Annals of Cytology and Pathology
Evaluation of angiogenesis in cervical cancer using CD34 as a biomarker and its correlation with pathoanatomical features
Mohsin Aijaz1*, Kiran Alam2 and Veena Maheshwari2
2Professor, Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh (202002), U.P, India
Cite this as
: Aijaz M, Alam K, Maheshwari V (2021) Evaluation of angiogenesis in cervical cancer using CD34 as a biomarker and its correlation with pathoanatomical features. Ann Cytol Pathol 6(1): 007-011. DOI: 10.17352/acp.000023Aim: CD34 is an antigen found in hematopoietic progenitor cells and endothelial cells. Anti-CD34 antibody is a highly sensitive marker for neovascularisation and has also been studied as a marker for vascular tumours. However, there are few studies relating it to cervical carcinoma. The aim of our study is to evaluate the CD34 expression as a marker of angiogenesis in cervical cancer and to correlate the microvessel count and Microvessel Density (MVD) with histopathological type and grade of tumour and lymph node metastasis.
Method: Total 75 malignant cases (65 cases of squamous cell carcinoma and 10 cases of adenocarcinoma) were stained with anti CD34 antibody to highlight the blood vessels. In each case, areas of highest neo-vascularization were identified using scanner lens. Blood vessels were counted in 10 microscopic fields at 400X magnification. Average number of non-canalized micro-vessels count per field was calculated (MVD).
Result: The total number of microvessels stained per case ranged from 54 to 216. No statistically significant differences were observed for MVD with respect to the age of patient and histological type of carcinoma (p > 0.05). The mean vascular density in differentiated carcinoma and undifferentiated carcinoma, was 10.56 and 14.14 respectively. This clearly shows that the distribution of microvessels was higher among undifferentiated carcinomas, as compared to differentiated carcinoma (p < 0.05). Furthermore, the MVD in lymph node positive cases was 12.84, slightly higher as compared to lymph node negative cases where it was 11.34. However, the relationship was statistically nonsignificant (p > 0.05).
Conclusion: CD 34 can be used as a novel marker for estimation of MVD in cervical cancer, however further studies are required on large group of patients to establish its role as a therapeutic target against angiogenesis.
Introduction
The cervical carcinoma represents one of the most frequent malignant tumours in female. Great advancements were made in the understanding of the mechanisms of the appearance and progression of the tumours, and also in the early diagnosis of them. In the present time, the discussions are focused on the angiogenesis and the neovascularisation. Angiogenesis is the growth of new blood vessels toward and within a tumour, and it has been shown that tumours do not proliferate beyond a size of 2 to 3 mm2 unless they are able to develop new capillaries from the existing vascular network [1]. Previous researches have demonstrated that microvessel density in carcinomas cervix is a factor associated with poor prognosis. It also tends to be related with pathological features indicative of a greater risk of recurrence of tumour [2]. The purpose of this study was to determine in patients with cervical cancer whether the intensity of tumour angiogenesis, expressed as microvessel density, is surely a significant parameter predicting lymph node metastasis.
Various researchers used different markers to highlight blood vessels in cervical carcinoma in order to measure angiogenesis. The different antibodies that were used are factor VIII or Von Willebrand factor, European I lecithin Ulex, and anti-CD31. Fewer studies were found using anti-CD34 in cancer of the uterine cervix, thus we used anti-CD34 as a marker of angiogenesis in our study, owing to the ability of anti-CD34 antibody to highlight blood vessels more strongly as compared to normal vessels.
CD34 is a transmembrane, highly glycosylated protein expressed by hematopoeitic stem/progenitor cells [3] and endothelial cells [4]. On endothelial cells, CD34 acts as a ligand for L-selectin [5], although this is not the case in HPSCs, where its function remains unclear.
Besides these, CD34+ fibrocytes also known to be present in the connective tissue compartment of various tissue such as the skin [6], gastrointestinal [7] and urogenital tracts [8], pancreas [9], breast [10], thyroid [11] and peripheral nervous system [12]. In addition, CD34 is known to be present in stromal fibroblasts of the uterine cervix [8]. Thus CD34 reactivity of normal endocervical and other uterine stromal tissues should be kept in mind while evaluating angiogenesis, as it may contribute to falsely elevated MVD. It is therefore necessary for the pathologist to try to identify the tissue morphology carefully on H & E stained slides, so that an adequate description of microvessels is possible.
Observations and results
The present study was conducted in the Department of Pathology, JNMCH, AMU, Aligarh. A total of 75 females ranging in age between 20 years and 75 years (average 51.14 years) who were diagnosed with stages I and II invasive cervical cancer undergoing radical hysterectomy and bilateral pelvic lymphadenectomy were evaluated. Informed or written consent was also taken from the patients. Detailed medical history, clinical examination and other relevant investigations were obtained from the Medical Records Department at our hospital and also retrieved from the pathology reports and recorded on a proforma. Diagnosis of malignancy, typing and grading of tumour was based on examination of hematoxylin and eosin stained tissue sections.
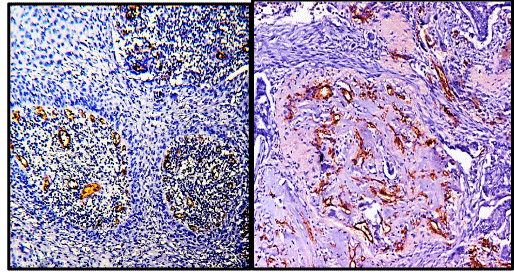
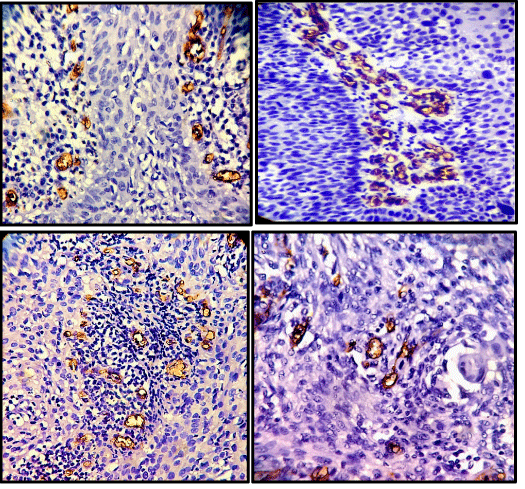
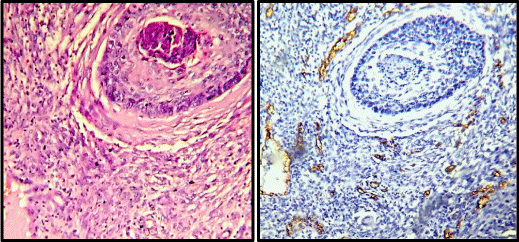
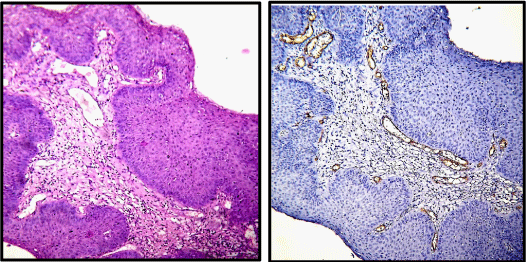
Immunohistochemistry for CD34 was performed on paraffin embedded sections using anti CD34 antibody (QBEnd/10) to highlight the blood vessels. All cases of cervical carcinoma were considered for this study (total 75 cases). Sections were examined and microvessel density and microvessel count was calculated for each case of cervical cancer. A vascular unit was identified according to criteria established by Weidner, et al. 1991 [13]. In each case tumour sections were screened under scanner to find out areas of highest neo-vascularization. Using 400X magnification blood vessels were counted in 10 microscopic fields (Figure 1A & 1B). Average number of non-canalized micro-vessels count per field was calculated. Any brown staining endothelial cell or endothelial cell cluster which was clearly separate from adjacent micro-vessels was considered a single, countable micro-vessel. Red blood cells in lumen were not used to define a micro-vessel. Statistical analysis was carried out using the Mann–Whitney test to compare the distribution function of microvessel density according to pathoanatomic features.
The mean age of patients with cervical carcinoma was 51.14 years with a SD of 11.34 years. Seventy-One percent of the patients had clinical stage I cancers. Out of 75 malignant cases, 65 cases were of squamous cell carcinoma and 10 cases were of adenocarcinoma (Figures 2,3). The total number of microvessels (which is a sum of microvessels in 10 hpf) stained per case ranged from 54 to 216 (Table 1).
Microvessel density was compared with respect to the age of patient and histological type of carcinoma. No statistically significant differences were observed for these categories (p > 0.05).
Mean vascular density was also compared with degree or grade of differentiation of tumour. For convenience, all cases of well differentiated and moderately differentiated squamous cell carcinoma were grouped as differentiated carcinoma (72.3% cases), whereas cases of poorly differentiated carcinoma were considered as undifferentiated carcinoma. The mean vascular density in differentiated carcinoma was 10.56, whereas in undifferentiated carcinoma, it was found to be 14.14. Thus it is clearly evident that the distribution of microvessel density is higher among undifferentiated carcinomas, as compared to differentiated carcinoma. (p < 0.05) (Table 1). All cases of adenocarcinoma showed glandular differentiation, so such comparison was not done in cases of adenocarcinoma.
In our study, thirty-six percent cases showed lymph node involvement. Furthermore, MVD was also compared between node negative and node positive cases in order to determine whether increased angiogenesis would increase the chances of metastasis to lymph nodes. The MVD in lymph node positive cases was found to be 12.84, whereas it was 11.34 in lymph node negative cases. It is slightly higher in node positive cases. However, the relationship is statistically insignificant. (p > 0.05) (Table1).
Discussion
Neo-angiogenesis or new micro-vessels formation is a complex process. It involves an interaction between pro- and anti-angiogenic signals which are released by endothelial and stromal tumour cells. These signals are critical for tumour growth, progression and metastases. The angiogenic activity, revealed by the development of novel micro-vessels in tumour tissue, can be quantified by intra-tumoral microvessel density.
Various studies were performed by different authors in the past to investigate the relationship between the parameters of angiogenesis in cervical carcinomas with the histopathological features of these tumours, and clinical outcome of the patients. Most of the authors used factor VIII, other studied CD 31, CD34 etc.
In our study, all 75 cases of malignancy were broadly classified into different categories (Table 1) depending upon mean age, histological type of carcinoma, degree of differentiation or lymph node positivity status. The total number of microvessels stained per case ranged from 54 to 216, similar to the findings of Vieira, et al. (2005) [9], where they also found, the total number of microvessels stained per case to range from 48 to 200. Hirakawa, et al. (1999) also used anti-CD34 antibody to determine microvessel density [17]. However, they counted the microvessels density in only three fields using 200X magnification, as against our study where we counted 10 fields using 400X magnification. They found the number of vessels to vary from 17 to 174.
The MVD in undifferentiated carcinoma was 14.14 whereas in differentiated carcinoma MVD was 10.56. It was observed that the distribution of MVD was higher in undifferentiated carcinoma as compared to differentiated carcinoma and this relationship was found to be statistically significant (p value < 0.05). In the study by Vieira, et al. (2005) the median vascular density in undifferentiated carcinoma was 10.8 whereas in differentiated carcinoma was 9.3 and this was statistically significant (p > 0.03) [18]. These results are in accordance with the findings of our study. Wiggens et al. (1995) compared the microvessel density in benign cervical tissue with invasive cervical carcinoma and observed a higher density in the invasive group, which was statistically significant (P = 0.013) [14].
Abulafia et al. (1996) analysed the microvessel count between normal cervical epithelium, in situ carcinomas and invasive cervical carcinoma. He also found a significantly lower microvessel density in the normal tissue (P < 0.005) [16]. Thus it can be inferred from these studies that with increase in invasive potential of tumour or decrease in grade of differentiation, the MVD is significantly increases.
We also observed high microvessels count per high power field in cases of squamous cell carcinoma as compared to adenocarcinoma but it was statistically non-significant (p value-0.7), similar to the study of Vieira et al. (2005) where the p value was 0.10 and Ancuta et al., (2010) (p value >0.05) [18,20]. Thus no significant relationship was established between intensity of angiogenesis (MVD) and histological type of tumour. This probably may be due to difference in sample size or the cases of adenocarcinoma were more differentiated.
We did not find any relationship between mean vascular density and other pathoanatomical features like age of the patient, staging of tumour, as also by other authors like Wiggen, et al. (1995); Vieira, et al. (2005); Ancuta, et al. (2010) [14,18,20].
The prognosis of patients with cervical cancer stages IB and IIA is considered to be dependent on the presence or absence of pelvic lymph node metastasis. The patients with positive pelvic lymph nodes are found to be at risk for recurrence and are treated more aggressively (van Bommel, 1987) [22]. Hence we also studied correlation of microvessel density with lymph node metastasis. We observed that MVD in lymph node positive case is higher as compared to lymph node negative cases. However; the p value was found to be insignificant (p< 0.19) an observation similar to Tjalma, et al. (1998); Hirakawa, et al. (19990; Vieira, et al. (2005). This is in contrast to study by Bremer, et al. (1996) where a statistically significant relationship was found. (p < 0.002) [2,17,18,15].
When comparing the results of the different studies published about tumour angiogenesis and cervical carcinoma, a great variation was seen among them. We analysed that besides the choice of the endothelial cell antibody, the method of microvessel quantification is also important for angiogenesis study. In almost all the studies the selection area for tumour quantification was mainly based on the pioneering work of Weidner, et al. (1991) [13]. In most of the studies quantification was done manually, however few authors used computer method for quantification. In our study manual method was used. We also observed that in these studies the amount of fields counted varies from unknown to 15, the magnification also varies from 100 to 400. The vessel marker, methodology and the technique used are probably the explanations for the noticeably different micro vessel densities in our study compared with those in the literature.
Regardless of the methodology, with the present study we found that in most studies the microvessel density is an independent prognostic marker. CD 34 can be used as a novel marker for quantification of microvessel density. Few studies showed high MVD is associated with invasive and undifferentiated carcinoma similar to our study. Others showed association of high microvessel count with positive lymph node, higher stage, lymphovascular invasion. Some of the authors like Hirakawa et al. (1999) failed to establish any relationship with these parameters [17].
Conclusion
Our study suggests that CD 34 can be used as a novel marker for estimation of MVD in cervical cancer. It was seen that anti-CD34 antibody immunoreactivity in cervical carcinoma is associated with pathoanatomical features which are indicative of a poorer prognosis. Further studies can also be undertaken on large group of patients to establish its role as a therapeutic target against angiogenesis in cases of cervical cancer.
- Folkman J (1990) What Is the Evidence That Tumors Are Angiogenesis Dependent? JNCI: Journal of the National Cancer Institute 82: 4-7. Link: https://bit.ly/3xTjDxX
- Tjalma W, Van Marck E, Weyler J, Dirix L, Van Daele A, et al. (1998) Quantification and prognostic relevance of angiogenic parameters in invasive cervical cancer. British Journal of Cancer 78: 170–174. Link: https://bit.ly/35PtR6K
- Van de Rijn M, Rouse RV (1994) CD34. A review. Appl Immunohistochem 2: 71-80.
- Fina L, Molgaard H, Robertson D, Bradley N, Monaghan P, et al. (1990) Expression of the CD34 gene in vascular endothelial cells. Blood 75: 2417-2426. Link: https://bit.ly/3gXu8e9
- Lanza F, Healy L, Sutherland DR (2001) Structural and functional features of the CD34 antigen: an update. J Biol Regul Homeost Agents 15: 1–13. Link: https://bit.ly/35TxFns
- Kirchmann TT, Prieto VG, Smoller BR (1994) CD34 staining pattern distinguishes basal cell carcinoma from trichoepithelioma. Arch Dermatol 130: 589–592. Link: https://bit.ly/3w2komZ
- Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, et al. (2000) Differential expression of CD34 in normal colorectal tissue, peritumoral inflammatory tissue, and tumour stroma. J Clin Pathol 53: 626–629. Link: https://bit.ly/3gYHgQl
- Lindenmayer AE, Miettinen M (1995) Immunophenotypic features of uterine stromal cells. CD34 expression in endocervical stroma. Virchows Arch 426: 457–460. Link: https://bit.ly/3haMJCe
- Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in ductal adenocarcinoma of the pancreas, chronic pancreatitis and normal pancreatic parenchyma. Virchows Arch 440: 128–133. Link: https://bit.ly/3zYtrZB
- Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch 440: 298–303. Link: https://bit.ly/3dixshP
- Yamazaki K, Eyden BP (1997) Interfollicular fibroblasts in the human thyroid gland: recognition of a CD34-positive stromal cell network communicated by gap junctions and termineated by autonomic nerve endings. J Submicrosc Cytol Pathol 29: 461–467. Link: https://bit.ly/3dbqtqT
- Khalifa MA, Montgomery EA, Ismiil N, Azumi N (2000) What are CD34+ cells in benign peripheral nerve sheath tumors? Am J Clin Pathol 114: 123–126. Link: https://bit.ly/35QLRxs
- Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor Angiogenesis and Metastasis — Correlation in Invasive Breast Carcinoma. New England Journal of Medicine 324: 1–8. Link: https://bit.ly/3vXBcvA
- Wiggins DL, Granai CO, Steinhoff MM, Calabresi P (1995) Tumor Angiogenesis as a Prognostic Factor in Cervical Carcinoma. Gynecologic Oncology 56: 353–356. Link: https://bit.ly/3dfzsYe
- Bremer GL, Tiebosch AT, van der Putten HW, Schouten HJ, de Haan J, et al. (1996) Tumor angiogenesis: An independent prognostic parameter in cervical cancer. American Journal of Obstetrics and Gynecology 174: 126–131. Link: https://bit.ly/3jnqjjQ
- Abulafia O, Triest WE, Sherer DM (1996) Angiogenesis in squamous cell carcinoma in situ and microinvasive carcinoma of the uterine cervix. Obstet Gynecol 88: 927-32. Link: https://bit.ly/3vXbjw2
- Hirakawa T, Kamura T, Kaku T, Amada S, Ariyoshi K, et al. (1999) Prognostic Significance of Epithelial–Stromal Vascular Cuffing and Microvessel Density in Squamous Cell Carcinoma of the Uterine Cervix. Gynecologic Oncology 74: 369–374. Link: https://bit.ly/3jhJqfk
- Vieira SC, Silva BB, Pinto GA, Vassallo J, Moraes NG, et al. (2005) CD34 as a marker for evaluating angiogenesis in cervical cancer. Pathology - Research and Practice 201: 313–318. Link: https://bit.ly/3x20jyD
- Francu Dl, Francu Ll, Crauciuc E, Pandeli R, Cozma L, et al. (2008) The assessment of angiogenesis in the uterine cervix carcinogenesis, Analele Ştiinţifice ale Universităţii „Alexandru Ioan Cuza” din Iaşi. Secţiunea Genetică şi Biologie Moleculară 9: 11-15. Link: https://bit.ly/35T7Kw9
- Ancuţa C, Ancuţa E, Zugun-Eloae F, Carasevici E (2010) Neoangiogenesis in cervical cancer: focus on CD34 assessment. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie 51: 289–294.
- Mondal S, Dasgupta S, Mandal P, Chatterjee S, Chakraborty D (2014) Is there any role of mast cell density and microvessel density in cervical squamous cell carcinoma? A histologic study with special reference to CD-34 immunomarker staining. Indian Journal of Medical and Paediatric Oncology 35: 165. Link: https://bit.ly/3x1ZJ3V
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
