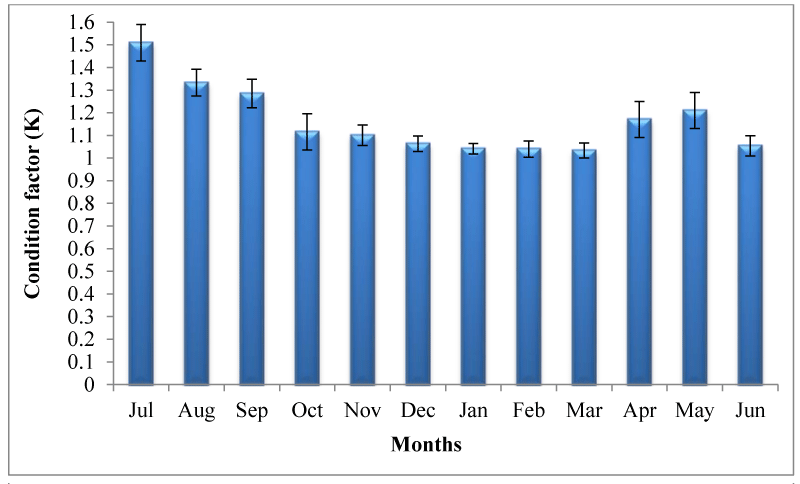
Growth performance of M. vittatus in terms of weight (g) and length (cm) is presented in figure 6. M. vittatus was attained average body weight 26.50±1.20 g (13.6±0.19cm) at harvest from 1.02±0.69g (4.3±0.14 cm) during 330 days of rearing. Mean daily weight gain recorded was 0.08±0.008 g day-1 which ranged between 0.05 (December) and 0.11g day-1 (March).Average specific growth rate calculated was 0.96±0.19 % day-1 which varied between 0.20 during final month and 2.5 % day-1 during early month of rearing. Exponential value (b) of Length-Weight Relationship (LWR) was recorded to be 2.981 indicating isometric growth (Figure 7). The Fulton’s condition factor (K) was noted 1.14±0.046 (Figure 8).
Discussion
Good water quality is very important factor to aqua-farming for optimum growth and survival of aquatic organism. Water temperature is a very important factor for fish distribution and aquatic organisms and their activity in the environment. Recorded water quality parameters in the present study were within optimum ranges in low saline polyculture pond of Indian Sundarban [28] and brackish water [29]. Salinity and temperature of Sundarban have most significant role as abiotic factors to regulate the fishery resources of this system [30]. Though M. vittatus is a freshwater catfish but growth and survival of fishes were good in low saline water as another freshwater catfish like Mystus tengara in such salinity regime at sundarban[28].Nitrite-nitrogen (NO2-N) and ammonia-nitrogen (NH4-N) concentrations obtained lower than the critical level and nitrate-nitrogen (NO3-N) and phosphate-phosphorous (PO4-P) concentrations were much lower than fertilized ponds reported from Sundarban [31,32].
Order of dominance of Planktonic groups in the pond water in the present study corroborated with that reported from Sundarban [33,34]. Estuarine and coastal regions receive inputs from several primary production sources and detritus food webs as results these areas are extremely productive [35].Being low nutrient input farming system depends on different natural resources might be considered as representative of the natural environment and existence of planktonic community structure resembling the natural environment is expected.
Higher feeding intensity in terms of stomach fullness in bigger fishes than smaller ones in the present study may be attributed to the fear of potential predators by the smaller fishes while feeding as they are more vulnerable and would rather feed more cautiously than their bigger counterpart [36]. Large fish may require more food to obtain the necessary energy for reproductive activity than smaller ones require for growth. Moreover, a wider mouth opening in larger fish helps to ingest relatively large quantity food items at a time [37]. Bhatt [38] has reported from Aligarh highest feeding intensity of M. vittatus has been observed during December- February but lowest feeding activity during March- June, prior to spawning. The feeding intensity is maximum during winter months and minimum in summer [15]. As earlier investigations, feeding intensity of M. vittatus was higher in winter and lowest in summer in present study.
Different types of food items are consumed by M. vittatus such as Copepods, Bacillariophyceae (diatoms), Fish parts and larvae, Insect parts and larvae, Unidentified organic matter, Chlorophyceae, Myxophyceae, Rotifers & Cladocera and Crustacean larvae and in their stomach indicated that fish preferred animal food materials. The present study revealed that the gut of this fish was mostly abundant with Copepods, Diatoms, Fish parts and larvae, Insect parts and larvae. Other food items eaten by M. vittatus were regarded as less abundantly. The study on the prey items of M. vittatus strongly suggested that this fish is an omnivorous. M vittatus is an omnivorous cat fish, mainly feeds on fish are fishes, diatoms, insects, green algae, crustaceans, blue green algae, plant matter, worms, copepods, mollusks [17].
M. vittatus is a carnivorous fish and its food consists mainly of fishes, insects, green algae, blue green algae, diatoms and crustaceans were reported throughout the study period [16]. Singh et al. [15], has reported that preferred foods of fish are Copepods, Insect, Insect larvae, Crustacean larvae, Rotifers, Eggs of invertebrates, Daphnids, Cypris, Algae, Sand and mud, Fish fry and Unidentified food items.
Dissimilar orders of dominance of prey groups in the environment and the stomach, in the present study, indicate that M. vittatus is a selective feeder. Considering the complex nature of fish feeding ecology, electivity index (E) analysis is necessary to throw some light on food preferences. As per Ivlev’s equation, E ranges from -1 to +1, where -1 to 0 stands for negative selection, while values 0 to +1 can be interpreted as positive selection of that prey item. Subsequent investigation [39] suggested that a true positive or negative prey selection can be interpreted only at values >+0.3 or <−0.3 respectively. In the present study M. vittatus actively selected Copepods (+0.53±0.05) as most preferred as well as most dominant food material in stomach although it ranked 7th in numeric order of dominance in the water column (Figure 5). Bacillariophyceae (+0.31±0.06) were found to be the truly selected second most preferred as well as second most numerically dominant prey group in stomach in spite of ranking 3rd in the numeric order of dominance in ambient water. Fish parts and larvae (+0.17±0.11), Insect parts and larvae (+0.06±0.07) and Crustacean larvae (0.02±0.17)were also positively selected but cannot be considered as true selection because E values were +0.03 and 0.Rotifers & Cladocera, Chlorophyceae and Myxophyceae were significantly negative selection and truly avoided by M. vittatus in the present study.
Food habits of a species are significant in relation to their growth and propagation under specific biological conditions. There is no available in formation on growth of M. vittatus in low salinewater, so it is not possible to compare the present findings on growth with previous reports.
However, growth of M. vittatus in the present observation can be compared with earlier studies reported on different freshwater dwelling species of the genus Mystus. In extensive polyculture trials of tengara (M.tengara) higher growth was reported at stocking density of 5000 nos ha-1 where fishes were grown from 1.38±1.02 g to 31.56±2.08 g (ADG: 0.09 g day-1; SGR: 0.95% day-1) in 360 days in low saline in low saline polyculture ponds of Indian Sundarbans [28]. In present study comparatively lower growth of M.vittatus may be attributed to insufficient food in such low input system.Growth performances of M. vittatusindicate that status is quite satisfactory.Length-weight relationships are of great importance in fisheries research because they provide information on population parameters [40] and also important in fisheries management for comparative growth studies [41]. Condition factor is used to compare the‘condition’, ‘fatness’ or ‘well-being’ of fish and are basedon the hypothesis that the heavier fish of a given length are in better condition. Condition factor data can be useful forproper management of culture systems as it provides theculturist with an indication of the conditions under which the organisms are growing. Exponent (b) value of length weight relationship in the present study indicated isometric growth of M vittatus. When the b parameter is equal to 3, growth is isometric and when it is less than or greater than 3, it is allometric [42]. More specifically, growth is positive allometric when organism weight increases more than length (b>3), and negative allometric when length increases more than weight (b<3) [43]. Isometric growth (b=2.981) and good condition factor (1.14±0.046) of M. vittatus in the present study indicated no severe competition for food and space in spite of being cultured with other fish. Exponent value (b) of LWR and condition factor in the present study corroborated with that reported from Indian Sundarban [28] indicating almost equal health status in low saline environment.
Conclusion
It may be concluded from the present study that M. vittatus can be reared along with other existing finfish in low saline ponds of Sundarbans for profitable polyculture and to improve fish production in homestead ponds. Growth and health conditions of freshwater catfish, M. vittatus were good in low saline water of Indian Sundarban. M. vittatus is an omnivorous small bagrid and it mostly preferred food as Copepods, Bacillariophyceae, Fish parts and larvae, Insect parts and larvae and Crustacean larvae.
The authors are extremely thankful to the authorities of Techno India University, Salt Lake, Kolkata, India for providing financial support laboratory facilities. The authors are deeply thankful to the faculty members, Professors, supporting staffs and colleagues for their continuous encouragement, priceless research comments, valuable guidance and important suggestions.
- Craig JM, Halls AS, Barr JJF, Bean CW (2004) The Bangladesh floodplain fisheries. Fish Res 66: 271-286. Link: https://goo.gl/dqcNzW
- Kibria G, Ahmed KKU (2005) Diversity of selective and nonselective fishing gear and their impact on inland fisheries in Bangladesh. NAGA ICLARM Quarterly 28: 43-48. Link: https://goo.gl/JAh1jo
- Hossain MY, Ahmed ZF, Leunda PM, Jasmine S, Oscoz J, et al. (2006) Condition, length-weight and length-length relationships of the Asian striped catfish Mystus vittatus (Bloch, 1794) (Siluriformes: Bagridae) in the Mathabhanga River, Southwestern Bangladesh. J Appl Ichthyol 22: 304-307. Link: https://goo.gl/jeZ84F
- Daniels RJR (2002) Freshwater fishes of Peninsular India. Universities Press Hyderabad India 288. Link: https://goo.gl/4WbRkA
- Chattopadhyay S, Nandi S, Saikia SK (2014) Mouth morphometry and architecture of freshwater cat fish Mystus vittatus Bloch (1974) (Siluriformes, Bagridae) in relation to its feeding habit. J Sc Res 6:169-174. Link: https://goo.gl/pYhzrA
- Gupta S, Banerjee S (2012) Indigenous ornamental fish: a new boon in ornamental fish trade of West Bengal. Fishing Chimes 32: 130-134. Link: https://goo.gl/cvAmQ4
- Gupta S, Banerjee S (2014) Indigenous ornamental fish trade of West Bengal. Narendra Publishing House New Delhi 63. Link: https://goo.gl/32EUH0
- Rao LM, Durga Prasad, NHK (2002) Comparative studies on the food and feeding habits of Therapon jarbua (Forskal) in relation to aquatic pollution. Ind J Fish 49: 199-203.
- Joadder Md AR (2014) Seasonal Occurence of Food and Feeding Habit of Labeo bata (Hamilton) (Cypriniformes :Cyprinidae). J Sc Found 12: 7-15. Link: https://goo.gl/Ik3TwY
- Sarkar UK, Deepak PK (2009) The diet of clown knife fish Chitala chitala (Hamilton–Buchanan) an endangered Notopterid from different wild population (India). Elec J Ichthyol 1: 11–20. Link: https://goo.gl/7UnhIh
- Adrian IDF, Barbieri G, Espectroalimentar, E, variaçãosazonale (1996) especialnacomposição da dieta de Parauchenipterusgaleatus L. (Siluriformes, Auchenipteridae) naregião do reservatório de Itaipu. Braz J Biol Form Revista.Bras Biolog 56: 409-422. Link:
- Al-Akel AS (2003) Selection of food on different size groups of Poecilialatipinna (Lesueur, 1821) from Alhasa of Saudi Arabia. Saudi J Biol Sc 10: 3-11.
- Azevedo P (1972) Principaispeixes das águasinteriores de São Paulo hábits de vida. In: ComissãoInterestadual da Bacia Paraná-Uruguai. Poluição e Piscicultura: NotasSobrePoluição, Ictiologia e Piscicultura. Facul deSaúd Públicada USP São.Paulo 109-112.
- Ageili IM, Al-Akel AS, Suliman EM (2013) Seasonal variations in food selectivity, condition factor and the hepatosomatic and gonadosomatic indices in the endangered killifish Aphaniusdispardispar (Teleostei: Cyprinodontidae) in Alhasa, Saudi Arabia. Life Sc J 10: 443-449.Link: https://goo.gl/KwDgc8
- Singh NP, Dwivedi RK, Tripathi VP, Tripathi PN (2015) Food and feeding habits of a fresh water catfish Mystus vittatus (Bloch.) in the Ghaghra belt of Eastern UP. J Exp Zool Ind 18: 489-493. Link: https://goo.gl/u5CVkP
- Victor Raj, M Sivakumar R, Mathialagan R (2014) Food and feeding habit and length-weight relationship of the Asian striped catfish Mystus vittatus (Bloch 1794) (Siluriformes: Bagridae) in the Vadavar River, Lower Anicut, Tamil Nadu. Ind J Sc 8: 55-64. Link: https://goo.gl/GnsUkZ
- Chaklader Md R, Nahar A, Siddik MAB, Sharker R (2014) Feeding Habits and Diet Composition of Asian Catfish Mystus vittatus (Bloch, 1794) in Shallow Water of an Impacted Coastal Habitat .World J Fish & Mar Sc 6: 551-556. Link: https://goo.gl/dVqtVX
- APHA (1998) Standard Methods for the Examination of Water and Wastewater 20th ed American Public Health Association Washington DC USA. Link: https://goo.gl/OuUXlJ
- Jhingran VG, Natarajan AV, Banerjee SM, David A (1969) Methodology on reservoir fisheries investigation in India. Central Inland Fisheries Research Institute Barrackpore India, Bulletin No: 12-10.
- Prescott (1961) Algae of the Western Great Lakes Area WMC Brown Company Publishers Dubuque IA USA 990. Link: https://goo.gl/DydyhJ
- Pillay TVR (1952) A critique of the methods of study of food of fishes. J Zool Soc Ind 4: 181-199.
- Hynes HBN (1950) The food of freshwater sticklebacks (Gasterosteusaculeatus and pygosteuspungitius) with a review of methods used in studies of the food of fishes. J Animal Ecol 19: 36-58. Link: https://goo.gl/SMv5ws
- Jacobs J (1974) Quantitative measurement of food selection – A modification of the Forage Ratio and the Ivlev’s Electivity Index. Ecologia 14: 413-417. Link: https://goo.gl/O12a9D
- Brown ME (1957) The Physiology of Fishes” Vol 1 Academic Press Inc New York pp 447. Link: https://goo.gl/49oPOl
- Le Cren ED (1951) The length-weight relationships and seasonal cycle in gonad weight and condition in the perch (Percafluviatilis)”. J Anim Ecol 20: 201–219. Link: https://goo.gl/31KQfD
- Chow S, Sandifer PA (1991) Differences in growth, morphometric traits, and male sexual maturity among Pacific white shrimp Penaeus vannamei from different commercial hatcheries. Aquacult 92 165-179. Link: https://goo.gl/9eDvPT
- Duncan DB (1955) Multiple range and multiple F-test Biometrics 11: 1–42. Link: https://goo.gl/qffzfI
- Mondal A, Sundaray JK, Bhattacharyya SB, Chakravartty D, Zaman, S (2017) Growth Performance, Feeding Ecology and Prey Preferenceof Bagrid Catfish, Mystus Tengara (Hamilton, 1822) in Low Saline Polyculture Ponds of Indian Sundarbans. Int J Aqua Fish Sc 3: 001-008. Link: https://goo.gl/2CuSx4
- Chakraborti RK, Sundaray JK , Ghoshal TK (2002) Production of Penaeus monodon in the tide fed ponds of Sundarban. Ind J Fish 49: 419–426. Link: https://goo.gl/68rzoe
- Gopalakrishnan V (1973) An assessment of the prawn fi shery of the seawardreaches of the Hooghly estuary. J Mar Biol Assoc Ind 15: 406-418.
- Saha, SB, Bhattacharyya SB, Mitra A, Choudhury A, (2001) Quality of shrimp culture farm effluents and its impact on the receiving environment. Bangla J Zool 29: 139-149.
- Biswas G, De D, Thirunavukkarasu AR, Natarajan M, Sundaray JK et al. (2012) Effects of stocking density feeding fertilization and combined fertilization-feeding on the performances of striped grey mullet (Mugil cephalus L.) fingerlings in brackish water pond rearing systems. Aquacult 338-341: 284–292. Link: https://goo.gl/AfUkfe
- Dutta S, Maity S, Bhattacharyya SB, Sundaray JK, Hazra S (2014) Diet composition and intensity of feeding of Tenualosa ilisha (Hamilton 1822) occurring in the Northern Bay of Bengal India. Proc Zool Soc Ind. Link: https://goo.gl/h3C5zk
- Mondal A, Chakravartty D (2016) Grow-out performance, length-weight relationship and variation in condition of all male Nile tilapia (Oreochromis niloticus Linnaeus 1758) from low saline fertilize earthen ponds of Indian Sundarbans. Int J Biol Res 1: 28-33. Link: https://goo.gl/JLvSv2
- Kennedy VS (1990) Anticipated effects of climate changes on estuarine and coastal fishes. Fisher 15: 16-24. Link: https://goo.gl/O3tFKR
- Akpan AW, Isangedighi IA (2004) Aspects of the Feeding Ecology of three Species of Pseudotolithus (Sciaenidae) in the inshore waters of Southeastern Nigeria East of the Niger Delta Nigeria. J Aqua Sc 192: 51-58. Link: https://goo.gl/RyOOD8
- Isangedighi I, Uudo PJ, Ekpo IE (2009) Diet composition of Mugil cephalus (pisces: mugilidae) in the Cross River estuary Niger delta Nigeria. Nig J Agricul Food &Env 5: 10-15. Link: https://goo.gl/PbSHkw
- Bhatt VS (1971) Studies on the biology of some freshwater fishes. Part V. Mystus vittatus (Bloch). J Bomb Nat Hist Soc 68: 556-572. Link: https://goo.gl/2IwOyi
- Lazzaro X (1987) A review of planktivorous fishes their evolution feeding behavior selectivities and impacts. Hydrobiol 146: 97–167. Link: https://goo.gl/jeNpPi
- Ecoutin JM, Albaret JJ, Trape S (2005) Length–weight relationships or fish populations of a relatively undisturbed tropical estuary: The Gambia. Fish Res 72: 347–351. Link: https://goo.gl/CjXkPs
- Moutopoulos DK, Stergiou KI (2002) Length–weight and length– length relationships of fish species from Aegean Sea (Greece). J Appl Ichthyol 18: 200-203. Link: https://goo.gl/zYd9s8
- Enin U (1994) Length-weight parameters and condition factor of two West African Prawns. Review Hydrobiol Trop 27: s121-127. Link: https://goo.gl/sHiF2o
- Wootton RJ (1992) Fish ecology tertiary level biology Blackie London 212.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
