Open Journal of Hepatology
Examination of Paraffin Sections of Different Rainbow Trout (Oncorhynchus mykiss) Tissues by Light and Scanning Electron Microscope
Beste Demirci1, Funda Terzi2 and Osman Sabri Kesbiç3*
2Department of Pathology, Faculty of Veterinary Medicine, Kastamonu University, 37200, Kastamonu, Turkey
3Department of Animal Nutrition and Nutritional Diseases, Faculty of Veterinary Medicine, Kastamonu University, 37200, Kastamonu, Turkey
Cite this as
Demirci B, Terzi F, Kesbiç OS (2022) Examination of Paraffin Sections of Different Rainbow Trout (Oncorhynchus mykiss) Tissues by Light and Scanning Electron Microscope. Open J Hepatol 4(1): 001-007. DOI: 10.17352/ojh.000007Copyright License
©2022 Demirci B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The current study aimed to highlight histopathological findings in paraffin block sections of the liver, gill kidney, and pyloric cecum of rainbow trout (Oncorhynchus mykiss) by different imagining devices such as Scanning Electron (SEM) and Light Microscope (LM). To determine the performance of different imagining methods two different thickness paraffin sections such as 5 and 15 µm about various rainbow trout tissue were prepared for imagining different devices. That sections were imagined by SEM and LM, both sections including 5 and 15 µm were imagined by SEM while just 5 µm was an image by LM. In LM imagining, it was detected that hydropic degeneration and vacuole formations in the liver hepatocytes of fish, as well as hyperplasia in bile ducts. Lamellar epithelial cell hyperplasia/hypertrophy was mild and histopathological findings such as secondary lamellar elevation and edema were more severe in rainbow trout gills. Glomerular atrophy/hypertrophy was moderately detected in the kidneys and hydropic degeneration of tubular epithelium was more severe. No degeneration or necrosis was observed in the lamina epithelium of the pyloric cecum. In SEM imagining of different thickness paraffin sections, cartilage and secondary lamellar structure in the gills, glomerulus, and Bowman’s capsule structure in the kidneys, and the structure of the pyloric cecum was observed. In the SEM imaging of the paraffin block sections of hepatocytes of the liver, the cell nuclei were determined, and also the grooves in the cytoplasm were thought to be vacuoles. As a consequence, the structural elements of the organ had higher clarity in SEM imaging from paraffin block sections, but the histopathological alterations remained unclear. As a result, SEM imaging of fish tissue is more suited for seeing tissue architecture, although LM imaging is better suited for determining and scoring histopathological variations.
Introduction
Aquaculture has become a highly productive industry for human consumption and animal protein production [1]. Rainbow trout (Oncorhynchus mykiss), one of the salmonids, is more preferred in this area due to its many features such as adaptation to the environment, reproductive efficiency, and disease resistance [2,3]. In scientific studies, organ anatomy, morphology, and histology of salmonid species are evaluated. Of these organs, the gills are anatomically located bilaterally on both sides of the pharynx in all fish. The gill filament is the basic functional unit or subdivision of the gill. Gill filaments, also called primary lamellae, consist of a series of pouch-like or arch-like structures that provide physical support [4]. Fish gills are an organ with many functions such as respiration, ion regulation, acid-base regulation, and nitrogenous waste excretion, and constitute more than 50% of the total surface area of the animal [5]. Salmonid and other fish species have morphologically and histologically all structural elements (liver cells-hepatocytes, blood vessels, and biliary tract) in their liver [6]. Fish liver morphology and histology, unlike mammalian liver structure, is the absence of its basic morphological unit, liver lobules, and portal triads [7]. Instead, fish hepatocytes display a diffuse or radial organization in the branching tubules that make up the liver parenchyma [8]. Additionally, the liver plays an important role in essential body functions such as regulation of metabolism, synthesis of plasma proteins, storage of energy, certain vitamins, and trace metals, conversion, and excretion of steroids, and xenobiotics [9].
The intestine is the main organ for digestion/absorption in fish and is critical for water and electrolyte balance, endocrine regulation of digestion and metabolism, and immunity [10]. Intestine histology consists of tunica mucosa, tunica submucosa, tunica muscularis, and tunica serosa of the intestinal wall. There are numerous villi and a simple columnar epithelium associated with the intestinal mucosa, goblet cells, and intraepithelial lymphocytes [11]. The pyloric caeca are quantitatively the most important part of the gastrointestinal tract concerning food digestion in salmonids [12,13]. The structure of the kidney in fish species is quite similar, and the kidneys of trout are fused, appearing as a single organ instead of two. Based on location as well as morphological differences, it is divided into a cranial or head kidney and a caudal or trunk kidney [14]. The histological structure of the salmonid kidney consists of a functional unit called the nephron, which includes the glomerulus, Bowman’s capsule, proximal, distal, and collecting tubules [15]. The kidney is the primary organ for water elimination and ion reabsorption mechanisms in the kidney minimize ion loss in these fish [16].
In fish, biochemical, growth, and histopathology, along with other methods, are biomarkers used to evaluate the effects of both internal (feed used) and external (aquatic) environmental conditions [12,17]. Rainbow trout (Oncorhynchus mykiss) histopathology can be used to provide a prognostic diagnosis of potential pathophysiological effects and to create specific models of both acute and chronic detrimental effects on tissues and organs (gills liver, kidney, intestine, pyloric caeca). These histopathological data are scored semi-quantitatively and quantitatively for statistical analysis. Semi-quantitative scoring is still the most preferred by pathologists today for incorporating histopathological information into biomedical research.
Among the more established morphological research techniques such as light microscopy and transmission electron microscopy, scanning electron microscopy (SEM) is also preferred today, which allows the examination of large surface areas at high resolution and magnification [18]. SEM is widely used to study the structural details on the surface of biological samples [19]. In addition, SEM enables the distinction between inorganic nanoparticles and the surrounding organic structure and demonstrates nanoparticles in cells and tissue [20]. In fish, the morphology of the tongue [21], kidney [22], skeletal muscles [23] and spleen [24], was demonstrated using the SEM.
From that perspective, the study aimed to determine to performance of different imagining devices such as SEM and LM in rainbow trout tissue paraffin sections.
Materials and methods
In this study, fifty healthy rainbow trout (51.22 ± 3.04 g), which were used in a study approved by the Kastamonu University Animal Experiments Local Ethics Committee (Decision no: 2021-4/32), without any experimental application, were used. The fish were kept in the closed circuit experiment system with 10-15% water change daily, dissolved oxygen 8.4 ± 0.2 mg/L, temperature 13.8 ± 1.2 °C, and pH 7.7 ± 0.1. No lesion was found in the macroscopic examination of rainbow trout. A systemic necropsy of rainbow trout was performed. After necropsy, liver, gill, kidney, and pyloric cecum tissue were fixed in 10% formaldehyde solution.
Histopathological analysis
Liver, gill, kidney, and pyloric cecum tissues were trimmed and taken into the cassettes and a routine histopathological method was applied with the following procedure; (1) graded alcohol series from 70 to 96%, (2) xylol for 2h in 30 min. periods. Then, the tissues were blocked with paraffin, and sections of 5 µm and 15 µm thickness were cut on the rotary microtome (Leica RM 2255). The sections were stained with hematoxylin-eosin. Sections were examined under a light microscope (Leica DM 400B) and photographs of the sections were imaged by a camera attached to the microscope. Scores were scored semi-quantitatively as indicated by the severity and extent of changes; none (-) mild (+) moderate (++) and severe (+++) [12].
Scanning electron microscopy (SEM) analysis
Five and fifteen µm thick sections from paraffin blocks of the liver, gill, kidney, and pyloric cecum of rainbow trout were taken on polylysine slides. The sections were first kept in xylol series (10 minutes each) and then in alcohol series (100%, 96%, 80%, and 70%) for five minutes and dried at ambient temperature. First, sections were coated with gold-palladium (Au-Pd) (Cressington, Sputter Coater 108 Auto). The ETD detector was then examined and imaged under a high vacuum at 5.00 kV with a scanning electron microscope (FEI, Quanta FEG 250). SEM analysis was performed at Kastamonu University Central Research Laboratory, Imaging Laboratory.
Results
Histopathological results
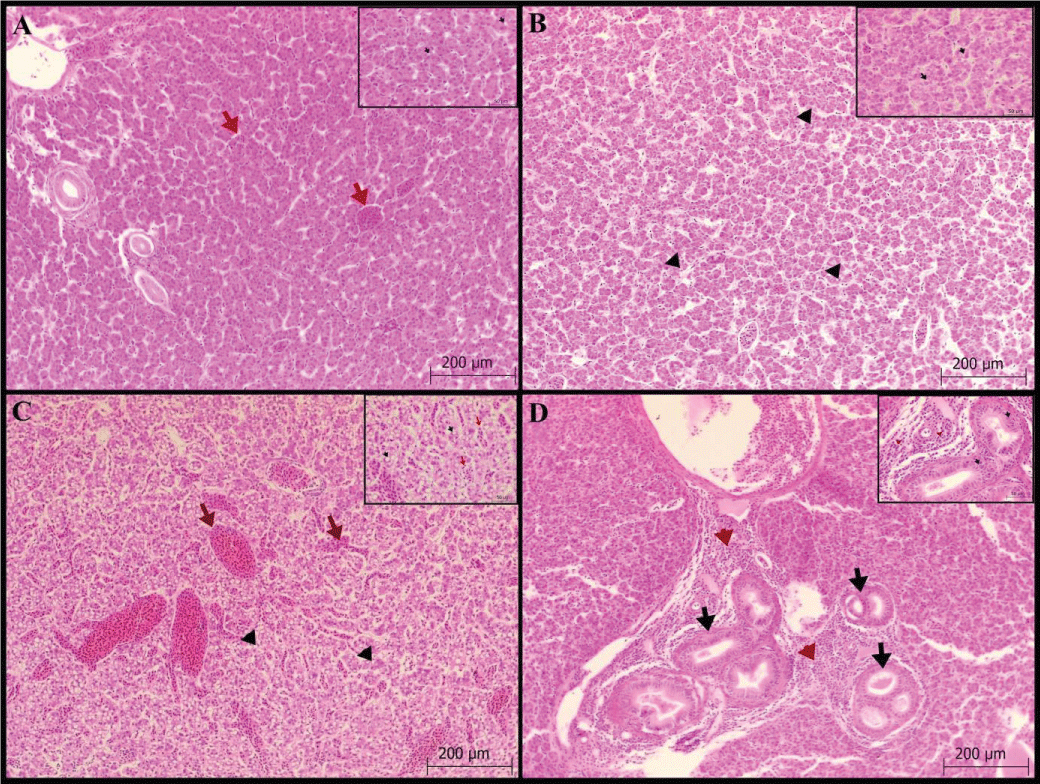
Liver: Hydropic degeneration and vacuolization in the hepatocyte cytoplasm, and congestion and dilation with hyperplasia in the bile ducts were scored. Vacuole formation was found in 34 fish (+1; 5 fish, +2; 10 fish and +3; 19 fish) in the hepatocyte cytoplasm (Figures 1A-C). Hydropic degeneration in hepatocytes was detected as mild in 21 fish and moderate and severe in 10 fish. In addition, congestion and dilatation were severe in all fish. Kupfer cells are commonly seen with hyperplasia in bile ducts (Figure 1D), Table 1.
Gill: Epithelial cell hyperplasia/hypertrophy in the lamellar, fusion in the secondary lamella, degeneration in the lamellar epithelial cells, lamellar epithelial lifting and edema, and telangiectasis/aneurysm in the lamella were scored. Lamellar epithelial cell hyperplasia/hypertrophy was mild in 18 fish, moderate in 4 fish, and severe in 1 fish. (Figures 2 A-C). In addition, secondary lamella lifting and edema (Figure 2D) were found in more than half of the fish (82%).
Kidney: Atrophy in the glomerulus was found to be mild in 20 fish, and moderate and severe in 3 fish each. Glomerulus hypertrophy was determined in 37 fish (+1; 22 fish, +2; 8 fish and +3;7 fish). Hydropic degeneration of the tubular epithelium was observed in 44 fish with mild to moderate severity (Figure 3A-B) and severe in 3 fish. In addition, pycnosis was found in tubular epithelial cell nuclei.
Pyloric cecum: No lamina epithelial degeneration or necrosis was observed. While no cell infiltration was detected in the lamina propria and submucosa, only mild hyperemia (Figure 3C-D) was observed.
SEM results
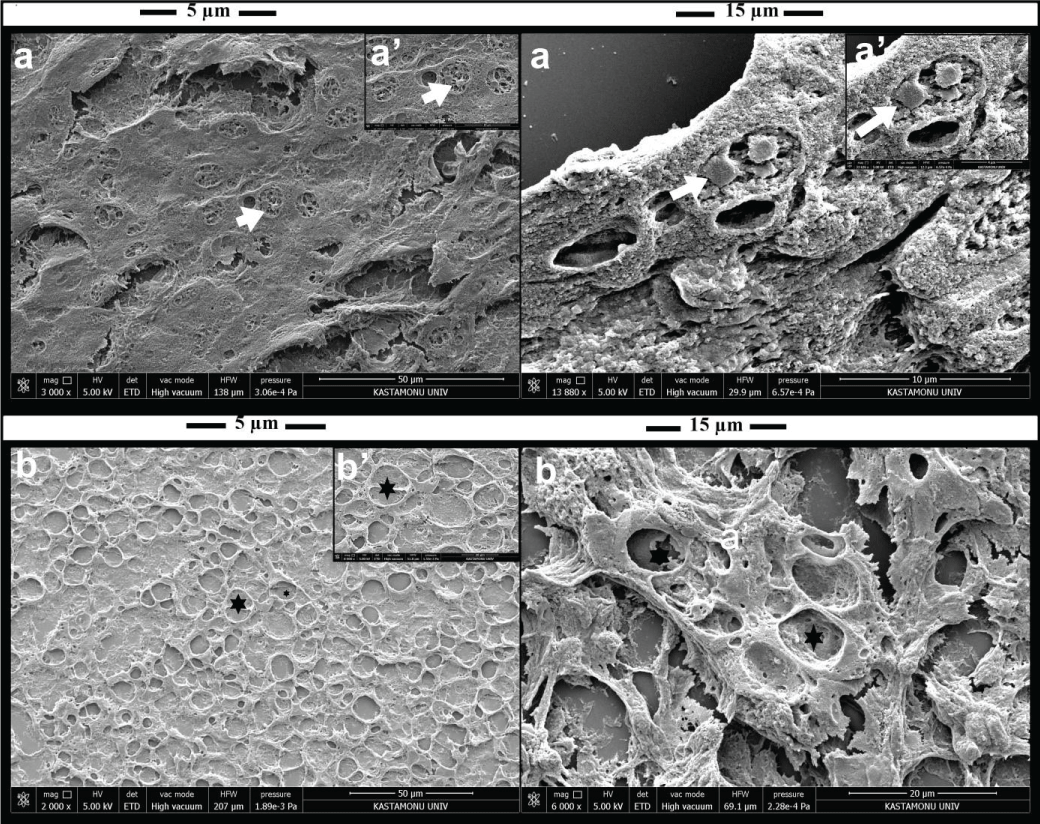
Paraffin block sections of 5 and 15 µm thickness taken from the gill, liver, kidney, and pyloric cecum of rainbow trout were demonstrated by SEM. In liver paraffin block sections, nuclei in hepatocytes and vacuoles in hepatocytes (Figure 4A-B) were determined. Gill cartilage and lamellar structure (Figure 5A) were seen. The kidney glomerulus structure and Bowman’s capsule structure (Figure 5B) were observed in both 5 µm and 15 µm thick paraffin block sections. The histological structure of the pyloric cecum (Figure 5C) was photographed.
Discussion
In the fish, many biomarkers (molecular, biochemical, physiological and histopathological, etc.) are used to determine the negative acute/or chronic effects of stress factors on tissues. Transmission Electron Microscopy (TEM) is used to determine the structure and changes of cell organelles, while Scanning Electron Microscopy (SEM), a surface imaging technique that provides a high depth of field, is used to view the 3D structure of cells [25,26]. This study, it was aimed to visualize the liver, gill, kidney, and pyloric caecum parts of rainbow trout in paraffin sections with light microscopy and SEM.
In SEM, tissue sample preparation is done by fixation, dehydration, drying, and conductive coating. Formaldehyde for Light Microscope, Glutaraldehyde (GA), and Osmium Tetroxide (OT) for SEM is used to fix tissue samples [27,28]. Nordestgaard and Rostgaard [29] determined that hepatocytes decreased in fixed volumes in the detection of glutaraldehyde and glutaraldehyde + OsO4. However, Maupin-Szamier and Pollard [30] state that osmium damages proteins and other components. The morphological structure of the gills in fish has been demonstrated in previous studies by Scanning electron microscopy [31,32]. Paruruckumani, et al. [33] determined the histopathological changes in the liver by SEM method with the detection of 2.5% glutaraldehyde and 1% osmium tetroxide in Lates calcarifer fish liver tissue. To demonstrate cell/tissue architectures by SEM in paraffin block sections, Sawaguchi, et al. (2018) examined rat organs from 30 µm thick paraffin blocks on New Silane II-coated microscope slides. In our study, we imaged paraffin sections of the liver, gill, kidney, and pyloric caecum with a Scanning electron microscope. We think that paraffin surface residues harm the detection of cells in SEM imaging. In addition, methods to completely remove paraffin should be developed for SEM imaging of paraffin block sections to be taken from archival materials.
The liver is the largest internal organ of the body and the organ where the nutrients absorbed in the digestive system are processed and stored for use by other organs of the body [34]. In the liver, hepatocyte vacuolization, fatty degeneration of the liver, changes in metabolic activity, changes in the liver parenchyma, and necrosis are the most common changes [6,35,36]. In our study, we detected hydropic degeneration and vacuole formations in the liver hepatocytes of fish, as well as hyperplasia in bile ducts. In our study, cytoplasmic vacuolizations in liver hepatocytes are one of the most prominent findings. We imaged fish liver paraffin sections by SEM and thought that the spaces in the cytoplasm of hepatocytes may be vacuoles in SEM imaging. The next generation of High-Resolution Field Emission Scanning Electron Microscopy (HRSEMs) not only has better performance characteristics but also has many applications that can be usefully used for diagnostic pathology and cell biology [37]. There is a need for studies using high-resolution SEM imaging techniques to detect pathological changes in liver hepatocytes.
In teleosts, the kidney, gills, and intestines are responsible for the excretion of body fluids and maintaining homeostasis [38]. In fish, the gills are an organ responsible for respiration and maintaining the optimal osmotic pressure and acid-base balance of body fluid [39]. In previous studies [40-44], rainbow trout gills were investigated histopathological lesions such as lamellar hyperplasia/hypertrophy, lamellar fusion, lamellar telangiectasia, and lamellar epithelial lift/edema, and lamellar clubbing. In fish, lifting of the lamellar epithelial cells from the basement membrane due to fluid penetration is the most common lesion and may result in decreased respiratory gas exchange (Salamat & Zarie, 2012). Hyperplasia and hypertrophy of epithelial cells and partial fusion of lamellae increase the distance between the blood and the environment and limit the entry of pollutants into the organism [45]. We found that while lamellar epithelial cell hyperplasia/hypertrophy was mild in fish, histopathological findings such as secondary lamellar elevation and edema were more severe. In the SEM imaging of the gills, the ultrastructural structure of the gills could be detected, but cell infiltration and edema that caused histopathological findings could not be detected.
The fish urinary system consists of the kidney, urethra, and bladder and plays a vital role not only in the excretion of urine but also in osmoregulation [46]. In fish, there is no metanephros formation, the pronephros and mesonephros are present as cephalic or head kidneys and exocrine or trunk kidneys, respectively [47]. In the head kidney, there is hematopoietic tissue, and this tissue is regarded as the teleost bone marrow [48,49]. In the trunk kidney, glomeruli, and proximal and distal tubules may be determined [50]. Researchers evaluate renal histopathological lesions such as enlargement of Bowman’s space in kidneys, glomerular atrophy and hypertrophy, degenerative changes in tubular epithelium, necrosis and/or epithelial desquamation, protein in tubule lumen, and interstitial hematopoietic necrosis in fish. In our study, glomerular atrophy/hypertrophy was moderately detected in the kidneys of the fish. In addition, hydropic degeneration of tubular epithelium was more severe in fish. The histopathological evaluation of the kidney suggests that it will contribute to the studies to be carried out with creatures living in the aquatic environment. In addition, glomerulus and Bowman’s capsule structure were determined in the imaging of kidney 5 and 15 µm paraffin sections with SEM, but the tubule structure could not be determined clearly. It is thought that studies should be done to show kidney structure in fish by SEM.
The general gastrointestinal morphology of fish relates to different dietary habits, including food content and frequency of food intake, as well as taxonomy, body size, and shape [52]. It promotes the absorption of nutrients, acting as a selective filter between the gastrointestinal tract (GI), lumen, and circulatory system, but also prevents the passage of harmful intraluminal xenobiotics [53]. In rainbow trout, the pyloric caecum is the site of digestion and absorption [54]. In the study, the pyloric cecum was examined and no lesion was found histopathologically, only hyperemia was detected in the lamina propria. In addition, the structure of the cells of the pyloric cecum could not be determined by SEM in paraffin incisions of the pyloric cecum, and it is thought that studies should be carried out to use special dyes to determine the cellular structures.
Conclusion
In rainbow trout, the structure of the liver, kidney, gill, and pyloric cecum on paraffin sections was demonstrated by SEM. In addition, it is thought that studies should be done to show the histopathological changes in fish species both in different thickness tissue and from paraffin block sections by SEM. As a result of the findings, SEM imaging is more effective in evaluating the structural differences of the tissues, while the conventional histopathologic method is more effective in evaluating and scoring the differences.
Declarations
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical approval and consent to participate: All the practices on rats were carried out with reference to European Union Directive and were approved by the local ethics committee of Kastamonu University (approval no:2021-4/32).
Authors’ contributions: All authors participated in the design of the study, interpretation of the findings, and analysis of and review of the manuscript. BD: SEM imaging and concentration, FT: histopathological analysis and draft preparation; OSK statistical analyses and proofreading.
- Caballero M, Obach A, Rosenlund G, Montero D, Gisvold M, Izquierdo M. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture. 2002; 214(1-4): 253-271.
- Crawford SS, Muir AM. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Reviews in Fish Biology and Fisheries. 2008; 18(3): 313-344.
- Penn MH, Bendiksen EÅ, Campbell P, Krogdahl Å. High level of dietary pea protein concentrate induces enteropathy in Atlantic salmon (Salmo salar L.). Aquaculture. 2011; 310(3-4): 267-273.
- Wilson JM, Laurent P. Fish gill morphology: inside out. Journal of Experimental Zoology. 2002; 293(3): 192-213.
- Nilsson S, Sundin L. Gill Blood Flow Control. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 1998; 119(1): 137-147. https://doi.org/https://doi.org/10.1016/S1095-6433(97)00397-8
- Rašković B, Stanković M, Marković Z, Poleksić V. Histological methods in the assessment of different feed effects on liver and intestine of fish. Journal of Agricultural Sciences (Belgrade). 2011; 56(1): 87-100. https://doi.org/https://doi.org/10.2298/JAS1101087R
- Brusle J, Anadon GG. The structure and function of fish liver. In Fish morphology. Routledge. 2017; 77-93.
- Rocha E, Monteiro RA, Pereira CA. The liver of the brown trout, Salmo trutta fario: a light and electron microscope study. J Anat. 1994 Oct;185 ( Pt 2)(Pt 2):241-9. PMID: 7961130; PMCID: PMC1166753.
- Salamat N, Zarie M. Fish histopathology as a tool for use in marine environment monitoring: a review. Comparative clinical pathology. 2016; 25(6): 1273-1278.
- Ringø E, Olsen RE, Mayhew TM, Myklebust R. Electron microscopy of the intestinal microflora of fish. Aquaculture. 2003; 227(1-4): 395-415.
- Khojasteh SB, Sheikhzadeh F, Mohammadnejad D, Azami A. Histological, histochemical and ultrastructural study of the intestine of rainbow trout (Oncorhynchus mykiss). World Applied Sciences Journal. 2009; 6(11): 1525-1531.
- Demirci B, Terzi F, Kesbic OS, Acar U, Yilmaz S, Kesbic FI. Does dietary incorporation level of pea protein isolate influence the digestive system morphology in rainbow trout (Oncorhynchus mykiss)? Anat Histol Embryol. 2021 Nov;50(6):956-964. doi: 10.1111/ahe.12740. Epub 2021 Sep 24. PMID: 34558733.
- Bakke-McKellep AM, Nordrum S, Krogdahl Å, Buddington R. Absorption of glucose, amino acids, and dipeptides by the intestines of Atlantic salmon (Salmo salar L.). Fish Physiology and Biochemistry. 22(1): 33-44.
- Anderson BG, Loewen RD. Renal morphology of freshwater trout. Am J Anat. 1975 May;143(1):93-114. doi: 10.1002/aja.1001430105. PMID: 1130299.
- Sarma D, Kohli V, Kushwaha SS, Pandey J, Mallik S, Shahi N, Das P, Srivastava S, Joshi V, Akhtar M. Histological alterations in gill, liver and kidney of rainbow trout following fungal infection. Journal of Ecophysiology and Occupational Health. 2014; 14(3/4): 123.
- Salamat N, Zarie M. Using of fish pathological alterations to assess aquatic pollution: a review. World Journal of Fish and Marine Sciences. 2012; 4(3): 223-231.
- Poleksic V, Lenhardt M, Jaric I, Djordjevic D, Gacic Z, Cvijanovic G, Raskovic B. Liver, gills, and skin histopathology and heavy metal content of the Danube sterlet (Acipenser ruthenus Linnaeus, 1758). Environ Toxicol Chem. 2010 Mar;29(3):515-21. doi: 10.1002/etc.82. PMID: 20821473.
- Bezerra W, Oliveira MS, Garcia Filho FC, Demosthenes LC, da Silva LC, Monteiro SN. Characterization of Arapaima Fish Scales and Related Reinforced Epoxy Matrix Composites by XRD, EDS, and SEM. In Green Materials Engineering. Springer, Cham. 2019.
- Zhang Y, Huang T, Jorgens DM, Nickerson A, Lin LJ, Pelz J, Gray JW, López CS, Nan X. Quantitating morphological changes in biological samples during scanning electron microscopy sample preparation with correlative super-resolution microscopy. PLoS One. 2017 May 31;12(5):e0176839. doi: 10.1371/journal.pone.0176839. PMID: 28562683; PMCID: PMC5451012.
- Koh AL, Shachaf CM, Elchuri S, Nolan GP, Sinclair R. Electron microscopy localization and characterization of functionalized composite organic-inorganic SERS nanoparticles on leukemia cells. Ultramicroscopy. 2008 Dec;109(1):111-21. doi: 10.1016/j.ultramic.2008.09.004. Epub 2008 Oct 2. PMID: 18995965; PMCID: PMC2650478.
- Abbate F, Guerrera MC, Levanti M, Laurà R, Aragona M, Mhalhel K, Montalbano G, Germanà A. Anatomical, histological and immunohistochemical study of the tongue in the rainbow trout (Oncorhynchus mykiss). Anat Histol Embryol. 2020 Nov;49(6):848-858. doi: 10.1111/ahe.12593. Epub 2020 Jul 24. PMID: 32705711.
- Cui K, Li Q, Xu D, Zhang J, Gao S, Xu W, Mai K, Ai Q. Establishment and characterization of two head kidney macrophage cell lines from large yellow croaker (Larimichthys crocea). Dev Comp Immunol. 2020 Jan;102:103477. doi: 10.1016/j.dci.2019.103477. Epub 2019 Aug 27. PMID: 31470020.
- Adam MA, Maftuch M, Kilawati Y, Risjani Y. The effect of cadmium exposure on the cytoskeleton and morphology of the gill chloride cells in juvenile mosquito fish (Gambusia affinis). The Egyptian Journal of Aquatic Research. 2019; 45(4): 337-343.
- He Y, Wang E, Wang K, Wang J, Fan W, Chen D, Yang Q. Morphology of the Spleen in Oreochromis niloticus: Splenic Subregions and the Blood-Spleen Barrier. Animals (Basel). 2021 Oct 11;11(10):2934. doi: 10.3390/ani11102934. PMID: 34679955; PMCID: PMC8532917.
- Cheville NF, Stasko J. Techniques in electron microscopy of animal tissue. Vet Pathol. 2014 Jan;51(1):28-41. doi: 10.1177/0300985813505114. Epub 2013 Oct 10. PMID: 24114311.
- Graham L, Orenstein JM. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat Protoc. 2007;2(10):2439-50. doi: 10.1038/nprot.2007.304. PMID: 17947985; PMCID: PMC7086545.
- Bell PB, Safiejko‐Mroczka B. Preparing whole mounts of biological specimens for imaging macromolecular structures by light and electron microscopy. International journal of imaging systems and technology. 1997;8(3): 225-239.
- Hopwood D. Fixatives and fixation: a review. The Histochemical journal. 1969; 1(4): 323-360.
- Nordestgaard B, Rostgaard J. Dimensional changes of isolated hepatocytes during processing for scanning and transmission electron microscopy. Micron and microscopica acta. 1985; 16(2): 65-75.
- Maupin-Szamier P, Pollard TD. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837-52. doi: 10.1083/jcb.77.3.837. PMID: 28332; PMCID: PMC2110151.
- Gregory M, George R, Marshall D, Anandraj A, Mcclurg T. The effects of mercury exposure on the surface morphology of gill filaments in Perna perna (Mollusca: Bivalvia). Marine Pollution Bulletin. 1999; 39(1-12): 116-121.
- Kumari U, Yashpal M, Mittal S, Mittal AK. Morphology of the pharyngeal cavity, especially the surface ultrastructure of gill arches and gill rakers in relation to the feeding ecology of the catfish Rita rita (Siluriformes, Bagridae). J Morphol. 2005 Aug;265(2):197-208. doi: 10.1002/jmor.10350. PMID: 15971265.
- Paruruckumani PS, Maharajan A, Ganapiriya V, Narayanaswamy Y, Jeyasekar RR. Surface Ultrastructural Changes in the Gill and Liver Tissue of Asian Sea Bass Lates calcarifer (Bloch) Exposed to Copper. Biol Trace Elem Res. 2015 Dec;168(2):500-7. doi: 10.1007/s12011-015-0370-z. Epub 2015 May 26. PMID: 26006096.
- Akiyoshi H, Inoue A. Comparative histological study of teleost livers in relation to phylogeny. Zoolog Sci. 2004 Aug;21(8):841-50. doi: 10.2108/zsj.21.841. PMID: 15333997.
- Ostaszewska T, Dabrowski K, Czumińska K, Olech W, Olejniczak M. Rearing of pike‐perch larvae using formulated diets–first success with starter feeds. Aquaculture Research.2005;36(12): 1167-1176.
- Reddy P, Rawat S. Assessment of aquatic pollution using histopathology in fish as a protocol. International Research Journal of Environment Sciences. 2013; 2(8): 79-82.
- Cohen Hyams T, Mam K, Killingsworth MC. Scanning electron microscopy as a new tool for diagnostic pathology and cell biology. Micron. 2020 Mar;130:102797. doi: 10.1016/j.micron.2019.102797. Epub 2019 Dec 4. PMID: 31862481.
- Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005 Jan;85(1):97-177. doi: 10.1152/physrev.00050.2003. PMID: 15618479.
- Strzyzewska E, Szarek J, Babinska I. Morphologic evaluation of the gills as a tool in the diagnostics of pathological conditions in fish and pollution in the aquatic environment: a review. Veterinarni Medicina 2016; 61(3).
- Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol. 2013 Jan 15;126:104-15. doi: 10.1016/j.aquatox.2012.10.005. Epub 2012 Oct 13. PMID: 23174144.
- Banaee M, Sureda A, Mirvagefei A, Ahmadi K. Histopathological alterations induced by diazinon in rainbow trout (Oncorhynchus mykiss). International Journal of Environmental Research. 2013; 7(3):735-744.
- Johari SA, Kalbassi MR, Yu IJ, Lee JH. Chronic effect of waterborne silver nanoparticles on rainbow trout (Oncorhynchus mykiss): histopathology and bioaccumulation. Comparative Clinical Pathology. 2015; 24(5): 995-1007.
- Khabbazi M, Harsuj M, Hedayati SAA, Gerami M H, Ghafari-Farsani H. Histopathology of rainbow trout gills after exposure to copper. Iranian Journal of Ichthyology.2015; 1(3); 191-196.
- Topal A, Oruç E, Altun S, Ceyhun SB, Atamanalp M. ]The effects of acute boric acid treatment on gill, kidney and muscle tissues in juvenile rainbow trout. Journal of Applied Animal Research. 2016; 44(1): 297-302.
- Poleksić V, Mitrović-Tutundžić V. Fish gills as a monitor of sublethal and chronic effects of pollution. Sublethal and chronic effects of pollutants on freshwater fish. Oxford: Fishing News Books. 1994; 339-352.
- Varsamos S, Nebel C, Charmantier G. Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol A Mol Integr Physiol. 2005 Aug;141(4):401-29. doi: 10.1016/j.cbpb.2005.01.013. Epub 2005 Feb 25. PMID: 16140237.
- Fernandes CE, Marcondes S F, Galindo GM, Franco-Belussi L. Kidney anatomy, histology and histometric traits associated to renosomatic index in Gymnotus inaequilabiatus (Gymnotiformes: Gymnotidae). Neotropical Ichthyology. 2019;17(4).
- Press CM, Evensen Ø. The morphology of the immune system in teleost fishes. Fish & shellfish immunology. 1999; 9(4): 309-318.
- Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Curr Top Microbiol Immunol. 2000;248:67-107. doi: 10.1007/978-3-642-59674-2_5. PMID: 10793475.
- Bjørgen H, Koppang EO. Anatomy of teleost fish immune structures and organs. Immunogenetics. 2021 Feb;73(1):53-63. doi: 10.1007/s00251-020-01196-0. Epub 2021 Jan 11. PMID: 33426583; PMCID: PMC7862538.
- Cinar K, Senol N. Histological and histochemical characterization of the mucosa of the digestive tract in flower fish (Pseudophoxinus antalyae). Anat Histol Embryol. 2006 Jun;35(3):147-51. doi: 10.1111/j.1439-0264.2005.00629.x. PMID: 16677207.
- Murray H, Wright GM, Goff G. A comparative histological and histochemical study of the post‐gastric alimentary canal from three species of pleuronectid, the Atlantic halibut, the yellowtail flounder and the winter flounder. Journal of Fish Biology. 1996; 48(2): 187-206.
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012 Oct 5;11(4):452-60. doi: 10.1016/j.stem.2012.09.009. PMID: 23040474.
- Mumford S, Heidel J, Smith C, Morrison J, MacConnell B, Blazer V. Fish histology and histopathology. US Fish and Wildlife National Conservation Training Center, Amerika Serikat. 2007.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
