International Journal of Pharmaceutical Sciences and Developmental Research
Efficacy of a new Nutraceutical Formulation in preventing acute intestinal inflammation: New therapeutic opportunities for the treatment of diverticulitis?
Silvia D’Alessio1, Arianna Vanelli2*, Stefania Murzilli2, Ilaria D’Augello2 and Silvio Danese3,4
2Research and Development, Nutrilinea SRL, part of Biofarma Group, Italy
3Department of Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele, Milan, Italy
4University Vita-Salute San Raffaele, Milan, Italy
Cite this as
D’Alessio S, Vanelli A, Murzilli S, D’Augello I, Danese S (2022) Efficacy of a new Nutraceutical Formulation in preventing acute intestinal inflammation: New therapeutic opportunities for the treatment of diverticulitis? Int J Pharm Sci Dev Res 8(1): 032-037. DOI: 10.17352/ijpsdr.000040Copyright License
© 2022 D’Alessio S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Acute diverticulitis is a painful, relatively sudden condition, characterized by the presence of low-grade inflammation in the colonic mucosa. Recent clinical trials supported the use of nutraceutical compounds in the treatment of patients with gastrointestinal disorders, including diverticulitis. To verify the hypothesis that nutraceutical ingredients my prevent diverticulitis development, we tested a combination of different natural active supplements, in a mouse model of acute intestinal inflammation. A nutraceutical formulation was administered daily in a prevention setting, by intrarectal enema. Inflammation severity was monitored using a Disease Activity Index (DAI) score, histological and endoscopic analysis, and quantification of Fecal Calprotectin (FC).
Results showed that a combination of cranberry juice, pomegranate extract fruit in ellagic acid and inulin Fibruline®, prevented the development of experimental acute intestinal inflammation. This was confirmed endoscopically, in terms of reduced colon thickness and granularity of mucosal surface. Results were also confirmed histologically, not only by significant reduction of ulcerations, compared to control groups, but also by significant prevention of flogosis and inflammatory infiltration. Nutraceutical ingredients were also able to significantly reduce FC levels, confirming the anti-inflammatory properties of these dietary supplements.
Our study supports the potential use of nutraceutical ingredients as a novel therapeutic approach to prevent acute diverticulitis.
Introduction
Diverticulosis is one of the most common conditions in Western countries, and its incidence is increasing worldwide [1]. About one-fourth of patients with diverticulosis will develop the Symptomatic Uncomplicated Diverticular Disease (SUDD), and about 20% - 25% of them will ultimately develop diverticulitis [2]. Acute diverticulitis is a serious and potentially life-threatening condition, characterized by the presence of low-grade inflammation in the mucosa [3]. Recent studies have confirmed the presence of microscopic colitis on human tissue specimens from diverticular disease, with the degree of inflammatory infiltration correlated with disease severity [4]. At the molecular level, increased amounts of matrix metalloproteinases and pro-inflammatory cytokines have been identified [5,6]. Inflammation persistence may also be a risk factor for acute diverticulitis recurrence, thus it is of major importance to prevent the inflammatory process in patients with diverticular diseases.
Recently, clinical studies showed that the use of nutraceutical compounds might play a role in the treatment of patients with gastrointestinal disorders, by reducing the inflammatory response and intestinal permeability [7]. In particular, a new association of natural active ingredients containing Boswellia serrata, inulin, niacin, cranberry, vitamin B, zinc, and folic acid (DIVER-100®) is well tolerated and effective in obtaining remission and symptom relief in patients with SUDD [8]. However, further studies are needed to confirm these data. To address the hypothesis that nutraceutical compounds may prevent the inflammatory process and diverticulitis development, we evaluated a prevention protocol using a combination of different natural active ingredients, in a mouse model of acute intestinal inflammation [9].
Materials and methods
Mice
Authorization to develop this study (1144/2020-PR (prot. DC8BD.226) has been obtained by the Italian Ministry of Health. Regular use of environmental enrichment has been made in order to decrease stress in animals. Mice used in this study were female C57BL/6N (Charles River Laboratories) and were maintained under pathogen-free conditions.
Induction of acute intestinal inflammation and treatments
To date, no validated models of acute diverticulitis are available and the current protocols require either a significant amount of time to develop the disease, present a relatively low success rate, or seriously deteriorate the animals' systemic health [10]. For this reason, and since microscopic colitis has been observed in biopsies from patients with diverticular disease [4], acute intestinal inflammation was induced by Dextran Sodium Sulfate (DSS) in 8-weeks-old C57Bl/6N mice, as this mouse strain is highly susceptible to DSS treatment, and closely resembles human colitis [11,12]. Animals (n = 8 per group) have been subjected to 1 oral cycle of 2.5% (weight/volume) DSS (molecular mass, 40 kDa; MP Biomedicals), characterized by 7 days of DSS exposure in drinking water followed by 3 days of filter-purified water. Starting from Day 0 (prevention protocol) and throughout the entire experiment, a nutraceutical formulation containing concentrated Cranberry juice (0,080 mg/day per mouse), Pomegranate extract fruit in ellagic acid (0,089 mg/day per mouse), and Inulin Fibruline® (0,159 mg/day per mouse) has been administered daily by intrarectal enema, upon anesthesia with 2% isoflurane. This dose range has been chosen on the basis of previous daily intake of the same ingredients in human and animal studies [8,13,14] and related to mouse weight. The control mice groups received water without DSS or DSS + vehicle enema (sterile water).
On day 10, mice were anesthetized and subjected to endoscopy, to confirm the inflammatory grade, as previously described [12] and stool have been collected to quantify Fecal calprotectin, a marker of mucosal inflammation representing leukocyte accumulation [15,16]. Next, mice have been euthanized by carbon dioxide, and colons were excised and collected to measure colon length and to perform histological analysis.
Evaluation of acute intestinal inflammation
DAI score: Inflammation severity was monitored using a disease activity index (DAI) score, based on daily evaluation of body weight, stool consistency and presence of blood in the stools; grading of intestinal inflammation, as previously described [12,17]. Grading of intestinal inflammation was confirmed also histologically on 2-μM paraffin-embedded colon sections stained with hematoxylin (Dako) and eosin (Diapath). A blinded pathologist evaluated the degree of inflammatory cell infiltration and mucosal damage, according to the RACHMILEWITZ score [18].
Endoscopic score: The experimental endoscopy setup denoted “Coloview system”, consisted of a miniature endoscope (scope 1.9 mm outer diameter), a xenon light source, a triple chip camera, and an air pump (all from Karl Storz, Tuttlingen, Germany) to achieve regulated inflation of the mouse colon. The endoscopic procedure has been viewed on a color monitor and a modified murine endoscopic index score of colitis severity has been assigned, as previously described [12].
Fecal calprotectin quantification
Freshly produced stools were collected on day 10, weighed, and immediately frozen at -80 °C for subsequent analysis. Fecal calprotectin concentration was determined using the S100A9/calprotectin, mouse, enzyme-linked immunosorbent assay (ELISA) kit [Hycult Biotech], according to the manufacturer’s instructions. Briefly, fecal sample aliquots were suspended in an extraction buffer, suspensions were thoroughly vortexed, filtered through a 100-μm cell strainer, and then incubated on a shaker on ice for 20 minutes. The homogenates were centrifuged for 20 minutes at 10,000 g at 4 °C. The upper portion of the supernatants was pipetted off and used for the quantification of calprotectin. Values have been expressed as mg/g of stool.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software). Data are presented as means ±SD or ± SEM and differences were considered statistically significant when p < 0.05. For experiments including more groups, a one-way or two-way ANOVA multivariate analysis has been performed accompanied by a post-hoc modification test.
Results
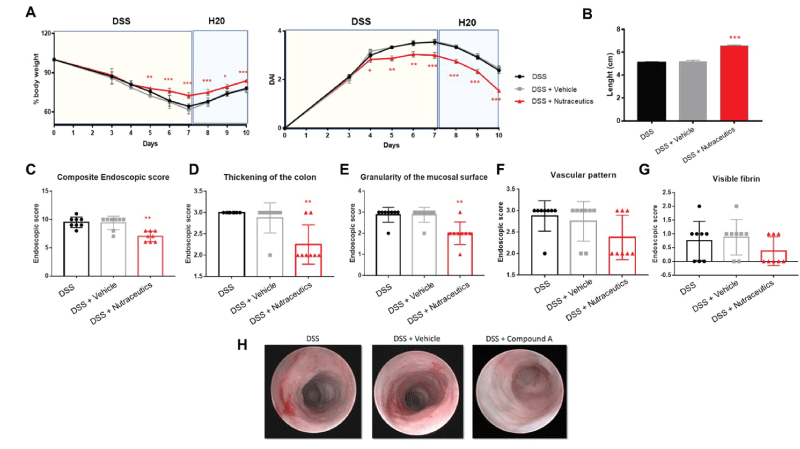
Results showed that active natural containing concentrated cranberry juice, pomegranate extract fruit in ellagic acid, and inulin Fibruline® significantly inhibited DSS-induced acute intestinal inflammation, in terms of percentage of body weight loss (Figure 1A, left panel), reduced DAI (Figure 1A, right panel), and colon length (Figure 1B). No differences were observed between DSS and DSS + vehicle.
As an additional clinical parameter of inflammation, on the day of sacrifice mice were anesthetized and subjected to endoscopy, to evaluate the inflammatory grade. Endoscopic score confirmed that these nutraceuticals can significantly inhibit experimental acute intestinal inflammation when compared to DSS or DSS + vehicle, in terms of composite endoscopic score (Figure 1C). Interestingly, they were more efficient in reducing the colon thickness (Figure 1D and H), and the granularity of the mucosal surface (Figure 1E and H), whereas no effects were observed on the vascular pattern (Figure 1F and H) and fibrin deposition (Figure 1G and H).
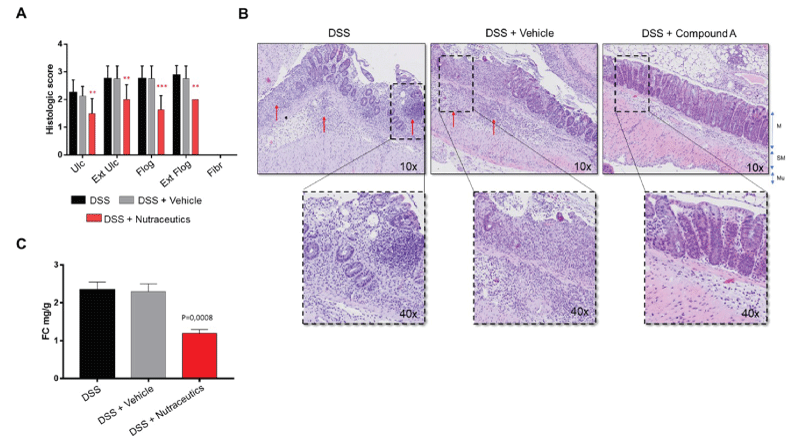
The anti-inflammatory effects of this formulation in DSS-induced acute intestinal inflammation were confirmed histologically by a blinded pathologist. According to the RACHMILEWITZ score [18], results show that this formulation was able to significantly inhibit experimental colon inflammation in comparison with the control group (DSS + vehicle). This was confirmed at all levels (ulceration, extension of ulcerations, flogosis, and extension of flogosis) (Figure 2A). Of note, in the DSS-induced model of acute intestinal inflammation, there is usually no development of fibrosis, and for this reason, no values are represented in the graph.
As expected, representative images of inflammatory conditions per group showed that in DSS-induced colitic mice (e.g. DSS and DSS + vehicle) layer stratification (mucosa, submucosa, and musculari propria) was not maintained, and a massive inflammatory infiltrate completely occupied both mucosa and submucosa, and the crypt was severely damaged in most parts of the colon. The epithelium was eroded indicating the presence of ulcerations (Figure 2B). Upon treatment with nutraceuticals, tissue layers were maintained, with only a slight infiltration of inflammatory cells within mucosa and submucosa, and both epithelium and crypts remained well structured.
We next determined the faecal calprotectin (FC) concentration, as a marker of mucosal inflammation representing leukocyte accumulation in the stool [15,19]. Results showed that these nutraceutical ingredients were able to reduce FC levels, expressed as mg/g of stool (Figure 2C), confirming the anti-inflammatory properties of these dietary supplements.
Discussion
Diverticular disease is represented by a large spectrum of manifestations varying from the presence of persistent abdominal symptoms with low-grade inflammation (symptomatic uncomplicated diverticular disease [SUDD]) to a symptomatic inflammatory process, which leads to acute diverticulitis [3]. Acute diverticulitis is a serious and potentially life-threatening condition and is considered the most common cause of colorectal perforation [3]. Fiber, non-absorbable antibiotics, and probiotics seem to be effective in treating symptomatic and uncomplicated patients [20] unfortunately, the medical strategy to prevent diverticulitis is still limited.
We here evaluate the beneficial effects of a combined formulation of nutraceutical ingredients in an experimental model of acute intestinal inflammation. Since the presence of microscopic colitis has been demonstrated on tissue biopsies from human diverticular disease, with the degree of inflammatory infiltration apparently correlated with the severity of the condition [4], we decided to use the dextran sodium sulfate (DSS)-induced mouse model of acute intestinal inflammation [21]. Oral administration of DSS to mice via drinking water induces severe inflammation characterized by weight loss, bloody diarrhea, ulcer formation, and infiltrations of neutrophils [21]. This model has been used to investigate not only the effects of the intestinal microbiome but also of dietary factors affecting the development of intestinal inflammation [22,23].
The development of appropriate animal models is essential to studying the diverticular disease. To date, is very difficult to obtain animal models which resemble completely this disease condition.
A new animal model of disease with features most similar to human diverticulitis has been described by Guo and colleagues in 2019 [24]. The successful creation of this swine model may impact the understanding of the pathogenesis of diverticula.
As for our animal model, the initial disease condition is characterized by the induction of mucosal damage by high inflammation. This may significate because in our model we are able to see only the initial development of the pathology, thus leading to the hypothesis that our treatment is beneficial in the very early stages of the disease progress.
Thus, we suggest undertaking a future study exploring the potential efficacy of our treatment in a more complex disease model, such as the one previously indicated.
Our findings indicate that a combination of cranberry juice, pomegranate extract fruit in ellagic acid, and inulin Fibruline®, may prevent experimental acute intestinal inflammation and possibly diverticulitis. This was confirmed endoscopically in treated mice upon DSS administration, particularly in terms of reduced colon thickness and granularity of mucosal surface; increased mucosal granularity and thickness represent edema and small erosions, whereas edema of the lamina propria is characterized by clusters of neutrophils and capillaries. This data indicates that these nutraceutical compounds may help reduce neutrophil infiltration and mucosal edema.
The beneficial effects of cranberry juice, pomegranate extract fruit, and inulin Fibruline® on preventing acute DSS-induced inflammation were confirmed histologically, not only by a significant reduction of ulcerations, compared to control groups, but also by significant prevention of flogosis. Flogosis represents the presence of inflammatory infiltrate and edema, whereas ulceration represent epithelial damage, thus suggesting that nutraceutical ingredients may impact on both recruitment of inflammatory cells and epithelial barrier integrity.
The relevance of diet in gastrointestinal disease prevention has long been recognized [25]. Dietary supplements, including ingredients from botanic extracts, have become a fast-growing market [25]; as an example Psyllium, a commonly used soluble dietary fiber, has shown beneficial effects in patients with colonic diverticula [26]. Similarly, Quercetin, a polyphenol widely distributed in many fruits and plants, has been shown to prevent diverticular disease [27]. Very recently, a multi-compounds nutraceutical formulation named Enteroflegin® has shown significant efficacy in inducing remission, symptom relief and reduction of faecal calprotectin in patients with SUDD [28].
In our study we used a nutraceutical formulation containing concentrated cranberry juice, pomegranate extract fruit in ellagic acid and inulin Fibruline®. Cranberry juice and inulin have already shown to induce remission in patients with diverticular disease [8]. In particular, cranberry constituents, such as the proanthocyanidins, flavonols, and hydroxycinnamic acids, displayed antimicrobial properties, acting against various pathogens (e.g. Helicobacter pylori and extraintestinal pathogenic Escherichia coli) by preventing bacterial adhesion, decreasing biofilm formation and/or reducing inflammation rather than via bactericidal activity [29]. Similarly, inulin, which is a nondigestible food ingredient fermented in the colon, was shown to exert its beneficial effects in the gut by modulating the intestinal microbiota [30].
On the other side, pomegranate’s metabolites were demonstrated to enhance gut barrier integrity [31], to ameliorates colitis and to lower inflammatory markers, with a potential mediating role of gut microbiome [32].
Conclusion
There is growing interest in the use of nutraceutical compounds to manage gastrointestinal disorders, including diverticular diseases. Our findings suggest that a combination of concentrated cranberry juice, pomegranate extract fruit and inulin Fibruline® may significantly prevent the acute low-grade intestinal inflammation characterizing acute diverticulitis, possibly impacting on recruitment of inflammatory cells and epithelial damage; this could lead to reduction of neutrophil infiltration and mucosal edema, ultimately leading to prevention of diverticulitis. Our study supports the potential use of nutraceutical ingredients as a novel therapeutic approach to prevent acute diverticulitis; however, there is still a long way to go before putting nutraceuticals as considerable alternative or complementation of conventional drugs, and more efforts should be made to confirm these preliminary data.
Fundings
This study was sponsored by Nutrilinea srl.
Conflict of interest
SDA has served as a speaker, consultant, and advisory board member for Schering Plough, Abbott (AbbVie) Laboratories, Merck and Co, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alfa Wasserman, Genentech, Grunenthal, Pfizer, AstraZeneca, Novo Nordisk, Vifor, and Johnson and Johnson.
SD has served as a consultant for Ferring.
AV, SM and IDA are Nutrilinea srl employees and have no conflict of interest to declare.
Author contribution
SD: Conceptualization and writing original draft; SD: data search and acquisition; SD: bioinformatics and statistical analysis; SD, AV, SM, IDA and SDA: review and editing; AV, SM, and IDA: supervision, resources, and funding acquisition.
- Tursi A, Elisei W. Role of Inflammation in the Pathogenesis of Diverticular Disease. Mediators Inflamm. 2019 Mar 14;2019:8328490. doi: 10.1155/2019/8328490. PMID: 31001067; PMCID: PMC6437747.
- Tursi A, Papa A, Danese S. Review article: the pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment Pharmacol Ther. 2015 Sep;42(6):664-84. doi: 10.1111/apt.13322. Epub 2015 Jul 22. PMID: 26202723.
- Ceresoli M, Lo Bianco G, Gianotti L, Nespoli L. Inflammation management in acute diverticulitis: current perspectives. J Inflamm Res. 2018 May 30;11:239-246. doi: 10.2147/JIR.S142990. PMID: 29881303; PMCID: PMC5985778.
- Tursi A, Brandimarte G, Elisei W, Inchingolo CD, Aiello F. Epithelial cell proliferation of the colonic mucosa in different degrees of colonic diverticular disease. J Clin Gastroenterol. 2006 Apr;40(4):306-11. doi: 10.1097/01.mcg.0000210093.54425.72. PMID: 16633102.
- Rosemar A, Ivarsson ML, Börjesson L, Holmdahl L. Increased concentration of tissue-degrading matrix metalloproteinases and their inhibitor in complicated diverticular disease. Scand J Gastroenterol. 2007 Feb;42(2):215-20. doi: 10.1080/00365520600960104. PMID: 17327941.
- Tursi A, Elisei W, Brandimarte G, Giorgetti GM, Inchingolo CD, Nenna R, Picchio M, Giorgio F, Ierardi E. Mucosal expression of basic fibroblastic growth factor, Syndecan 1 and tumor necrosis factor-alpha in diverticular disease of the colon: a case-control study. Neurogastroenterol Motil. 2012 Sep;24(9):836-e396. doi: 10.1111/j.1365-2982.2012.01946.x. Epub 2012 Jun 11. PMID: 22680042.
- Larussa T, Imeneo M, Luzza F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J Gastroenterol. 2017 Apr 14;23(14):2483-2492. doi: 10.3748/wjg.v23.i14.2483. PMID: 28465632; PMCID: PMC5394511.
- D'Amico F, Fiorini G, Tursi A, Saracino IM, Pavoni M, Danese S, Vaira D. Efficacy of a New Nutraceutical Formulation in Patients with Symptomatic Uncomplicated Diverticular Disease (SUDD): a Prospective Observational Study. J Gastrointestin Liver Dis. 2019 Dec 19;28(suppl. 4):49-52. doi: 10.15403/jgld-560. PMID: 31930222.
- Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017 Sep 7;23(33):6016-6029. doi: 10.3748/wjg.v23.i33.6016. PMID: 28970718; PMCID: PMC5597494.
- Patel B, Guo X, Noblet J, Chambers S, Kassab GS. Animal Models of Diverticulosis: Review and Recommendations. Dig Dis Sci. 2018 Jun;63(6):1409-1418. doi: 10.1007/s10620-018-5071-y. Epub 2018 Apr 20. PMID: 29679297.
- Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017 Jul;12(7):1295-1309. doi: 10.1038/nprot.2017.044. Epub 2017 Jun 1. PMID: 28569761.
- D'Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, Fiocchi C, Danese S. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest. 2014 Sep;124(9):3863-78. doi: 10.1172/JCI72189. Epub 2014 Aug 8. PMID: 25105363; PMCID: PMC4151217.
- Parisio C, Lucarini E, Micheli L, Toti A, Khatib M, Mulinacci N, Calosi L, Bani D, Di Cesare Mannelli L, Ghelardini C. Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions. Int J Mol Sci. 2020 Jun 17;21(12):4304. doi: 10.3390/ijms21124304. PMID: 32560291; PMCID: PMC7353021.
- Guerrero-Solano JA, Jaramillo-Morales OA, Velázquez-González C, De la O-Arciniega M, Castañeda-Ovando A, Betanzos-Cabrera G, Bautista M. Pomegranate as a Potential Alternative of Pain Management: A Review. Plants (Basel). 2020 Mar 30;9(4):419. doi: 10.3390/plants9040419. PMID: 32235455; PMCID: PMC7238014.
- Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008 Jan;103(1):162-9. doi: 10.1111/j.1572-0241.2007.01556.x. Epub 2007 Oct 4. PMID: 17916108.
- Sekiya S, Murata M, Arai S, Murayama H, Kawasaki A, Ashida N, Okada K, Ikemoto M. Enzyme-linked immunosorbent assay for S100A9 in the stool of rats with dextran sulfate sodium-induced colitis. J Immunol Methods. 2016 Dec;439:44-49. doi: 10.1016/j.jim.2016.09.009. Epub 2016 Sep 28. PMID: 27693389.
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993 Aug;69(2):238-49. PMID: 8350599.
- Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, Lee J, Leoni LM, Raz E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002 May;122(5):1428-41. doi: 10.1053/gast.2002.32994. PMID: 11984528.
- Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE. Calprotectin: from biomarker to biological function. Gut. 2021 Oct;70(10):1978-1988. doi: 10.1136/gutjnl-2021-324855. Epub 2021 Jun 18. PMID: 34145045; PMCID: PMC8458070.
- Elisei W, Tursi A. Recent advances in the treatment of colonic diverticular disease and prevention of acute diverticulitis. Ann Gastroenterol. 2016 Jan-Mar;29(1):24-32. PMID: 26752946; PMCID: PMC4700842.
- Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014 Feb 4;104:15.25.1-15.25.14. doi: 10.1002/0471142735.im1525s104. PMID: 24510619; PMCID: PMC3980572.
- Choi EK, Aring L, Das NK, Solanki S, Inohara N, Iwase S, Samuelson LC, Shah YM, Seo YA. Impact of dietary manganese on experimental colitis in mice. FASEB J. 2020 Feb;34(2):2929-2943. doi: 10.1096/fj.201902396R. Epub 2019 Dec 29. PMID: 31908045; PMCID: PMC8103308.
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011 May 27;145(5):745-57. doi: 10.1016/j.cell.2011.04.022. Epub 2011 May 12. PMID: 21565393; PMCID: PMC3140910.
- Guo X, Patel B, Han L, Al-Dulaimi H, Van Alstine WG, Noblet JN, Chambers S, Kassab GS. Novel swine model of colonic diverticulosis. Am J Physiol Gastrointest Liver Physiol. 2019 Jul 1;317(1):G51-G56. doi: 10.1152/ajpgi.00408.2018. Epub 2019 May 15. PMID: 31091148.
- Gao X, Liu J, Li L, Liu W, Sun M. A Brief Review of Nutraceutical Ingredients in Gastrointestinal Disorders: Evidence and Suggestions. Int J Mol Sci. 2020 Mar 6;21(5):1822. doi: 10.3390/ijms21051822. PMID: 32155799; PMCID: PMC7084955.
- Carabotti M, Annibale B, Severi C, Lahner E. Role of Fiber in Symptomatic Uncomplicated Diverticular Disease: A Systematic Review. Nutrients. 2017 Feb 20;9(2):161. doi: 10.3390/nu9020161. PMID: 28230737; PMCID: PMC5331592.
- Sierżant K, Orda J, Korzeniowska M. Effect of dietary supplementation with extracts of rosemary, olive leaves, pine bark and quercetin on selected performance indices of broiler chickens and microbiological status of their ileum. Med Weter. 2019; 75: 6143–2019.
- Brandimarte G, Frajese GV, Bargiggia S, Castellani D, Cocco A, Colucci R, Evangelista E, Gravina AG, Napoletano D, Nardi E, Maisto T, Morabito A, Pianese G, Romano A, Sacco R, Sediari L, Sinnona N, Tifi L, D'Avino A, Elisei W, Tursi A. Performance of a multicompounds nutraceutical formulation in patients with symptomatic uncomplicated diverticular disease. Minerva Gastroenterol (Torino). 2022 Jun;68(2):216-222. doi: 10.23736/S2724-5985.22.03132-1. Epub 2022 Mar 8. PMID: 35262307.
- Blumberg JB, Basu A, Krueger CG, Lila MA, Neto CC, Novotny JA, Reed JD, Rodriguez-Mateos A, Toner CD. Impact of Cranberries on Gut Microbiota and Cardiometabolic Health: Proceedings of the Cranberry Health Research Conference 2015. Adv Nutr. 2016 Jul 15;7(4):759S-70S. doi: 10.3945/an.116.012583. PMID: 27422512; PMCID: PMC4942875.
- Akram W, Garud N, Joshi R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov Ther. 2019;13(1):1-8. doi: 10.5582/ddt.2019.01000. PMID: 30880316.
- Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, Hiwale AA, Saiyed T, Patel P, Vijay-Kumar M, Langille MGI, Douglas GM, Cheng X, Rouchka EC, Waigel SJ, Dryden GW, Alatassi H, Zhang HG, Haribabu B, Vemula PK, Jala VR. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019 Jan 9;10(1):89. doi: 10.1038/s41467-018-07859-7. PMID: 30626868; PMCID: PMC6327034.
- Yang J, Germano PM, Oh S, Wang S, Wang J, Lee R, Paige H, Yang S, Henning SM, Zhong J, Jacobs JP, Li Z. Pomegranate Extract Improves Colitis in IL-10 Knockout Mice Fed a High Fat High Sucrose Diet. Mol Nutr Food Res. 2022 Mar;66(5):e2100730. doi: 10.1002/mnfr.202100730. Epub 2022 Jan 5. PMID: 34932869.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
