Journal of Gynecological Research and Obstetrics
Establishment and validation of a rodent model of endometriosis to evaluate the effect of new therapeutic strategies
Rana Assaly1*, Sandrine Compagnie1, Laurine Allimonnier1, Manon Bracconi1, Francois Giuliano2 and Delphine Behr-Roussel1
2AP-HP, Neuro-Uro-Andrology, Department of Physical Medicine and Rehabilitation, Raymond Poincaré hospital, Garches, France
Cite this as
Assaly R, Compagnie S, Allimonnier L, Bracconi M, Giuliano F, et al. (2022) Establishment and validation of a rodent model of endometriosis to evaluate the effect of new therapeutic strategies. J Gynecol Res Obstet 8(3): 036-042. DOI: 10.17352/jgro.000114Copyright License
© 2022 Assaly R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Objectives: Endometriosis is a common disease that affects about 10% - 15% of women in their reproductive years worldwide with no curative treatment. The most common symptom of endometriosis is debilitating pelvic/abdominal pain. Current therapeutic options have limited insight into the disease mechanism and include drugs and/or surgery, which may be ineffective over the long term with unwanted side effects. We aimed at establishing a translational rodent endometriosis model that can be used to identify novel therapies. The validity of the model was confirmed by investigating the effect of the clinically-used GnRH agonist, leuprolide.
Methods: Endometriosis was induced by a surgical procedure in adult non-pregnant female Sprague Dawley rats in the diestrus or estrus stage (cycle determination by vaginal smear). One group of rats received a subcutaneous injection of leuprolide at 1mg/kg, every 4 weeks. Following the treatment period, we performed a direct assessment of the endometriosis-induced abdominal pain using the Von-Frey method and spontaneous pain using the abdominal licking test. Then, the lesions were excised and measured.
Results: Abdominal pain threshold was decreased by more than 2 fold in rats with surgically-induced endometriosis compared to sham rats. Leuprolide treatment significantly increased the threshold force required to elicit a behavioral withdrawal response in rats suffering from endometriosis. The observed pelvic floor mechanical hyperalgesia has not been correlated to the growth of endometriosis lesions. The hormonal cycle at the surgery induction influenced the endometriosis lesions growth. Leuprolide significantly inhibited the growth of endometriosis-like lesions.
Conclusions: we have established, based on previously reported rodent models, a model of endometriosis-associated pain that responds to clinically active drugs and can, therefore, be used to identify novel therapies and investigate some of the pathophysiological mechanisms involved in endometriosis.
Introduction
Endometriosis is the presence of ectopic endometrial tissue outside the uterine cavity and is a common gynecological disease reported in 10% - 15% of women during their reproductive years in the world [1]. The most common symptom of endometriosis is debilitating pelvic/abdominal pain. In fact, up to 70% of women suffering from pelvic pain are affected by endometriosis. Other symptoms include dyspareunia, severe dysmenorrhea, and dysuria. Women with the condition also suffer from co-occurring painful conditions, including interstitial cystitis/painful bladder symptoms and irritable bowel syndrome and 50% of these women suffer from infertility [2].
The disease has a huge negative impact on women’s quality of life, work productivity, sexual relationship and self-esteem [3]. The most common theory proposed to explain the pathophysiology of the endometriosis is retrograde menstruation hypothesis but also other hypotheses have been discussed like inflammatory factors, dysregulated immunity, hormones and genetic and epigenetic factors [4]. None of these mechanisms could explain alone the different types and symptoms of endometriosis. Current therapeutic options have limited insight into the disease mechanisms and include drugs and/or surgery to reduce symptoms and manage complications. These available treatments provide short-term solutions but tend to be ineffective over the long term with a high incidence of unwanted side effects such as premature bone loss and vaginal dryness. The most common medical treatment for endometriosis is gonadotropin-releasing hormone (GnRH) agonists and estrogen/progestin combinations [5,6]. In recent years, the search for more effective and less invasive therapeutic curative strategies to reduce years of suffering in women with endometriosis is receiving increased research attention. The development and validation of In vivo models are necessary to allow the preclinical testing of the efficacy of potential new treatment options.
Non-human primates are considered the most physiologically relevant model of endometriosis since these animals develop spontaneous endometriosis [7]. However, their use is limited by cost and ethical concerns. Thus, rodent models of endometriosis [8] have been developed to investigate the effect of the presence of endometriosis lesions on functional outcomes and the extent of its reversal by new compounds. However, it is difficult to develop a unique model that replicates all symptoms and aspects of a complex disease such as endometriosis. Indeed, endometriosis is a heterogeneous disease with poorly known etiology and several phenotypes such as ovarian endometrioma, superficial peritoneal disease and deep infiltrating endometriosis [4]. In addition, endometriosis is multifactorial and has been associated with environmental, genetic, immunological and hormonal factors [9]. Different models have been developed in mice and rats and recently reviewed by Bruner-Tran, et al. [8], each model emphasizing one or several features to explore disease-related mechanisms, to identify therapeutic targets and/or to provide insight into the development of co-morbidities. Pain is considered a significant contributor to endometriosis morbidity and pelvic pain is one of the most described symptoms [10], even though asymptomatic cases are described [11], this study aimed at establishing a rodent model of endometriosis to be a useful tool in evaluating endometriosis-related pelvic pain. To that end, we developed a rodent model of endometriosis in immunocompetent rats based on surgically-induced endometriosis originally described by Vermon and Wilson [12] to evaluate endometriosis-induced pain by assessing specifically pelvic pain while others extensively evaluated generalized pain behavior. In addition, endometriosis diagnosis is based on the analysis of the lesions collected during laparoscopic surgery [13] and the American Society of Reproductive Medicine classified the disease into 4 stages according to the evaluation of the endometriotic lesions [14]. Thus, this study also described the proliferation of endometriosis lesions being a major feature of endometriosis. Even though the model described in this study is based on previously published studies reviewed by others [8,15], very few authors reported the impact of the estrous stage on endometriosis-induced models in rodents. Hence, this study was designed to investigate if any influence of the estrous stage at induction of endometriosis on both primary endpoints i.e., pelvic pain and endometriosis lesions size. To validate the translational value of this model, we tested the effect of one of the most clinically used medical options, a GnRH agonist on pelvic pain and lesions size.
Materials and methods
Endometriosis-induced surgery and treatment administration
Adult non-pregnant female Sprague Dawley rats (Elevage Janvier, Le Genest-St-Isle, France, 6-8 weeks old) were housed at least 10 days prior to the beginning of the experiments with free access to standard chow (Chow M20, 841201, SDS, UK) and water and maintained on an inversed 12h dark/light cycle (10:00/22:00). All procedures were approved by the local ethical committee (CEE47) and performed in accordance with the legislation on the use of laboratory animals (NIH publication N° 85 - 23, revised 1996) and Animal Care Regulations in force in France as of 1988 (authorization from competent French Ministry of Agriculture - Agreement No. B78-423-1, July 2017).
After the acclimation period, the reproductive status was determined by vaginal lavage using traditional nomenclature for the 4 estrous stages (proestrus, estrus, metestrus and diestrus). After the confirmation of one full cycle by daily vaginal lavage, the rats were identified with a unique identification number; then using a random number table, they were randomly allocated to different experimental groups (n = 12 rats per group): sham group (SHAM), endometriosis group (ENDO) and treated group (ENDO+leuprolide).
When in the diestrus stage, all animals underwent either sham the surgical procedure or endometriosis induction by a surgical procedure based on the initial protocol described by Vernon and Wilson in rats [12] and Cummings and Metcalf [16].
In addition, in order to evaluate the influence of the estrous stage on pelvic pain and lesions size, a group of rats (n = 12) that underwent endometriosis induction when in the proestrus/estrous stage was added to this study.
Whether surgical induction was conducted in the diestrus or proestrus/estrus stage, the rats were anesthetized with isoflurane (1% - 1.2%, Centravet, France) under aseptic conditions and using a heating pad to maintain a body temperature of the animal at 37 °C. A midline incision was made through the skin and muscle layer to expose the pelvic and abdominal organs. A segment of the mid-left uterine horn was excised, six 2x2 pieces of the excised uterus were prepared and each piece was sutured onto alternate mesenteric arteries using 4.0 nylon sutures. The same surgical procedure was applied for the sham group except for the step of suturing uterine pieces. Then, the muscle layer and the skin were closed using polyester suture (Vetsure© Bond) and the animal was closely monitored during recovery. Following surgery, animals were carefully monitored for health status and weighed twice per week. One week after surgery, the rats allocated to the treated group received a single subcutaneous injection of the GnRH agonist, leuprolide at 1mg/kg repeated once on day 28 [17]. All endpoints were reported 6 weeks post-surgical procedure.
Pelvic pain assessments
A vaginal cytology smear was performed to determine the estrous stage at the time of pain assessment.
A widely accepted method to evaluate mechanical hyperalgesia in conscious animals is the Von Frey filaments. This method is based on behavioral observations of animal response to mechanical stimuli of increasing forces applied to the plantar area by means of filaments with grading forces and consists of determining the hind paw withdrawal threshold as a referred pain-associated behavior. In this study, we adapted the Von Frey method to apply to the pelvic area and to determine a pelvic pain nociception threshold. Briefly, a few days before the Von Frey experiments, the rat abdomen was shaved under light isoflurane anesthesia (Centravet, France). On the day of assessment, the rat bladder was emptied by manual compression and the rat was placed in a metabolic cage for a minimum 30-minute acclimatization period before evaluation.
Monofilaments of differing forces ranging from 0.008 to 4g (Bioseb, Vitrolles, France) were applied in increasing order of force and rat behavior was recorded according to a pain behavior rating scale (score 1 to 4). The forces of the von Frey fibers used in this study were determined in a preliminary test based on the modified up-and-down method [18]. Five repeated stimulations per filament with 5 - second intervals were performed, followed by 3-minute intervals before applying the next higher force filament. Care was taken to stimulate different areas within the lower abdominal region to avoid desensitization. The pain threshold was determined by the lower force evoking a pain reaction of the rat characterized by abrupt retraction of the abdomen, jumping, and/or immediate licking of the site of application (score 3 and above).
Also, spontaneous pain was evaluated by the abdominal licking test which is used as an indicator of abdominal discomfort in various pelvic pain models [19,20]. This test was performed in an open-field home cage and consists of observing, after allowing the rat to acclimate to the cage, the number of times the rat licked the abdominal region [21]. The average number of abdominally-directed licking was recorded over two 10 min periods by an experimenter blinded to the groups.
Lesions measurement
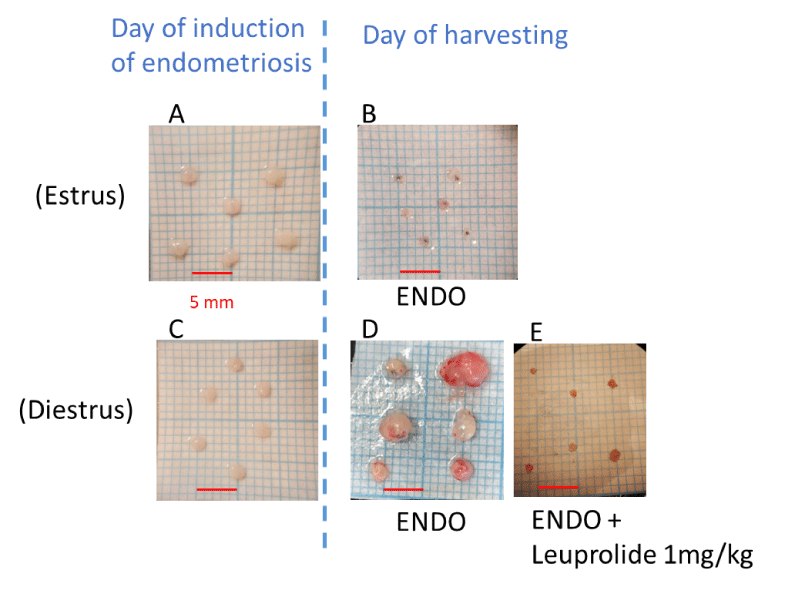
Following behavioral assessments, the rats were anesthetized and then euthanized by an intracardiac injection of pentobarbital (Eutasol®, Centravet, France), the abdominal cavity opened and the lesions measured by an in-house developed method adapted from a described method by Becker, et al. [22]. Briefly, the harvested lesions were aligned on graph paper, and the image was captured and analyzed using Image J software by measuring for each lesion two perpendicular diameters (D1 and D2) and calculating a cross-sectional area (CSA) using the formula for an ellipse: (D1xD2x π/4). Then, the CSA of the lesions harvested from the same animal was averaged to calculate a mean CSA for each animal and finally to compute the average CSA per experimental group for comparison between groups.
Statistical analysis
All results are presented as mean ± SEM: Some rats were excluded if the endometriosis-like lesions were not present at harvesting or if the behavior of the rat during pain evaluation was agitated not allowing assessment. A final n = 10 rats/group was included in the final analysis.
Student’s t-test or one-way ANOVA followed by Dunnett’s multiple comparisons post-test were used when appropriate to compare the means of two groups. Statistical analysis was performed with GraphPad Prism® 6.05 software. p values < 0.05 were considered significant.
Results
Influence of estrous cycle on pelvic pain and lesions size
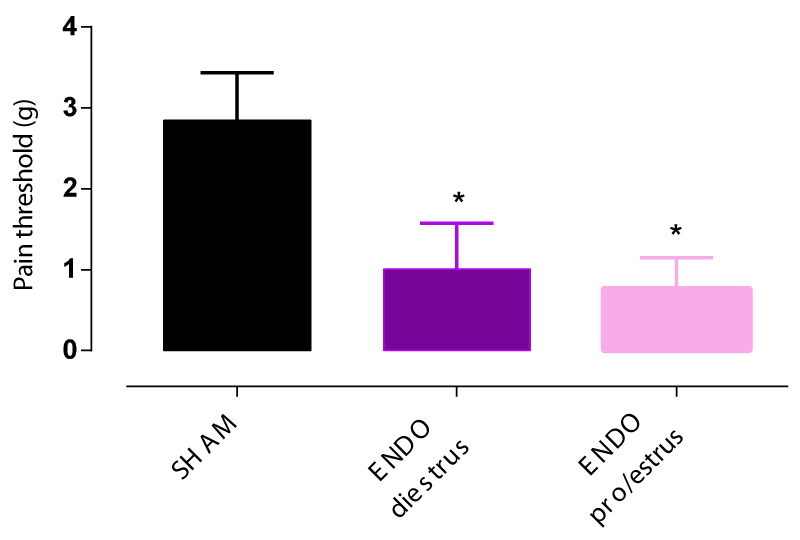
Pelvic pain threshold determined by Von Frey filaments in endometriosis rats significantly decreased by 2 fold compared to the SHAM group 6 weeks post-induction surgery independently of the estrous stage at the time of endometriosis induction (Figure 1).
However, the mean cross-sectional lesion area was significantly larger compared with the mean area of the lesions at the time of the surgical induction of endometriosis (3.8 ± 0.8 mm2 vs. 2.2 ± 0.1 mm2, respectively, Figure 2) when the surgical induction of endometriosis was achieved in diestrus stage but not in proestrus/estrus stage. In fact, when the surgery was conducted in the proestrus/estrus stage, the mean cross-sectional lesion area was smaller compared with the mean area of the lesions at the time of the surgery (1.7 ± 0.2 mm2 vs. 3.0 ± 0.1 mm2, respectively, Figure 2).
Of note, the lesions prepared from the uterus when the rat was in the estrus/proestrus stage were larger than the lesions prepared when the rat was in the diestrus stage.
Consequently, the establishment and validation of the model were continued using rats in the diestrus stage at the induction of endometriosis and presented here.
Body weight evolution
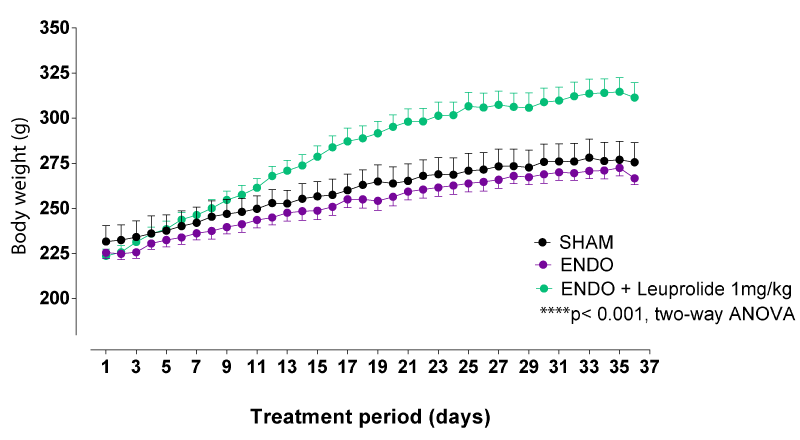
The body weight progressively increased, starting from the first-week post-surgery until the end of the experimental period for all groups of rats. The rats in the ENDO group showed the same body weight evolution compared to sham-operated rats. However, the leuprolide-treated endometriosis rats (ENDO+leuprolide) showed significantly higher body weight evolution than ENDO rats (Figure 3). Indeed, the rats that received leuprolide showed a sharp increase in body weight gain the first 2 weeks after treatment administration compared to the day of starting the treatment (12 ± 1% the first week and the second week) compared to the ENDO group (5 ± 1% the first week and the second week)
Of note, the body weights, before starting the treatment period, were comparable between all groups of rats (232 ± 9 g, 226 ± 3 g, and 224 ± 4 g for SHAM, ENDO and ENDO+leuprolide groups, respectively; One-Way ANOVA, ns, p > 0.05).
Pelvic pain assessments
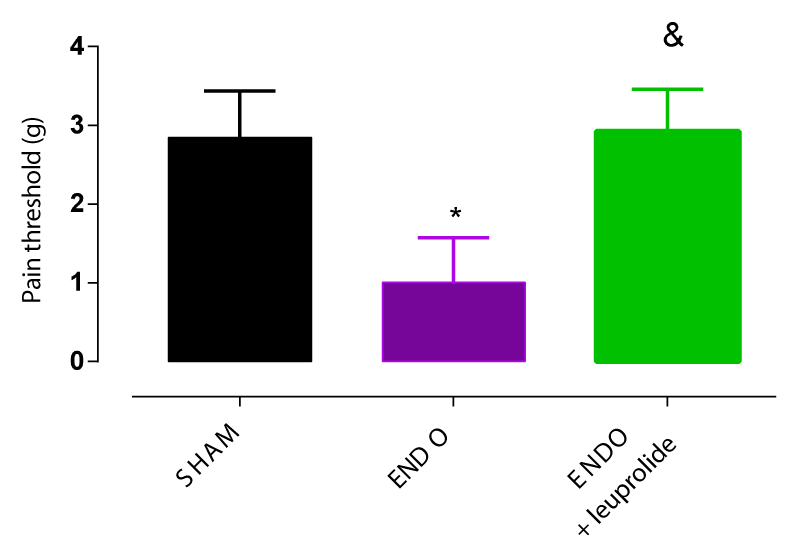
As mentioned earlier, pelvic pain threshold in the ENDO group was significantly decreased by more than 2 fold compared to the SHAM group 6 weeks post-induction surgery (Figure 1). Leuprolide at 1mg/kg administered subcutaneously once every 28 days shifted pain responses towards higher forces indicating that rats in the ENDO+leuprolide group are less sensitive to pain compared to rats from ENDO group. Thus, the GnRH agonist leuprolide decreased abdominal hyperalgesia induced by endometriosis surgery by significantly increasing the pain threshold (Figure 4).
Licking behavior remained almost unchanged in SHAM rats before and after surgery (+5%) while a slight increase in abdominal-directed licking was observed in ENDO rats after surgery (+15%), however, this increase did not reach statistical significance (Student’s t-test, ns, p > 0.05). Consequently, the abdominal-directed licking behavior assessment design should be reconsidered with some modifications to the experimental conditions to allow the evaluation of the effect of potential therapies. Indeed, some authors reported abdominal direct licking behavior in mice and rats using different conditions compared to the ones used in this study such as increasing the number of animals [21], increasing the observation window [19] and taking into account abdominal directed licking when part of the normal grooming behavior [20].
Lesions measurement
The presence of lesions was confirmed in 90% of endometriosis rats (ENDO and ENDO+leuprolide groups). The mean cross-sectional lesion area was significantly larger compared with the mean area of the lesions at the time of the surgical induction of endometriosis (3.8 ± 0.8 mm2 vs. 2.2 ± 0.1 mm2, respectively). Treatment with leuprolide suppressed by 60% the growth of endometriosis-like lesions (p < 0.05, Student’s t-test).
Discussion
This study described a translational rat model of endometriosis-associated pain. The validity of the model was confirmed by investigating the effect of the clinically-used GnRH agonist, leuprolide.
First, this study investigated the potential influence of the hormonal cycle on the growth of endometriosis lesions. Some authors used uterine tissues at the proestrus/estrus phase [12,23] since the endometrium is thicker compared to other stages providing enough tissue for suturing in line with our observations. However, a correlation between the volume of endometriosis lesions and the estrous cycle has been described [24] while others reported that lesion size is independent of the estrous stages [25]. Thus, the first aim of this study was to examine the influence of the estrous stage and to set the stage to be used in subsequent experiments.
Endometriosis is an estrogen-dependent and dynamic disorder. Indeed, endometriosis lesions’ appearance and progression/regression are dependent on hormonal levels. It has been reported that endometriosis lesions growth is triggered by high estrogen levels while low estrogen levels lead to lesions regression [26]. Besides, it is known that estrogen level starts to decrease at late proestrus corresponding to the follicular phase in women that is characterized by the release of luteinizing hormone and follicular-stimulating hormone [27]. The increase in follicular stimulating hormone induces ovulation matching the estrus stage of the cycle in rodents during which the estrogen levels decrease and prolactin levels peak. During the metestrus/diestrus stage, progesterone levels increase, corresponding to the luteal phase in women, followed by a peak in estrogen levels during the late luteal phase, a decline in progesterone levels, and regression of the corpus luteum, leading to menstruation in women. The late luteal phase and the regression of the corpus luteum phase correspond to the diestrus stage in rodents [28,29].
Taken together, it is plausible to suggest that the diestrus stage in rodents is comparable to the menstrual phase in women and the changes in estrogen/progesterone levels during the estrous cycle may influence the endometriosis lesions growth. Accordingly, this study is to our best knowledge among the few studies showing by direct comparison that the estrous cycle significantly affected the size and the macroscopic growth of endometriosis lesions using an autologous endometriosis rat model without ovariectomy. Indeed, significant growth of endometriosis lesions was observed when the preparation of the lesions and the induction of the surgery was done when the rats were in the diestrus stage but not when in the proestrus/estrus stage.
If the main characteristic of endometriosis is the presence of tissue lesions containing stromal, epithelial and inflammatory cells, the main clinical sign of endometriosis is pain, even though asymptomatic endometriosis has been reported [4,6]. Women suffering from endometriosis described different intensities and types of pains ranging from severe dysmenorrhea to chronic pelvic pain accompanied or not with other comorbid pain conditions leading to a significant impact on the quality of life of patients [30]. Recent studies have illustrated that women with endometriosis develop significant sensitivity to any stimuli as a consequence of central sensitization [31]. Thus, the development of new and effective therapies is a research priority that requires the development of appropriate pre-clinical models of pain-associated endometriosis and validated tools for pain assessment which can advance our understanding of this condition of central sensitization. The first publications describing surgically induced rodent models of endometriosis focused mainly on lesion description [12,16,22,32]. More recently, investigators developing rodent models of endometriosis models have given more focus on pain endpoint but not specifically pelvic pain and rather generalized pain by performing paw withdrawal behavioral tests as an indicator of stimulus-evoked pain [21,33,34]. Indeed, only a minority of studies measured pelvic pain [20,35-37]. Thus, we aimed in this study to set up a specific pelvic-pain-associated endometriosis model to allow in the future to understand the related mechanisms. Thus, we investigated both phenotypes of pain, the stimulus evoked pelvic pain response and the spontaneous pain as indicators of chronic intermittent pain reported by patients suffering from endometriosis.
Indeed, this study showed a significant increase in pain perception in rats suffering from endometriosis. Measurement of pain was performed by evaluation of behavioural responses stimulus- dependent using Von Frey filaments. Furthermore, rats suffering from endometriosis showed exacerbated spontaneous pain behavior compared to sham rats, even though the design of this study did not allow to reach a statistical significance in the number of times the rat licked the abdominal region, and thus it needs to be fine-tuned in the future experiments. In fact, very few authors reported both phenotypes of pain in a rodent model. Indeed, pelvic and spontaneous pain was recently reported in a non-surgical mice model [36] but not yet in a rat model of endometriosis.
It is now well established that the association between the severity of pelvic pain and the endometriosis lesions growth is inconsistent [38]. Stratton and Berkley [39] have shown that a major contributing factor to endometriosis-associated pain is not the ectopic lesions growth but rather the development of nerve supply creating a direct interaction between lesions and the central nervous system. Another feature that has been associated with the severity of endometriosis pain is the vascularity of the lesions and the development of new blood vessels. This coordinated growth of blood vessels and nerves has been termed “neuroangiogenesis” [40]. In line with these suggestions, the model described in this study mimics pain-associated endometriosis and the non-correlation between the severity of pelvic pain and the growth of the endometriosis lesions.
The validation of the translational value of this model was confirmed by testing one of the most clinically-used compounds, leuprolide, a GnRH agonist. Subcutaneous leuprolide administered in a dosage regimen similar to its clinical use significantly decreased pain sensation in rats suffering from endometriosis as well as the endometriosis lesions growth. Indeed, in clinical practice, leuprolide and more broadly the GnRH agonist are prescribed as second-line therapy in endometriosis-related pain with a satisfactory result on pain and on decreasing lesions volume when the first-line such as combined oral contraceptives and progestin fail or due to intolerance or contraindications which represent 1/4 to 1/3 of patients [41]. However, the GnRH agonists induce an artificial menopause state and thus add-on therapy is needed to limit some related risks such as premature bone mass density loss. Moreover, this type of treatment can only be prescribed to patients who do not wish to become pregnant [4].
In conclusion, there is a need to develop more effective therapeutic and curative strategies with fewer unwanted effects. To this end, it is necessary to establish a reliable and standardized animal model of endometriosis. In this study, we have established a model of endometriosis-associated pain that responds to clinically active drugs and can, therefore, be used to identify novel therapies. It should be noted, however; no model is expected to mimic all aspects of the women’s disorder and it is probable that the new therapies have to be tested in several models, each replicating some features of the disease to improve the transitional value and to increase the success rate in clinical trials.
This work was supported by a restricted grant from the « Plan France 2030 » National Program.
- Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr Obstet Gynecol Rep. 2017 Mar;6(1):34-41. doi: 10.1007/s13669-017-0187-1. Epub 2017 Jan 27. PMID: 29276652; PMCID: PMC5737931.
- Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010 Aug;27(8):441-7. doi: 10.1007/s10815-010-9436-1. Epub 2010 Jun 25. PMID: 20574791; PMCID: PMC2941592.
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D'Hooghe T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012 May;27(5):1292-9. doi: 10.1093/humrep/des073. Epub 2012 Mar 14. Erratum in: Hum Reprod. 2014 Sep;29(9):2073. PMID: 22422778.
- Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019 Nov;15(11):666-682. doi: 10.1038/s41574-019-0245-z. Epub 2019 Sep 5. PMID: 31488888.
- Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016 Sep;152(3):R63-78. doi: 10.1530/REP-16-0052. Epub 2016 May 10. PMID: 27165051; PMCID: PMC4958554.
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018 Jul 19;4(1):9. doi: 10.1038/s41572-018-0008-5. PMID: 30026507.
- Simitsidellis I, Gibson DA, Saunders PTK. Animal models of endometriosis: Replicating the aetiology and symptoms of the human disorder. Best Pract Res Clin Endocrinol Metab. 2018 Jun;32(3):257-269. doi: 10.1016/j.beem.2018.03.004. Epub 2018 Apr 6. PMID: 29779580.
- Bruner-Tran KL, Mokshagundam S, Herington JL, Ding T, Osteen KG. Rodent Models of Experimental Endometriosis: Identifying Mechanisms of Disease and Therapeutic Targets. Curr Womens Health Rev. 2018 Jun;14(2):173-188. doi: 10.2174/1573404813666170921162041. PMID: 29861705; PMCID: PMC5925870.
- Malvezzi H, Marengo EB, Podgaec S, Piccinato CA. Endometriosis: current challenges in modeling a multifactorial disease of unknown etiology. J Transl Med. 2020 Aug 12;18(1):311. doi: 10.1186/s12967-020-02471-0. PMID: 32787880; PMCID: PMC7425005.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005 Jun 10;308(5728):1587-9. doi: 10.1126/science.1111445. PMID: 15947176.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Aug;51:53-67. doi: 10.1016/j.bpobgyn.2018.01.014. Epub 2018 Feb 15. PMID: 29525437.
- Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985 Nov;44(5):684-94. PMID: 4054348.
- Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005 Oct;20(10):2698-704. doi: 10.1093/humrep/dei135. Epub 2005 Jun 24. PMID: 15980014.
- Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997 May;67(5):817-21. doi: 10.1016/s0015-0282(97)81391-x. PMID: 9130884.
- Greaves E, Critchley HOD, Horne AW, Saunders PTK. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet Gynecol Scand. 2017 Jun;96(6):644-658. doi: 10.1111/aogs.13119. Epub 2017 Apr 5. PMID: 28233896; PMCID: PMC5485163.
- Cummings AM, Metcalf JL. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol. 1995 May-Jun;9(3):233-8. doi: 10.1016/0890-6238(95)00004-t. PMID: 7579907.
- Ercan CM, Kayaalp O, Cengiz M, Keskin U, Yumusak N, Aydogan U, Ide T, Ergun A. Comparison of efficacy of bromocriptine and cabergoline to GnRH agonist in a rat endometriosis model. Arch Gynecol Obstet. 2015 May;291(5):1103-11. doi: 10.1007/s00404-014-3524-x. Epub 2014 Nov 4. PMID: 25367601.
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994 Jul;53(1):55-63. doi: 10.1016/0165-0270(94)90144-9. PMID: 7990513.
- Craft RM, Carlisi VJ, Mattia A, Herman RM, Porreca F. Behavioral characterization of the excitatory and desensitizing effects of intravesical capsaicin and resiniferatoxin in the rat. Pain. 1993 Nov;55(2):205-215. doi: 10.1016/0304-3959(93)90149-J. PMID: 7508591.
- Greaves E, Horne AW, Jerina H, Mikolajczak M, Hilferty L, Mitchell R, Fleetwood-Walker SM, Saunders PT. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci Rep. 2017 Mar 10;7:44169. doi: 10.1038/srep44169. PMID: 28281561; PMCID: PMC5345039.
- McAllister SL, Sinharoy P, Vasu M, Gross ER. Aberrant reactive aldehyde detoxification by aldehyde dehydrogenase-2 influences endometriosis development and pain-associated behaviors. Pain. 2021 Jan;162(1):71-83. doi: 10.1097/j.pain.0000000000001949. PMID: 32541390; PMCID: PMC7718385.
- Becker CM, Rohwer N, Funakoshi T, Cramer T, Bernhardt W, Birsner A, Folkman J, D'Amato RJ. 2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008 Feb;172(2):534-44. doi: 10.2353/ajpath.2008.061244. Epub 2008 Jan 17. PMID: 18202195; PMCID: PMC2312351.
- Rudzitis-Auth J, Körbel C, Scheuer C, Menger MD, Laschke MW. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum Reprod. 2012 Jun;27(6):1735-44. doi: 10.1093/humrep/des095. Epub 2012 Mar 23. PMID: 22447626.
- Uchida M, Kobayashi O. Sequential observation of implanted endometriosis by laparoscopy in rats: correlation between the prevalence rate and the estrous cycle. J Pharmacol Sci. 2013;121(4):299-304. doi: 10.1254/jphs.12180fp. Epub 2013 Mar 29. PMID: 23538674.
- Kiani K, Movahedin M, Malekafzali H, Mirfasihi F, Sadati SN, Moini A, Ostad S, Aflatoonian R. Effect of the estrus cycle stage on the establishment of murine endometriosis lesions. Int J Reprod Biomed. 2018 May;16(5):305-314. PMID: 30027146; PMCID: PMC6046203.
- Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003 Aug;44(2):123-31. doi: 10.1016/s0018-506x(03)00121-1. PMID: 13129484.
- Hawkins SM, Matzuk MM. The menstrual cycle: basic biology. Ann N Y Acad Sci. 2008;1135:10-8. doi: 10.1196/annals.1429.018. PMID: 18574203; PMCID: PMC2913133.
- Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011 Apr;124(3-4):229-36. doi: 10.1016/j.anireprosci.2010.08.030. Epub 2010 Sep 3. PMID: 20869180.
- Rudolph M, Döcke WD, Müller A, Menning A, Röse L, Zollner TM, Gashaw I. Induction of overt menstruation in intact mice. PLoS One. 2012;7(3):e32922. doi: 10.1371/journal.pone.0032922. Epub 2012 Mar 7. PMID: 22412950; PMCID: PMC3296749.
- Maddern J, Grundy L, Castro J, Brierley SM. Pain in Endometriosis. Front Cell Neurosci. 2020 Oct 6;14:590823. doi: 10.3389/fncel.2020.590823. PMID: 33132854; PMCID: PMC7573391.
- McKinnon BD, Bertschi D, Bersinger NA, Mueller MD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. 2015 Jan;26(1):1-10. doi: 10.1016/j.tem.2014.10.003. Epub 2014 Nov 19. PMID: 25465987.
- Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. Am J Physiol Regul Integr Comp Physiol 2008; 294:R162-R171.
- Ge P, Ren J, Harrington AM, Grundy L, Castro J, Brierley SM, Hannig G. Linaclotide treatment reduces endometriosis-associated vaginal hyperalgesia and mechanical allodynia through viscerovisceral cross-talk. Pain. 2019 Nov;160(11):2566-2579. doi: 10.1097/j.pain.0000000000001657. PMID: 31335750.
- Davenport AJ, Neagoe I, Bräuer N, Koch M, Rotgeri A, Nagel J, Laux-Biehlmann A, Machet F, Coelho AM, Boyce S, Carty N, Gemkow MJ, Hess SD, Zollner TM, Fischer OM. Eliapixant is a selective P2X3 receptor antagonist for the treatment of disorders associated with hypersensitive nerve fibers. Sci Rep. 2021 Oct 6;11(1):19877. doi: 10.1038/s41598-021-99177-0. PMID: 34615939; PMCID: PMC8494816.
- Escudero-Lara A, Cabañero D, Maldonado R. Kappa opioid receptor modulation of endometriosis pain in mice. Neuropharmacology. 2021 Sep 1;195:108677. doi: 10.1016/j.neuropharm.2021.108677. Epub 2021 Jun 19. PMID: 34153313.
- Fattori V, Franklin NS, Gonzalez-Cano R, Peterse D, Ghalali A, Madrian E, Verri WA Jr, Andrews N, Woolf CJ, Rogers MS. Nonsurgical mouse model of endometriosis-associated pain that responds to clinically active drugs. Pain. 2020 Jun;161(6):1321-1331. doi: 10.1097/j.pain.0000000000001832. PMID: 32132396.
- Arosh JA, Lee J, Balasubbramanian D, Stanley JA, Long CR, Meagher MW, Osteen KG, Bruner-Tran KL, Burghardt RC, Starzinski-Powitz A, Banu SK. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc Natl Acad Sci U S A. 2015 Aug 4;112(31):9716-21. doi: 10.1073/pnas.1507931112. Epub 2015 Jul 21. PMID: 26199416; PMCID: PMC4534219.
- Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996 Feb;65(2):299-304. PMID: 8566252.
- Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011 May-Jun;17(3):327-46. doi: 10.1093/humupd/dmq050. Epub 2010 Nov 23. PMID: 21106492; PMCID: PMC3072022.
- Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163-82. doi: 10.1146/annurev-physiol-012110-142158. PMID: 21054165.
- Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother. 2018 Jul;19(10):1109-1125. doi: 10.1080/14656566.2018.1494154. Epub 2018 Jul 5. PMID: 29975553.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
