Global Journal of Cancer Therapy
Essentially new inhibitors of metastasis of malignant tumors for chemotherapy-sparing treatment
ISh Nadiradze* and NSh Chigogidze
Cite this as
Nadiradze I, Chigogidze N (2021) Essentially new inhibitors of metastasis of malignant tumors for chemotherapy-sparing treatment. Glob J Cancer Ther 7(1): 016-030. DOI: 10.17352/2581-5407.000038The purpose of this work was
1. Processing a new concept of specific inhibitors for metastasis to mercy cancer chemotherapy for oncology.
2. A chemical synthesis of Multinucleated Anion active Metastasis inhibitors based on processed conception.
3. Trial them in D60p4 cell line culture of atypical fibroblasts of different concentrations.
Georgia has received a patent for the first medicine (Amphicezine) of this class, which does not damage the normal healthy cells in the body. There are presented the synthesis schemes and methods of prospective metastasis inhibitors by the authors. The second-generation works of Multinucleated Anion active Metastasis inhibitors have been completed, there is also discussed and shown multifunctional action on tumor cells, connections between oncodrugs activity and their ligand properties.
Introduction
Most patients with malignant tumors die from metastases, which penetrate the vital organs and are difficult to respond to the definitive therapy. Therefore, the fight against tumor metastasis is the primary task of modern cancer chemotherapy [1-10].
Traditional methods of therapy for cancer patients, despite the constant improvement and increase in the effectiveness of treatment, still do not provide the desired result. It is necessary to have new cognitive approaches to the problems of metastasis and methods of obtaining a therapeutic effect, taking into account all the variety of naturally occurring properties in tumor cells:
1. Modification of the cell surface;
2. Changes in the structure of the membranes of tumor cells;
3. Adsorption on surfaces;
4. Increase in the mobility and adhesiveness of tumor cells;
5. Activation of the production of intracellular enzymes;
6. The course of enzymatic reactions, the work of intracellular ribosomes, and the regulation of the structures of nucleic acids are determined by the nature of the metal-complexing ions and the surrounding conditions;
7. The formation of lipids in malignant cells is significantly reduced, the specificity of phospholipids is gradually lost;
8. For tumor cells it is characterized to contain an abnormally larger number of ATP molecules than in the original normal cells;
9. In malignant cells, there is changed the course of metabolic processes, the intensity of oxidative processes is reduced in them;
10. As bioinorganic chemists, we consider it necessary to pay attention to the unusual stereo chemical specificity of blood plasma in cancer patients, as well as to the fact that the dextrorotatory D-glutamic acid was isolated from tumor cells. It is known that optical antipodes of the same substance indistinguishable in their physicochemical properties differ sharply from each other in their physiological action. For example, the levorotatory form of the medicine Sarcolysin is active in the therapy of certain types of tumors, and the dextrorotatory form is inactive.
The reason for the stereo-specificity of biological action is that living organisms are themselves built of optically active, asymmetric material. Moreover, living organisms are capable of constantly producing asymmetric material, converting optically inactive substances into optically active ones. On our own, we add that the more asymmetric carbon atoms in a molecule of a chemical compound, the higher its biological activity [11].
Let us note, unfortunately, that s lot of interesting facts related to oncological diseases, obtained by scientists of various specialties, have remained unnoticed and unresolved up to the present.
According to the available long-term observations, the process of metastasis of the malignant tumors in the body consists of a complex chain of successive steps:
1. Separation of single tumor cells or their groups from the primary tumor;
2. Transfer of these cells by blood or lymph into the smallest vessels;
3. Fixation of tumor cells in organs;
4. Observing their proliferation with the subsequent autonomous growth of metastasis under the favorable environment
The separation of tumor cells is a continuous process. Fibrin is formed on the plasma membranes of such cells. Yet in 1903, M. Schmidt showed for the first time that tumor cells adhere to the endothelium of the pulmonary capillaries and here they are surrounded by a thin fibrin web.
The adhesion (stickiness) of tumor cells is due to a layer of fibrin covering their surface. Two surface glycoproteins, laminin, and fibronectin are directly involved in tumor cell adhesion. Two surfaces of glycoprotein - laminin and fibronectin - are directly involved in the adhesion of tumor cells. Namely, fibrin occupies the main place in the mechanism of tumor metastasis. Histological studies have shown that the endothelium loses its normal structure when a tumor cell adheres to it in the form of a parietal thrombus [1,3-10].
The interrelation between the endothelium and tumor cells is a central problem in the development of metastases. The cells that adhered to the vascular endothelium sometimes destroy spontaneously or remain inactive for many years. The latter type of cells is called “dormant”. The reason for the reactivation of dormant tumor cells is stress, trauma, and changes in hormonal balance, exposure to carcinogenic substances, and physical agents (various types of radiation). The enzyme hyaluronidase (spreading factor) secreted by tumor cells helps to separate them from the primary tumor node and fix them in other places.
The transformation of a healthy cell into a tumor cell is accompanied by distinct changes in the structure of its surface. Healthy cells form ordered tissue due to their ability to sense the presence of neighboring cells and communicate with them through plasma membranes. However, malignant cells lack a mechanism to regulate cell growth.
Sialic acid (NeuNAc)
Increased amount of sialic acid (N - acetylneuraminic acid) on the surface of tumor cells leads not only to a change in their glycoprotein composition, but also to visible structural changes [12].
Malignant cells are usually hypoxic (completely anaerobic) cells that are resistant to both chemotherapy medicines and radiation. However, there is a group of substances - nitroimidazole, which have a cytostatic effect on them. Besides, and very importantly, these substances make tumor cells sensitive to radiation. In tumor cells, the nitro group is probably reconstructed to the nitroxyl anion radical (NO2-). In healthy (Aerobic) cells this process does not take place, and therefore they are not damaged by nitroimidazoles [12-15].
The group of nitroimidazoles itself may be divided into two subgroups of medicinal products:
I subgroup – 2-nitroimidazole produces, an old medicine of this series
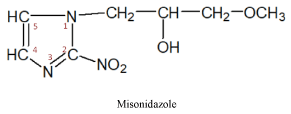
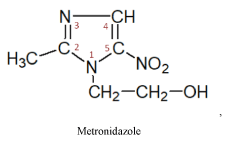
II subgroup – 5-nitroimidazole produces three medicines of this series
These medicines have a radiosensitizing effect on malignant cells and are prescribed to increase their sensitivity to radiation therapy. It must be noted that the 2-nitroimidazole produced medicines -misonidazole, directly exerts a cytostatic effect on tumor cells [12,15].
The era of modern chemotherapy for oncological diseases begins in 1942 [16-18] when they began clinical trials of the nitrogen analog of mustard gas (dichloroethyl sulphide),
Which had a specific cytotoxic effect on lymphoid tissues and showed antitumor activity in lymph-sarcoma in mice [16].
Soon, several derivatives of nitrogen mustard were synthesized, some of which found application as pharmaceutical products. According to the mechanism of action, medicines of this group are considered as alkylating substances. Subsequently, cytotoxic alkylating compounds were obtained of other chemical groups. Over the next six decades up to the present, all discovered and obtained cancer medicines include medical products of various chemical structures and different pharmacological (biological) actions, which can be classified into the following groups [8,13-16].
1. Alkylating substances;
2. Antimetabolites;
3. Synthetic anticancer drugs of different chemical groups;
4. Enzymes applied for the treatment of the oncological diseases;
5. Antitumor antibiotics;
6. Alkaloids, polyphenols, and other substances of plant origin having a cytostatic effect;
7. Interferons and interleukins;
8. Hormonal medicines and their antagonists.
Each cancer patient’s tumor process has individual specificities. Depending on the characteristics of the oncological disease, its course, efficacy, and tolerability of anticancer medicines, the scheme of their use, dose, combination with other medicines, etc. is selected [17]. Chemotherapy is more commonly used after surgery and radiation therapy. To increase the therapeutic efficiency, anticancer medicines are often used as a combination of agents with different mechanisms of action [2].
Antitumor medicines cause various side effects: nausea, vomiting, anorexia, diarrhea, and other effects that interfere with conducting the chemotherapy. The main side effect of most cancer medicines is the suppression of hematopoiesis, which is enhanced by combination therapy. Antitumor antibiotics are cardiotoxic and nephrotoxic, and hormone medicines, their analogs, and antagonists can cause hormonal imbalance. In addition, a characteristic feature of some cancer medicines is their immunosuppressive effect, which weakens the body’s immunity and relieves the development of infectious complications. Therefore, in the last decade, some auxiliary medicines have been produced and released that contribute to increase in the tolerance and effectiveness of the main oncological medicines [15].
The variety of tumors, as well as many chemotherapeutic drugs, with the different mode of action, cause the necessity to use a large number of experimental methods and a wide range of reference tumors in research practice. Despite the real success, chemotherapy for oncological diseases nowadays still faces many unresolved problems:
1. There are no universal medicines for the treatment of many forms of cancer;
2. The tumor acquires resistance to the action of a specific medicine during the treatment;
3. Poor blood circulation in the tumor tissue complicates the delivery of the medicine to the tumor;
4. Most anticancer medicines inhibit the growth of any tissue, therefore, due to their weak specificity, they are not very effective in the case of slowly growing tumors;
5. Such medicines also have a damaging effect on those healthy tissues, which are characterized by rapid cell division, for example, bone marrow.
As a result, a new situation has arisen, when for the synthesis of effective anticancer medicines, a significantly new approach to their creation is required.
To improve the chemotherapy of oncological diseases, it is necessary to make the existing methods of treatment more selective and to find new groups of specific anticancer medicines. The more we know about the subtle differences between the tumor and normal cells, the easier it is to design schemes for the synthesis of chemical compounds having selective effects. We have revised the whole complex of differences in physicochemical factors between the tumor and healthy cells. These differences represent the real targets for the targeted control of tumor cells. The formation of metastases is closely related to the interrelation between the tumor cells and the vascular endothelium at the physicochemical level. Cells of various human tumors differ from the original normal cells in their electrical charge. The normal membrane potential in healthy cells is 90-100 mV, in tumor cells, it decreases to 40 mV. Such a decrease in potential in malignant cells allows them to limit sharply the penetration of foreign substances into them. This greatly complicates the action of antitumor agents. Normal cells can regulate their membrane potential [8,18-22].
An increase in the malignancy of tumor cells is accompanied by an increase in their negative electrical charge. Carcinogenic substances facilitate the increase of the negative charge of cells [8]. Carcinogens can be defined as the substances that promote the formation of tumors, regardless of their mechanism of action.
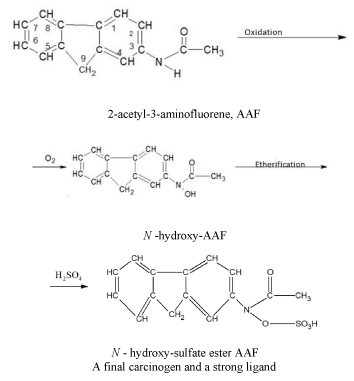
Some carcinogens are capable of acting as ligands (chelating agents) and bind metal ions in aqueous or non-aqueous environment. Other carcinogens, being themselves incapable of coordinating with metal ions, are metabolized into typical ligands. Other carcinogens, being themselves incapable of coordinating with metal ions, which are metabolized into typical ligands. As an example, let us give a scheme for the chemical conversion of an aromatic amide (2-acetyl-3-aminofluorene, AAF) through oxidation to N-hydroxy-sulfuric acid ether AAF, which is a final carcinogen and a strong ligand [18].
A final carcinogen and a strong ligand. It should be noted that the malignant cells compete with the normal cells for essential nutrients, including the metal ions. In addition, tumor cells win this competition for metals since they use stronger ligands than healthy cells [21,23-25].
Antitumor medicines are typical ligands and can form complexes with metal ions in organisms. The antitumor activity of many of these medicines increases if they are injected into the body as part of coordination compounds with metals.
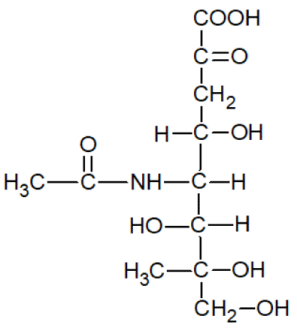
Here are the structural formulas of some known anticancer medicines [15]:
As you can see, all the above-mentioned formulas are typical ligands that can coordinate with metal ions in the body.
Antitumor medicines that are unable to form strong connections with metal ions are metabolically converted in the body into compounds with properties characteristic for ligands.
It should also be suggested that potential anticancer medicines could be sought among ligands as well that form sufficiently strong bonds with the low-charged metal ions.
Let us note that according to Martell’s concept, the carcinogenicity of metal is associated with the degree of its electropositivity [26], i.e. the concept of “ carcinogenicity of a metal “ does not refer to the element as such, but its specific physicochemical state (the oxidation degrees of the complexing ion).
Electropositive metal ions form labile complexes, which are mostly non-carcinogenic. Metal ions with low electropositivity form bonds with donor groups of bioligands and are capable of undergoing only very slow exchange reactions with other ligands in biological systems, which causes the carcinogenic effect of these cations.
Therefore, the use of chelating agents in medicinal products is justified and prospective. Besides, the addition of chelates during the treatment of oncological diseases increases the effectiveness of the action of anticancer chemotherapy medicines.
Based on the above-mentioned, a very important conclusion can be made that the activity of anticancer medicines is associated with their ligand properties or with the ability to transform into effective ligands in the body.
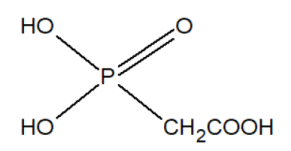
The group of inhibitors of nucleic acid synthesis includes phosphonates. For example, phosphonoacetic acid is capable of inhibiting viral DNA polymerases.
In the last decade, several diphosphonates have been proposed in the world as pharmaceuticals for certain types of tumors with a risk of bone metastases. By regulating membrane penetration, diphosphonates facilitate the transport of anticancer medicines into the cell.
We offer a general scheme for the chemical synthesis of diphosphonates:
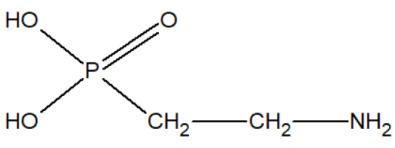
In our opinion, aminoethylphosphonic acid AEF (ciliatin) is of particular interest since it has been found in biological organisms:
Aminoethylphosphonic acid is a white crystalline substance, very well soluble in water and slightly soluble in organic solvents. A very stable chemical substance, not subject to hard hydrolytic conditions, for example, withstands heating for 8 hours at 120ºC in an environment or 48 hours at 150ºC in an environment [27,28]. AEF has been found in anemones from the Sea of Japan and in some mollusks. In the body of Tetrahymena, 15% of all phosphorus is present in the form of AEF.
The chemical synthesis of AEF is carried out in two stages:
Based on aminoethylphosphonic acid, we have developed methods for the synthesis of prospective transport chelating agents.
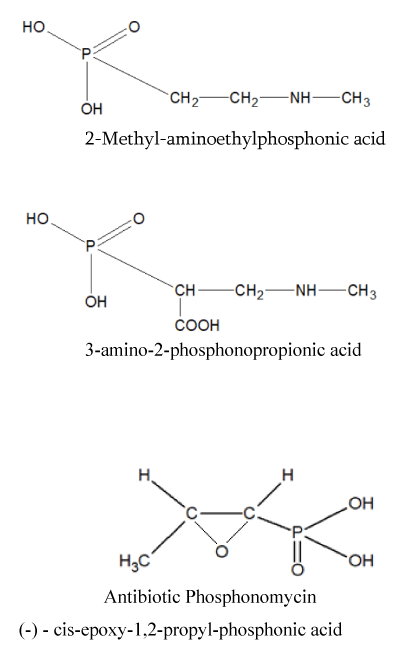
Other phosphonates, rarely found in nature, the synthesis of derivatives of which is of practical interest to us, are:
Since 1964, after the discovery by Rosenberg [24] of the efficacy of simple platinum complexes concerning some tumors, a rapid stream of studies began on the use of metal coordination compounds as antitumor medicines. Coordination compounds of bivalent platinum turned out to be effective, the physiological action of which depends on their structure: only cis-isomers are active, trans-isomers have no antitumor effect. Subsequently, complexes of nickel, palladium, rhodium, and iridium were discovered with similar effects [13,14,29,30].
It should be underlined that the stereochemical features of complex compounds are largely determined by the coordination number of the central complexing atom [25,31,32].
Besides, it should be noted that in recent years the authors have obtained coordination compounds of copper (II) and zinc with some antimycotic and antiprotozoarian drugs. Because of biomedical studies of these complexes, it was found that along with an increase in the antiprotozoarian and antibacterial activity of these compounds, a completely new cytotoxic activity concerning the malignant cells were also observed, which is not typical for the ligands themselves [33-37].
New concept
In connection with the above, it was of interest to approach the fight against metastasis in a complex manner, using the difference in the electrical potentials of the tumor and homologous normal cells. At the turn of 2009-2010, we had an idea, taking into consideration the negative electrical potential of malignant cells, to use negative multi-charged long-chain organic ions as a competitor and blocker of tumor cells. At the same time, it was supposed to synthesize and insert fragments of heparin molecules into macromolecules.
This would increase the sensitivity of tumor cells due to fibrinolytic action, which would prevent the adhesion of metastatic cells to the endothelium. Besides, such macromolecular polyanions compete with tumor cells themselves for metal cations and make them starve for nutrients.
The basis for the creation of a new class of synthetic inhibitors of tumor metastasis, discovered by professors I.Sh. Nadiradze and N.Sh. Chigogidze [38,39], a combination of the principle of competitive starvation of a cancer cell with the principle of multilevel (multilayer) chemotherapy, which involved the launch of cytolytic mechanisms with their selective localization in tumor cells [37,40-42].
This class of synthetic compounds is called multi-charged anionic inhibitors of tumor metastasis (MAITMs) [43,44].
The term “multi-charged” means the presence in the macromolecule of several functional groups –COOH, -OSO3H, -SO3H, -PO(OH)2 and others capable of forming organic salts of strong alkali metals cesium> rubidium> potassium. The hydrophilicity of MAITM macromolecules is caused by the presence of these functional groups. In the biological environment, these substances can dissociate with the formation of long-chain organic anions, as well as cations Cs+ and rubidium Rb+. MAITMs can simultaneously interact with both lipophilic and hydrophilic structures of cells, which determines their biological activity and value.
There are wide opportunities for the synthesis of numerous anionic active macromolecules, the variety of therapeutic properties, which can vary by the spatial structure of the hydrophobic region of the macromolecule, and the combination of functional groups along the length of its hydrophilic part [45,46].
Through the targeted synthesis, MAITMs can be imparted with antiplatelet, anticoagulant, or fibrinolytic properties to prevent tumor cell adhesion. The adhesion of tumor cells to the endothelium is also hindered by the equal negative charge of both.
The strong alkali metals cesium and rubidium are synergistic, i.e. they enhance the mutual action of each other. At the same time, cesium and rubidium [47] belong to the subgroup of potassium, being its analogs. This allows them to penetrate tumor cells, unlike other elements.
MAITMs can be synthesized both in the form of mono salts of cesium or rubidium and in the form of binuclear complex molecules, the structure of which simultaneously includes cesium and rubidium atoms in a certain ratio. The medicines are highly soluble in water, which ensures the high availability of medicines in the biological environment of the body.
As known, water molecules are linked by hydrogen bonds and substances dissolve in water due to their ability to break these bonds and form new bonds with water molecules. Amphiphilic molecules in water are in a state of dynamic equilibrium since their hydrophobic region is continuously displaced by water molecules striving to combine. These properties of water underlie nonspecific adsorption, since amphiphilic substances occupy any surface accessible to them, regardless of its chemical nature [48,49].
Specific adsorption plays a more important role in the action of medicines. It is characteristic of hydrophilic substances that tend to leave the water and settle on a surface that has a chemically complementary character. The simplest example of such complementarity is the attraction of an anion to a positively charged part of the surface, and a cation - to a negatively-charged one. In such cases, the ion will be absorbed more strongly than the non-ionized molecule.
Shifts in the physicochemical conditions of the internal environment of the cell have a significant effect on its subsequent transformation into a tumor.
For normal healthy cells, the value pH=7,35 is optimal. Normally, the pH of the extracellular fluid also lies in the range of 7, 36-7, and 44. The constancy of pH is maintained by the buffer systems of the body [19,22]. The exceptions are the pH values of the microenvironment inside the cells of the gastric glands – 1, 7-2, 0; prostate gland – 4, 5; in osteoblasts - up to 8, 5 [18-20].
Tumor cells are characterized by a more acidic environment with a gradual decrease in the pH value from 7, 2 to 6, 5. At the later stages of cancer, with an increase in the malignancy of tumor cells, their pH can drop to 5, 7-6, 0. The low pH=4, 5 value in the intracellular fluid of the prostate gland suggests the reason for the relatively easy and frequent degeneration of these cells into tumor cells.
Along with nutrition, which is necessary for the construction and growth of cellular structures, such an exchange process as respiration constantly occurs in cells, as a result of which the cell receives the energy it needs [50,51].
Cellular respiration is associated with the use of free oxygen in the air (this type of respiration is called aerobic). The respiration process allows cells to obtain the necessary energy by splitting glucose into simple compounds - water and carbon dioxide [18-20,22,50,51].
Aerobic oxidation of one gram-molecule (180g glucose) C6H12O6 can be represented by the equation:
C6H12O6+6O2 →6Co2↑+6H2O+674 kcal.
As can be seen from the equation, with an aerobic breakdown of g/mol of glucose, a large amount of caloric energy is released - 674 kcal.
A decrease in oxygen consumption by a healthy cell by one third leads to the degeneration of a normal cell into a tumor cell. To maintain its life support in conditions of lack of oxygen, the cell is forced to switch to the splitting of substances under anaerobic conditions. A normal cell returns to evolutionarily more primitive forms of nutrition, growth, and reproduction due to complex intramolecular chemical transformations. The latter is provided by a specific enzyme system. A reborn (tumor) cell becomes anaerobic (hypoxic).
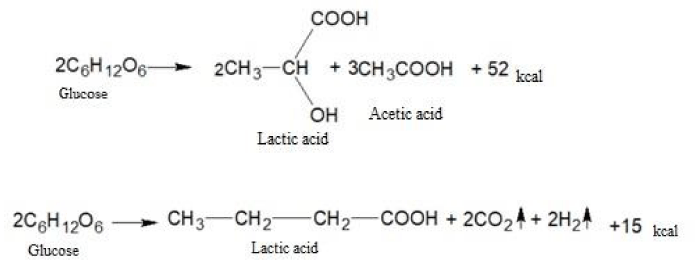
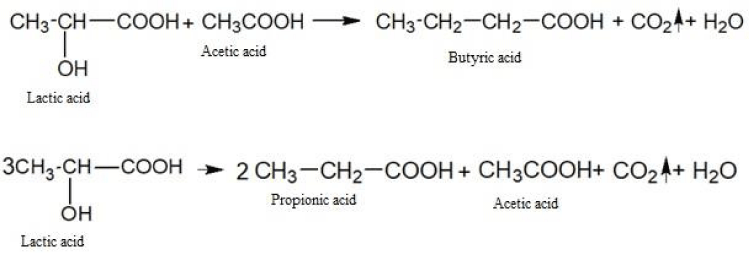
The fermentation breakdown of carbohydrates under anaerobic conditions is a fermentation process. In the process of fermentation, not only water and carbon dioxide are formed, but also a number of more complex substances: lactic, butyric, propionic, and other acids.
The fermentation of glucose in the absence of oxygen leads to the formation of lactic and a number of other acids.
Let us give examples of similar chemical transformations:
As you can see, the amount of energy released during anaerobic respiration is much less than during aerobic respiration. It is this circumstance that leads to the fact that tumor cells are forced to consume huge amounts of glucose and potassium ions to ensure their vital activity. In this case, the produced lactic acid lowers the pH value inside the tumor cells to the acidity and at the same time has a damaging effect on the genetic apparatus. The ability of nucleic acids to control cell division is destroyed and tumor cells can multiply uncontrolled. Besides, lactic acid causes severe local pain and also destroys cellular enzymes necessary for the normal oxygenation process.
Here are some examples of chemical transformations with the participation of lactic acid:
The butyric, propionic, acetic and other acids formed as a result of biochemical transformations also contribute to the acidification of the intracellular environment of cancer cells and metastases.
a) The MAITMs we offer have a bi-directional target effect on malignant cells:Blocking from protective fibrin enveloping of blastoma cells detached from the primary tumor focus;
b) Transport and delivery of alkalizing cations Cs+ and Rb+ into “naked” tumor cells, which leads to the complete death of these cells. Note that healthy cells prefer to absorb cations K+.
This selectivity of MAITMs’ action distinguishes favorably from the traditional chemotherapy medicines used, which are highly cytotoxic for healthy cells as well.
The search for and creation of prospective medicines based on new chemical compounds is a very long and expensive process that requires years of hard work and joint efforts of specialists in various fields. Therefore, molecular modification of existing biologically active substances can become a useful and economically available alternative that can provide more effective medicines. Molecular modification seems to us to be a rather attractive strategy in the development of analogs of cancer medicines with better bioavailability, higher pharmacological activity, and lower toxicity.
The strategy of creating multifunctional chemical compounds (“hybrid” compounds) involves the combination and optimization of two or more pharmacological effects in a new chemical object.
The first representative of a new class of inhibitors of tumor metastasis is the medicine Amphicezin synthesized by us [56]. As a delivery means (carrier) of cations Cs+ and Rb+, we used 2-amino-3-hydroxypropionic acid (hydroxyamino acid serine), which has optical isomers:
Here is a scheme of the chemical synthesis of serine from glycolaldehyde:
Serine is now being produced in large quantities in a more accessible and economical biotechnological way.
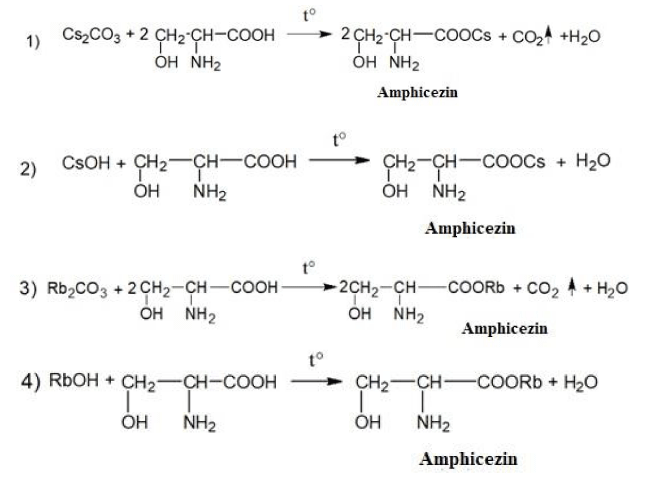
Please find the schemes of the chemical synthesis of the medicine Amphicezin [38,52-60]:
The pharmaceutical composition of the medicine Amphicezin contains 70% of cesium serinate + 30% rubidium serinate. Amphicezin can be taken orally (per os) in the form of tablets and aqueous solutions. Intramuscular and intravenous administration of Amphicezin is unacceptable! Not a toxic medicine, well tolerated by the human body.
Although, strictly speaking, serine is not a long-chain macromolecule, in order to test the very concept of MAITMs, our choice fell exactly on it. This was facilitated by a number of considerations before the first stage of research:
1) the serine residue is involved in the formation of active centers of a number of important enzymes: trypsin, chymotrypsin, thrombin, cholinesterase, liver carboxylesterase, etc., ensuring their function;
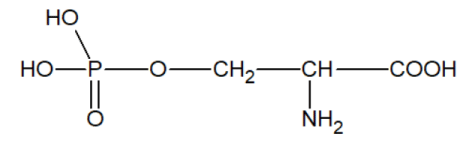
2) the hydroxyl group of the serine residue easily forms hydrogen bonds, which are necessary for maintaining the catalytically active macrostructure of enzymes.
3) phosphoryl serine can be easily obtained from serine
which contains phosphorus bound by a covalent PO bond;
4) serine strengthens the body’s immune system by participating in the production of antibodies, and also stimulates the formation of immune γ-interferon by lymphocytes;
5) serine can act as a chelating agent;
6) participates in the mechanisms of intercellular signal transfer;
7) serine - an essential amino acid for cellular energy production;
8) serine is involved in the formation of cell membranes.
Aims
Further biomedical tests of Amphicezin at various concentrations in the culture of atypical fibroblasts of the D60p4 cell line showed that our choice was justified with a high result. Amphicezin, as the simplest model of MAITMs, has positively proved itself in in vitro studies, which were carried out in the summer of 2018, at the Latvian International Virotherapy Center.
Materials
• Amphicezine
• Cell line D60 p4 (primary dermal atypical fibroblasts)
• FBS superior (Millipore cat. no. S0615)
• DMEM (Merck cat. no. FG 144)
• Penicillin-Streptomycin 10,000U/mL (Millipore cat. no. 15140 -122)
• Trypsin - EDTA (Thermo Fisher Scientific, cat. no. 25050 - 014)
• 10 x PBS (Thermo Fisher Scientific, cat. no. 70013016)
• Costar 24 well plate (Sigma - Aldrich, cat. no. CLS3527, cell cultivation area 1.9cm2)
• 75cm2 tissue culture flasks (Sarstedt cat. no. 83.3911)
• 15ml tubes (Sarstedt cat. no. 62.554.002)
Methods
Thawing
1. D60 p4 cell vial was removed from -80 °C freezer and immediately transferred to 37 °C incubator. When completely thawed, the content of the vial was transferred to a 15ml tube and 14ml warm cell culture medium (DMEM+20% FBS+ 1% penicillin/streptomycin) was slowly added.
2. Cells were centrifuged at 300xg for 5 minutes. The supernatant was discarded, and cells were resuspended in 1ml cell culture medium.
3. Cells were seeded onto 75cm2 cell culture flasks with total of 10ml of cell culture medium (DMEM+20% FBS+ 1% penicillin/streptomycin). Growth conditions: 37 °C, 5% CO2.
Cultivation
1. Cells where cultivated until confluent monolayer (>90%) was microscopically observed.
2. Cell culture medium was removed, and cells were washed once with 5ml of 1xPBS.
3. 5ml of pre-warmed Trypsin - EDTA solution was added to the flask with a gentle rock of the flask to get complete coverage of the cell layer. Cells were incubated for 5 min, 37 °C, 5% CO2.
4. Cell detachment reaction was stopped by adding 2 volumes (twice the volume used for the dissociation reagent) of pre-warmed DMEM+20% FBS+ 1% penicillin/streptomycin.
5. Cells were centrifuged at 300 x g for 5 minutes.
6. Cells were resuspended in 1ml of pre-warmed complete growth medium, and the total number of cells were determined using a hemocytometer.
Treatment of cells
10,000 cells were seeded (DMEM+20% FBS+ 1% penicillin/streptomycin) per 1cm2 in a 24 well plate. After 24h cultivation (to ensure complete cell attachment to plate surface) cell medium was removed and new (DMEM+10% FBS+ 1% penicillin/streptomycin) was added. Four concentrations of Amphicezine were added: 1%, 2.5%, 5% and 10% (v/v) of the total volume of the medium. An equal volume of DMEM with PBS (as diluent) medium was added to the control cells. Each test group was run in triplicate (n=3). Cell proliferation was monitored for 96 hours with a live cell imaging system (Cell-IQ®). Phase contrast microscopy images after 0, 3, 6, 12, 24, 48, 72 and 96 hours of cell culturing were collected. The inhibitory effect of Amphicezine was calculated at the end of culturing time (96h), using the formula: Inhibition (%) =100-(100xA/B), where A stands for cell number with Amphicezine at the end of culturing time, and B is control (control with PBS) the cell number at the end of the culturing time.
Results and discussion
The results of the tests of Amphicezin showed that its cytolytic activity on tumor cells depends on the doses used and the time of contact with malignant cells. The maximum effect of inhibition of the growth of atypical cells (93.5%) was observed in comparison with the control (healthy cells) during a cultivation time of 96h at a medicine concentration of 0.237%.
Currently, we have completed work on the second generation of MAITMs. As new carriers of strong alkali Cs+ and Rb+cations, we offer a very prospective class of nitrogen-containing substances - amidines, which exhibit high multifunctional biological.
Here is a two-step scheme for the chemical synthesis of amidines:
1) obtaining acid imidchlorides
2) obtaining amidines
In the second generation of MAITMs, we use thrombin enzyme inhibitors and antiplatelet agents containing a free amidine end-group.
We offer specific examples of carriers of Cs+ и Rb+ cations of the second generation of MAITMs:
I. Thrombin enzyme inhibitors
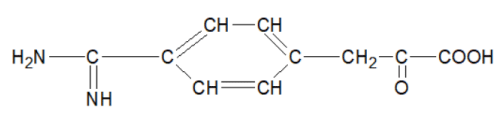
4-amidinophenylpyruvic acid
II. Synthetic antiplatelet agent
The latter substance can exhibit antibacterial activity.
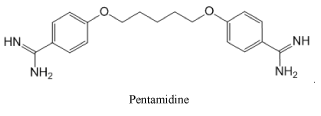
Representing the class of amidines, we consider it necessary to show the strongest antimicrobial medication Pentamidine used for the treatment of African trypanosomiasis, leishmaniasis, babesiosis, as well as for the prevention and treatment of Pneumocystis pneumonia in people with HIV infection:
Conclusions
1) In the final part of the article, we will consider the significant advantages of MAITMs over other anticancer medicines:Increasing the selectivity and specificity of the action of new inhibitors of malignant cells.
2) Reducing the toxicity of these chemotherapy medicines.
3) The possibility of their use in low concentrations for preventive purposes for a long time.
4) Good compatibility with other anticancer medicines and therapy methods.
5) Practically unlimited possibilities of their modification and chemical synthesis.
Accessibility for various segments of the population around the world.
- Balitsky K, Vorontsova A, Lisnyak I (1991) Tumor Metastasis: Pathogenic Aspects. Kiev: Naukova Dumka.
- Gershanovich M (1982) Complications during Chemotherapy and Hormone Therapy of Malignant Tumors. Moskow: Medicine.
- Andreenko G (1979) Fibrinolysis. Moscow: State University Publishing House.
- Zubairov DM (1978) Biochemistry of Blood Coagulation. Moscow: Medicine.
- Chazov EI, Lakin KM (1977) Anticoagulants and Fibrinolytic Agents. Moscow: Medicine.
- Gaffney PJ, Balkuv-Ulyutina S (Eds.) (1982) Fibrinolysis: Modern Fundamental and Clinical Concepts. Moscow: Medicine
- Gavrilov OK (Eds.) (1981) Problems and Hypotheses in the Studies of Blood Coagulation. Moscow: Medicine.
- Wolf M, Ransberger K (1976) Enzyme Therapy. Moscow: Mir.
- Nadiradze ISh (2000) Machabeli Syndrome in Oncology. Tbilisi: Chronograph.
- Nadiradze IS (1982) Intravascular Blood Coagulation in Patients with Malignant Neoplasms. Surgery 131-134.
- Potapov VM (1976) Stereochemistry. Moscow: Chemistry.
- Pomogailo AD, Uflyand IE (1991) Macromolecular Metal Chelates. Moscow: Chemistry.
- Mokrushin VS, Vavilov GA (2018) Fundamentals of Chemistry and Technology of Bioorganic and Synthetic Drugs. St. Petersburg, Science Avenue.
- Tyukavkina NA, Baukov YuI (1991) Bioorganic Chemistry. Moscow: Medicine.
- Albert A (1989) Selective Toxicity. The Physico-Chemical Bases of Therapy (Vols.1-2). Moscow: Medicine.
- Vardanyan RS (2004) Synthesis of Essential Drugs. Moscow: Medical Information Agency.
- Shelley K, Eckhardt Sh, Nemeth L (1975) Medicinal Treatment of Tumor Diseases. Budapest: Publishing House of the Hungarian Academy of Sciences.
- Nikolaev AYa (1989) Biological Chemistry. Moscow: Higher School.
- Ovchinnikov YuA (1987) Bioorganic Chemistry. Moscow: Education.
- Mashkovsky MD (2005) Drugs. (15 ed.) Moscow: New Wave.
- McAuliffe C (Eds.) (1978) Techniques and Topics in Bioinorganic Chemistry. Moscow: Mir.
- Yatsimirsky KB (Eds.) (1979) Biological Aspects of Coordination Chemistry. Kiev: Naukova Dumka.
- Hughes M (1983) The Inorganic Chemistry of Biological Processes. Moscow: Mir.
- Boldyrev AA (1986) Biological Membranes and Ion Transport. Moscow: Science
- Basolo F, Johnson R (1996) Coordination Chemistry. Moscow: Mir.
- Dyatlova NM, Temkina VYa, Popov KI (1988) Complexones and Complexonates of Metals. Moscow: Chemistry.
- Corbridge D (1982) Phosphorus: Chemistry, Biochemistry and Technology. Moscow: Mir. Griffith, E., Biton, A., Spenser, J., & Mitchell, D.(Eds). (1977) Fosfor v okruzhayushchei srede (Phosphorus in Environment). Moscow: Mir.
- Los K (1963) Synthetic Poisons. Moscow: Foreign Languages Publishing House.
- Granik VG (2001) Fundamentals of Medicinal Chemistry. Moscow: University Book.
- Spitsyna VI, Martynenko LI (Eds.) (1979) Coordination Chemistry of Rare Earth Elements. Moscow: Moscow State University.
- Ryles A, Smith K, Ward R (1983) Essential Organic Chemistry. Moscow: Mir.
- Tsivtsivadze T, Machkhoshvili R, Chigogidze N, Kldiashvili R, Skhiladze R, et al. (2012) Synthesis, Structure and Properties of Anti-Cancer Biocompatible Compounds with Plant Alkaloids. Proceedings of the Academy of Sciences of Georgia. Chemical series. 38: 317-324.
- Chigogidze N, Japaridze R (2015) Synthesis of New, Promising Compounds for Chemotherapy of Oncological Diseases. International Scientific-Practical Conference, Innovative Technologies for the Production of Functional Use Food Products. Kutaisi.
- Chigogidze NSh, Tsivtsivadze TI, Nadiradze ISh, Kldiashvili RSh, Petriashvili ZhD, et al. (2016) Antitumor Activity of Copper Complexes with Clotrimazole as a Result of the Formation of a Specific pentacoordinated structure. Georgian Engineering News. 77.
- Chigogidze NSh, Tsivtsivadze TI, Nadiradze ISh, Petriashvili ZhD, Japaridze RJ (2016) Influence of Specific Conformational Structure on Enhancing the Cytotoxic Activity of Clotrimazole Biocomplexes. Georgian Engineering News 78: 112-119.
- Tsivtsivadze T, Petriashvili Zh, Japaridze R, Chigogidze N, Kldiashvili R (2017) Complexes of Some Biometals with the Antifungal Medication Clotrimazole. Tbilisi: GTU.
- Chigogidze NSh, Petriashvili JD, Japaridze RJ (2015) Searching and synthesis of new chelating derivatives of the thiourea as antitumor remedies. 3Rd International conference on Pharmaceutical sciences. Looking towards the future, honoring the past. Tbilisi, Georgia.
- Nadiradze I, Chigogidze N (2019) Georgia Patent No. P2019 7005B.
- Nadiradze I, Chigogidze N (2019) Negative Multi-charged Long-chain Organic Ions as Inhibitors of Metastasis in Cancer Therapy. Certificate of Deposit 6811.
- Nadiradze Ish, Chigogidze NSh (2019) Amphicezin – in principle new inhibitor of malignant tumors metastasis. International Clinical Conference, Modern Approaches of diagnostics and Treatment. Batumi, Georgia.
- Nadiradze I, Chigogidze N (2016) Development of a New Class of Metastasis Inhibitors for Chemotherapy of Oncological Patients. Karozi 30-32.
- Nadiradze I, Chigogidze N (2015) Real Prospects for Cancer Defeat. Karozi 26-28.
- Chigogidze N (2019) Properties of Tumor Cells and a New Approach to Chemotherapy of Tumors. Ojakhis Mkurnali 32-35.
- Nadiradze I, Chigogidze N (2019) An Essentially New Inhibitor of Postoperative Malignancy of Malignant Tumors. Ojakhis Mkurnali. 8-12.
- Siegel HM (Eds) (1982) Metal Ions in Biological Systems. Moscow: Mir.
- Williams D (1975) The Metals of Life. Moscow: Mir.
- Ripan R, Chetyanu I (1971) Inorganic Chemistry. Moscow: Mir 1.
- Adamson AW (1979) Physical Chemistry of Surfaces. Moscow: Mir.
- Friedrichsberg DA (1984) Course of Colloid Chemistry course. Leningrad: Chemistry.
- Goodman M, Morehouse F (1977) Organic Molecules in Action. Moscow: Mir.
- Hauptmann S, Grefe Yu, Remane X (1979) Organic Chemistry. Moscow: Chemistry.
- Nesmeyanov AN, Nesmeyanov NA (1969) Beginnings of Organic Chemistry. Moscow: Chemistry 1.
- Nesmeyanov AN, Nesmeyanov NA (1970) Beginnings of Organic Chemistry. Moscow: Chemistry 2.
- Charlot G (1969) Methods of Analytical Chemistry. Part Two. Quantitative Analysis of Inorganic Compounds. Moscow: Chemistry.
- Poludek-Fabini R, Beirich T (1981) Organic Analysis. Leningrad: Chemistry.
- Gorelov IP (Eds.) (1984) Chemistry of Surfactants and Chelators. Kalinin: KSU.
- Fayngold S, Kuusk A, Kiik H (1984) Chemistry of Anionic and Amphoteric Nitrogen-containing Surfactants. Tallinn: Valgus.
- Abramzon AA (1981) Surfactants. Leningrad: Chemistry.
- Abramzon AA, Zaychenko LP, Fayngold SI (1988) Surfactants. Leningrad: Chemistry.
- Abramzon AA, Shchukin ED (Eds.) (1984) Surface Phenomena and Surfactants. Book of Reference. Leningrad: Chemistry.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


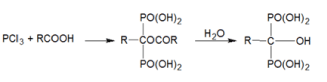

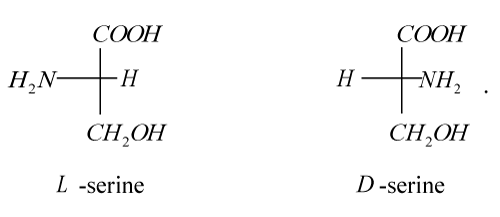


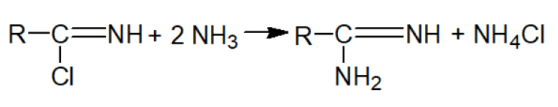

 Save to Mendeley
Save to Mendeley
