Open Journal of Plant Science
Effect of tryptophan and glutamic acid on morphological traits of Iranian and Afghan saffron
Naseer Mokhles1*, Azizollah Kheiry2*, Mohsen Sani Khani2 and Dawlat Sha Poyesh3
2Associated Professor, Horticultural Department, College of Agriculture, University of Zanjan, Iran
3Associated Professor, Horticultural Department, College of Agriculture, University of Bamyan, Afghanistan
Azizollah Kheiry, Associated Professor, Horticultural Department, College of Agriculture, University of Zanjan, Iran, E-mail: kheiry@znu.ac.ir
Cite this as
Mokhles N, Kheiry A, Khani MS, Poyesh DS (2023) Effect of tryptophan and glutamic acid on morphological traits of Iranian and Afghan saffron. Open J Plant Sci 8(1): 020-026. DOI: 10.17352/ojps.000053Copyright
© 2023 Mokhles N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.In order to investigate the effect of amino acids tryptophan and glutamic acid on the morphological traits of the saffron medicinal plant, a factorial experiment was conducted in the form of a randomized complete block design in three replications in 2018 in the research farm of Zanjan University. The experimental treatments include three genotypes (Iranian, Afghani 1, and Afghani 2) as the main treatment and tryptophan amino acid at two levels (1 and 2 mM) and glutamic acid at two levels (1 and 2 mM) as secondary treatments. They were considered as testify. The results showed that tryptophan and glutamic acid treatments had a significant effect (p ≤ 0.01) on most of the studied traits including the number of flowers, dry weight of flowers, and vegetative body. The highest content of the number of flowers and dry weight of flowers were observed, respectively, 34.6 and 37.36 mg of dry weight. Also, the performance characteristics of the fresh weight of the flower and the dry weight of the stigma showed a significant difference (p ≤ 0.05) under the treatment of two amino acids. In total, different levels of tryptophan and glutamic acid can have an effective role in improving the morphological traits and production of this product. The use of 1 mM glutamic acid to produce the maximum vegetative body of the plant, the treatment of 1 and 2 mM of both amino acids to improve morphological indicators, and 1 mM of glutamic acid for the performance of secondary metabolites is desirable and recommended for the purpose.
Introduction
Saffron with the scientific name Crocus sativus is a perennial plant belonging to the Iridaceae family. The Crocus genus has about 85 - 100 species and its global distribution is mainly in Mediterranean Europe and Western Asia. The entire range of this genus is located in the longitude of 10 degrees west to 80 degrees east and latitude of 30 - 50 degrees north. In terms of plant geography, most of the species in the Mediterranean floristic region spread to the east in the Iranian Turani region (Mathew, 1999). The origin of this plant is unknown. The probable center of origin of this plant is Asia Minor (Greece) or the Middle East (Iran) [1]. Currently, saffron is cultivated more or less in Iran, Spain, India, Greece, Morocco, Italy, Afghanistan, Turkey, France, Switzerland, Israel, Pakistan, Azerbaijan, China, Egypt, United Arab Emirates, and Japan. It has been introduced to countries such as Australia, New Zealand, the United States of America, Argentina, and Chile (Fernandez, 2004). Saffron stigma is a rich source of active chemicals related to primary and secondary metabolites, which is very remarkable due to the antioxidant effects of these chemical compounds. Among them, the most important compounds that play a role in the sensory quality of saffron are crocin, picrocrocin, and safranal [2]. Saffron has been considered in various foods due to its unique color, taste, and aroma (Ahmed, et al. 2021). This plant is not only used as a spice but because of its therapeutic potential, it has long been known as a medicinal plant (José Bagur, et al. 2018). All bioactive chemicals in saffron have anti-cancer, antioxidant, anti-depressant, and anti-tumor properties and reduce insomnia and anxiety (Imran, et al. 2018) [3]. Phytochemical compounds in saffron include vitamins (A, B1, B2, B6, and C) (Antonio & Maggi., 2017) [4], minerals (calcium, magnesium, iron, and phosphorus) and potassium) [5,6], carotenoids (beta-carotene, alpha-carotene, crostin and crocin) [7], monoterpenes (safranal and picrocrocin) [8,9], and isofrons (Li & Wu., 2002). Many studies have been conducted to evaluate the phenotypic and genotypic diversity of saffron among the samples collected from different growing regions [10] (Grilli, et al. 2001; Izadpanah, et al. 2015). This research shows phenotypic differences in terms of flower and leaf characteristics such as pistil weight, petal shape, number of spots, and leaf length. Also, significant differences in quantitative and qualitative traits have been reported by measuring flower and leaf characteristics along with the three main metabolites crocin, picrocrocin, and safranal between different oxygenates grown in different regions of Iran (Kalantari, et al. 2012; Izadpanah, et al. 2014) [11], Plant growth regulators are biologically active small signaling molecules, of organic nature, effective at very low concentrations and vital in controlling plant physiological processes [12]. The external application of these hormones and/or their precursors improves the growth and performance of plants by changing the level of internal hormones [12] (Zahir, et al. 2005). Amino acids are essential for plant growth and development [13] and are one of the most effective metabolites in plants. The presence of amino acids affects the physical and chemical properties of plant cells, tissues, and organs (Bashir, et al. 2018). Since tryptophan amino acid is an essential amino acid for the synthesis of proteins, it plays the main role in all living organisms from bacteria and fungi to plants and animals. In addition, tryptophan is involved in the synthesis of many essential compounds [14]. In plants, tryptophan plays a direct role in the regulation of plant growth by providing precursors for several pathways of indole acetic acid hormone synthesis that controls several physiological processes (Woodward & Bartel., 2005). Saremi, et al. 2022 reported that the highest amount of ascorbic acid (2.24 mg/g fresh weight of leaves) and alkaloid (25.42%) was obtained in the treatment of 8 mg/l tryptophan in the plant of the behind-the-scenes doll. The concentration of 100 ppm tryptophan significantly improved the parameters of plant height, number of branches, plant fresh and dry weight, seed yield, and phytochemical traits (the content of fatty acids and caffeic acid derivatives) in nettle plants [15]. The positive effects of the external application of tryptophan on the improvement of growth and functional characteristics of Abu Jahl watermelon [16] and marigold (Fouad, et al. 2022) have been reported.
Glutamic acid is an essential amino acid in plant physiology that plays a pivotal role in various metabolic processes, including nitrogen absorption pathways [17]. The external treatment of glutamic acid in the seedling stage improves the functional characteristics of the plant such as biomass, lipids, and the level of soluble protein and starch, and helps the natural process of plant strengthening [18] (Barros). Galvao, et al. 2017). Amin et al. found that foliar application of glutamic acid significantly improved plant growth, onion yield, and quality, as well as soluble sugars with an increase of 200 mg/liter of glutamic acid (Amin, et al. 2011). The external application of glutamic acid at a concentration of 10 mM improved the activity of antioxidant enzymes in sorghum plants [19]. Considering the economic importance of saffron, as well as the wide genetic diversity of the genus Crocus, as well as the important role of amino acids in improving the growth and phytochemical traits of plants, the necessity of investigating this research to determine the superior genotype in terms of the production of medicinal metabolites and the response of the genotype It describes the use of amino acids.
Materials and methods
In order to investigate the effect of tryptophan and glutamic acid on the morphological and phytochemical traits of experimental Iranian and Afghan saffron genotypes in a factorial manner: the first factor (Iranian genotype, Afghan genotype 1 and Afghan genotype 2) and the second factor (amino acids tryptophan(204 and 408 milligrams) and glutamic acid(147 and 294 milligrams) in concentrations of one and two mM) in the form of randomized complete block design in three replications in the research farm of Zanjan University with latitude 35 degrees and 25 minutes and longitude 47 and 10 minutes and It was grown at an average temperature of 18 degrees Celsius in August, approximate height of 1663 meters above sea level. Revenue implementation. Before planting, saffron seeds with the same weight and size were treated by immersion method with amino acid glutamic acid and tryptophan at two levels each (1 and 2 mM). To sample the soil of the test site from a depth of 0 - 30 cm, it was transferred to the soil science laboratory of Zanjan University to determine the physical, chemical, and texture characteristics of the soil. The physical and chemical properties of farm soil are listed in Table 1. Preparing the farmland, plowing and disking operations, leveling the land, removing stones and clods, weeds, and plotting were done. Planting distances included plot length and width of 2 x 5 meters, distance between rows 50 cm, distance between tubers 20 cm, and planting depth of tubers between 15 and 20 cm.
In each plot, 10 rows with a length of 2 meters were planted with a density of 100 tubers with three replications and a total of 45 experimental units. The first irrigation was done after cultivation. Then the cell-breaking operation took place. Irrigation was done in two stages during the growing season. Of course, according to the climate of the region, the second irrigation was done after collecting the flowers. Weed control and weeding were done manually. No chemical pesticides or herbicides were used during the experiment. The appearance of saffron flowers occurred on the first of November and flowering continued for 30 - 50 days after the appearance of the first flowers. The flowers are collected daily (between 6 am and 8 am), counted, and transported to the laboratory to measure the wet and dry weight of the stigma, and the flower head part of the stigmas is separated, and then the stigmas are placed in the oven (at a temperature of 40 degrees Celsius for 24 hours) dry and their dry weight was weighed with a sensitive scale with an accuracy of 0.0001 g. To calculate the length of the flower and stigma, the flowers harvested from each repetition were randomly selected daily and measured in centimeters using calipers. And finally, the average length of flowers and stigmas during the flowering period was considered as the average length of flowers and stigmas for each plot.
Results and discussion
Morphological traits
According to the results presented in Table 2, it was found that the genotype factor for the traits of number of flowers (p < 0.01), fresh weight of flowers (p < 0.05), dry weight of flowers (p < 0.05) and dry weight of stigma (p < 0.01) was statistically significant. On the other hand, amino acids treatment was significant only for the number of flowers and stigma dry weight traits (p < 0.05). The results of the analysis of variance of genotype interaction effects in amino acids showed that except for the flower number trait (p < 0.01), the rest of the measured traits did not show significant differences (Table 2).
Number of flowers
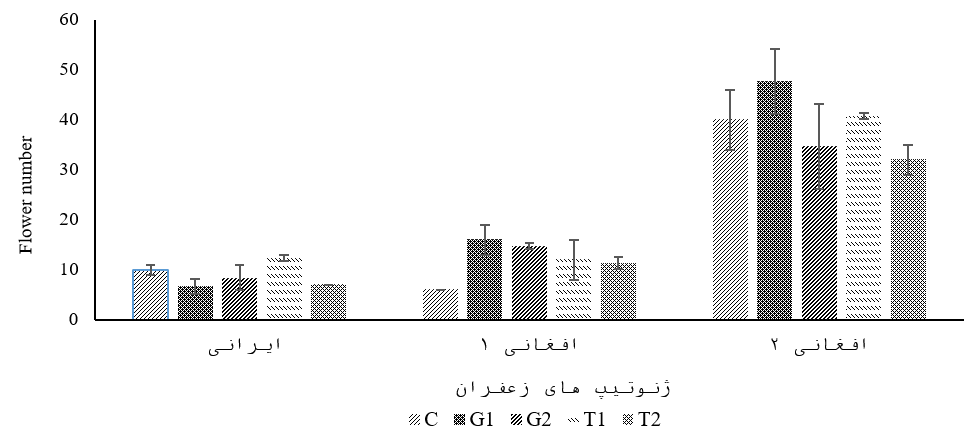
The results obtained from the analysis of the variance table (Table 2) showed that the trait of flower number was affected by the mutual effects of genotype and amino acids. According to the results of comparing the average of mutual effects, the highest number of flowers in Afghani genotype 2 was obtained in the treatment of 1 mM glutamic acid (47.6 flower number). According to the data presented in (Figure 1), in the Iranian genotype, only one mM tryptophan treatment increased the number of flowers compared to the control sample. While in Afghani genotype 1, all treatments showed an increase in the number of flowers compared to the control, and the highest number of flowers was obtained in the 1 mM glutamic acid treatment. In Afghani, genotype 2, only one mM glutamic treatment increased the number of flowers.
Flower fresh weight
Based on the results of the analysis of variance (Table 2), showed a completely significant difference in the trait of flower fresh weight in different genotypes. A low percentage of variation and a high test error indicates the insignificant effect of the environment on this trait (Figures 2,3). The results of the average comparison between genotypes indicated that Afghani genotype 1 with a weight of (295.53) mg per plot row was the best genotype in terms of this trait. Afghan 2 and Iranian genotypes with lower weight in each row of plots were placed in the second group. Glutamic acid and tryptophan treatments significantly affect the fresh weight of saffron flowers. It increased the fresh weight of flowers compared to the control. So that the highest flower fresh weight (291.52) mg was observed due to the treatment of glutamic acid and 2 mM tryptophan. Glutamic acid and tryptophan level treatments of 1 mM showed the lowest fresh weight of flowers in the control treatment. This research, comparing the average, showed that the amino acid glutamic acid and tryptophan 2 mM had the greatest effect, which caused an increase in the fresh weight of the flower and plant yield. It has been reported in many studies that foliar spraying with amino acids increases the growth and development of plants [20].
Dry weight of the flower
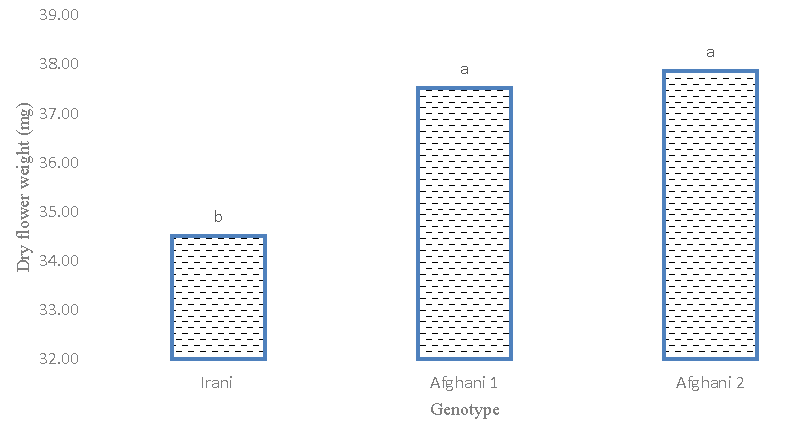
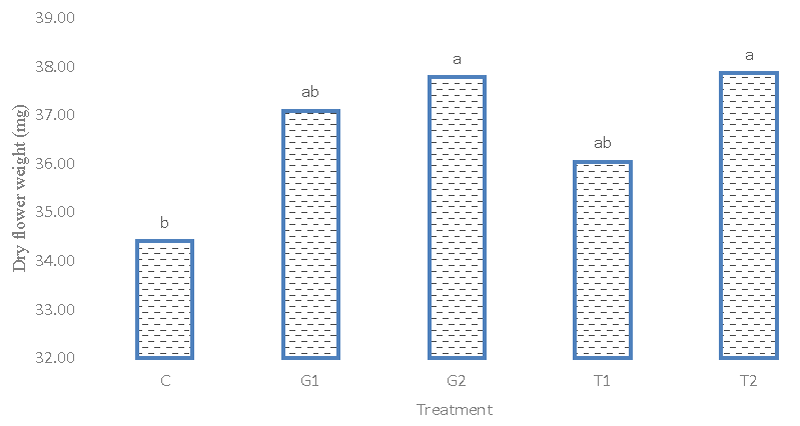
Based on the results of variance analysis (Table 2), it was found that the difference between genotypes for flower dry weight traits is significant at the 1% probability level. But the percentage of phenotypic and genetic variation was low for this experiment. This indicates low diversity in terms of flower dry weight. The results of the average comparison between different genotypes indicated that the Afghani genotype 1 with a weight of (37.584) mg for each flower took the highest amount and was placed in group a (Figures 4,5). These results show the positive effect of different treatment levels of glutamic acid and tryptophan and their effect on dry flower weight. The highest average dry weight of flowers was obtained with (37.364) mg in the simple effects treatment of 2 mM tryptophan level. The lowest dry flower weight is 34.397 and 34.36 mg respectively in the control treatment. The highest level of each treatment of glutamic acid and tryptophan improved the dry flower weight compared to the control.
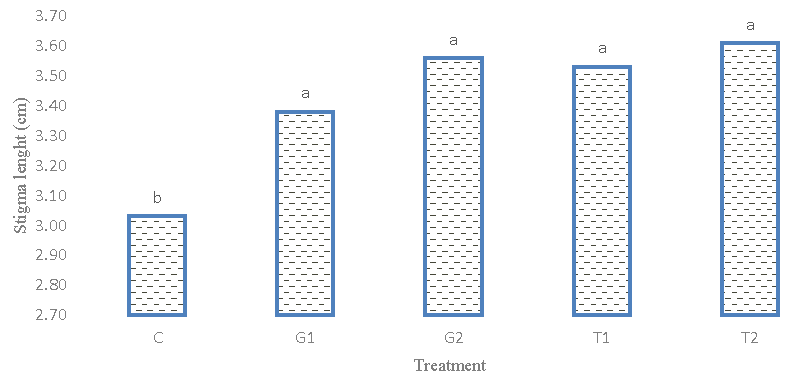
Stigma length
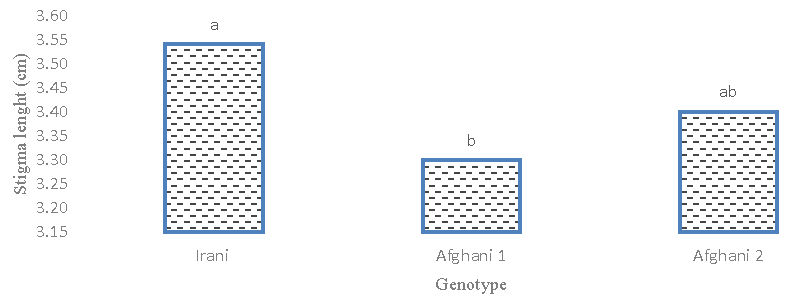
The result of the analysis of variance (Table 2) showed that the stigma length of different genotypes had no statistically significant difference. The coefficient of phenotypic and genotypic variation is low, which indicates low diversity in terms of stigma length. Figures 6,7 comparing the average length of the stigma showed that the Afghani genotype 1 with a length of (5.17) cm had the highest amount and there was no significant difference between the Afghani 2 and Iranian genotypes. The results of the average comparison (Figure 7) of the treatments showed that the effect of 1- and 2-mM glutamic acid increased the length of the stigma compared to the control and other treatments. Therefore, the diversity for this trait in the test was low and it did not show a relationship with yield, so it cannot be a suitable trait for saffron breeding.
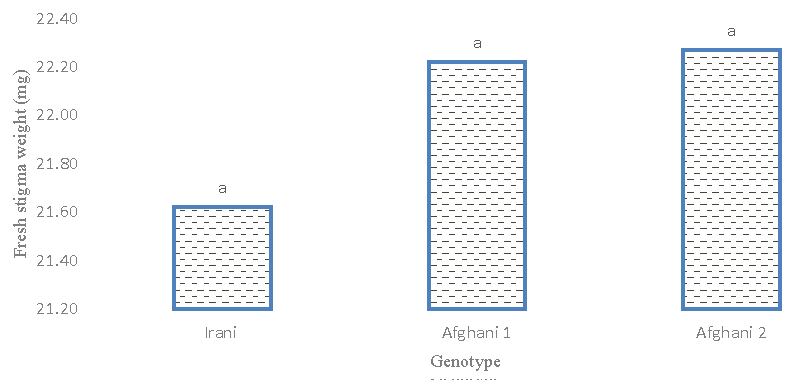
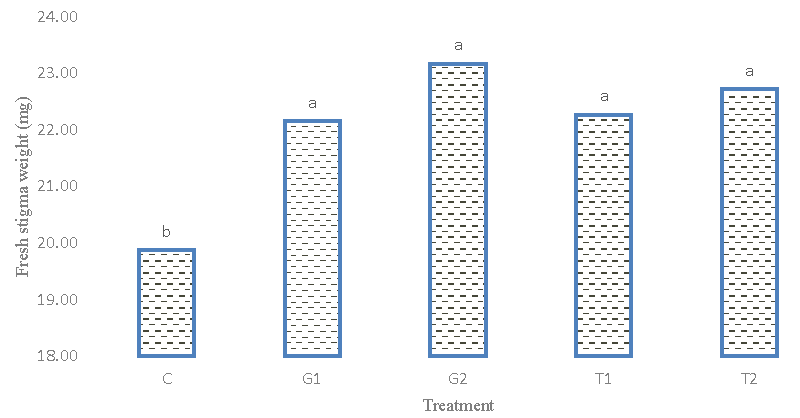
Stigma wet weight
The results of the analysis of variance (Table 2) of the observations indicated that there are no significant differences among the genotypes. The percentage of phenotypic and genetic variation coefficient is medium, which indicates the absence of high variation in terms of stigma weight trait. The results of the average comparison (Figure 8) between the genotypes showed that the Afghani genotype 1 had the highest stigma weight (20.815 mg). In this respect, they have a significant difference only with the Afghani 2 genotype and the Iranian genotype. The results of the average comparison (Figure 9) of the treatments, despite the fact that the treatment of glutamic acid and tryptophan significantly had a positive effect on the fresh weight of the stigma. But while the control showed the highest weight in the growth level compared to the treatments and there was a significant difference in this respect. The comparison of this trait with flower dry weight and stigma dry weight was positive and significant, but it did not show a significant relationship with yield in the unit area.
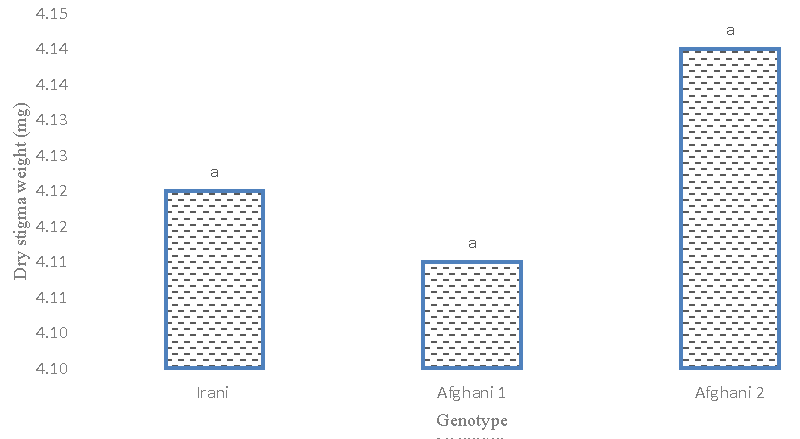
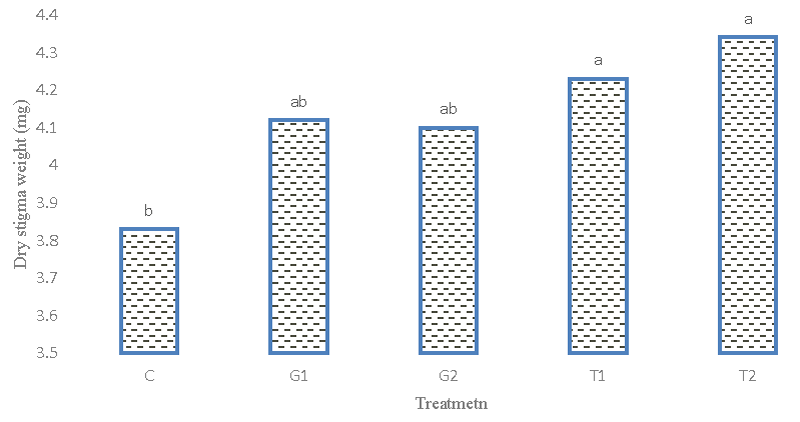
Stigma dry weight
The results of the analysis of variance (Table 2) revealed the observations. The difference between genotypes was significant at the 5% probability level. Figure 10 is the highest dry weight of the stigma of the Afghani 1 genotype. In this respect, there was a significant difference between the Afghani 2 genotype and the Iranian genotype. The average comparison results (Figure 11) showed that the effects of glutamic acid and tryptophan treatment on saffron genotypes were significant at the 5% probability level. Also, the highest yield of stigma dry weight was obtained in the simple effects treatment of 2 mM tryptophan level and the lowest in the 1 mM tryptophan treatment level. Small logs with lower density had lower dry weight.
Flower length
Treatment of glutamic acid and tryptophan significantly affected the flower length in saffron and caused an increase in flower length compared to the control. Thus, the maximum leaf length (5.21) cm was observed in the treatment of the effects of 2 mM glutamic acid. Surface treatments of 1 mM glutamic acid with a length of (5.16) cm and 2 mM tryptophan treatment with a flower length of (5.07) cm were placed in the next categories, the lowest leaf length was (5.05) cm in the treatment of 1 mM tryptophan. became. In this sense, amino acids are biological stimulants that increase the absorption of nutrients and improve plant growth and elongation (Abo sedera, et al. 2010) (Figures 12,13).
Morphological traits
Amino acids are substances that stimulate metabolism and metabolic processes to increase plant efficiency (Dewic, 2009). Based on the results of the analysis of variance (Table 2) and the results of the mean comparison (Figure 1), amino acid 1 mM glutamic acid and tryptophan increased the vegetative growth characteristics and increased the number of saffron plants. The amino acid has increased the number of leaves in plants by accelerating the absorption of water and nutrients and increasing vegetative growth. This result was similar to the results of the amino acid effect on mint plants (Dinoo et al., 2009). Therefore, in the first year, the number of flowers in a saffron plant depends on different factors such as weather conditions, farm management, and stresses in the origin areas of the genotypes. The number of flowers was considered the most effective trait in determining yield (Armenia, et al. 2014). Reports showed that the use of amino acid foliar spray in different concentrations improved the vegetative growth of Gladiolus grandiflora, and increased the quality of flowering [21].
Based on the research done, analysis of variance (Table 2), and the results of simple effects comparing the averages of 2 mM tryptophan amino acid and glutamic acid increased fresh and dry weight of flowers in plants. Because amino acids are biological stimulators that increase the absorption of nutrients, especially nitrogen, and increase photosynthesis, this improves the growth and weight of the plant (Abo Sedera, et al. 2010). Among them, the amino acid tryptophan is a precursor for the production of the auxin hormone. which causes an increase in cell elongation and, as a result, an increase in the height and weight of the seedling (Tarek and Hassan, 2014). The results of this research are the same as the findings of researchers such as (Saburi, et al. 2014) in evergreen plants, (Golzadeh, et al. 2012) in chamomile plants, and [22] in sweet plants, increasing the height, volume, and Elongation of the root of the plant. In another study, the use of tryptophan amino acid increased the wet and dry weight of nettle (Utrica pilulifera) [15]. Also, the average comparison (Figure 9) of the simple effects of 2 mM glutamic acid treatment has increased the stigma fresh weight of the saffron plant. Therefore, the results of the average comparison (Figure 11) of the simple effects of 2 mM tryptophan amino acid treatment caused an increase in the dry weight of saffron plant stigma. Therefore, the amino acid foliar application provides the basis for the absorption of nutrients in plants, which, as a result, increases the weight and volume of the plant (Porcel and Ruiz Lozano, 2004). The use of amino acids not only increases growth but also increases the quality and quantity of the product (Belal, et al. 2016). Amino acid tryptophan and glycine in different concentrations cause cell division and growth, so the height of Gladiolus grandifloras can be increased [23]. Based on the average comparison results (Figure 7), the treatment of 1 mM glutamic acid increased the stigma length in saffron plants. In the thyme plant (Thymus vulgaris L), the treatment of amino acid phenylalanine and tryptophan in different concentrations has caused a significant increase in plant height compared to control plants [24-38]. In another study, the use of the amino acid tryptophan increased fresh and dry weight in a type of nettle (Urtica pilulifera L) (Wahba, et al. 2015). In research, Filner (1966) found that some amino acids reduce the growth of tobacco plants. Gamborg (1970) also showed that the amino acid glutamine increases dry matter in soybeans. It has been reported in many studies that foliar spraying with amino acids increases the growth and development of plants ( [20]. In general, the use of amino acid tryptophan and glutamic acid has increased photosynthetic organic compounds and increased their transfer from leaves to fruits, thereby increasing crop production.
Conclusion
Glutamic acid and tryptophan have a positive and significant effect on the growth, and morphological characteristics of the saffron plant. has it. Different levels of two amino acids (glutamic acid and tryptophan) investigated had different effects on the growth pattern and development of morphological traits. The concentration of one mM glutamic acid and two mM tryptophan, in addition to improving growth and development indicators, also increased flower and stigma performance. Amino acid foliar application (1 mM glutamic acid and 2 mM tryptophan) led to increased growth and production of vegetative bodies. Also, the concentration of one mM glutamic acid accounted for the highest content of secondary metabolites. In general, it can be said that the application of amino acids, directly and indirectly, affects the morphological activities and improves the growth and development of plants and their application as foliar spraying can be effective and recommended.
- Shokrpour M. Saffron (Crocus sativus L) Breeding: Opportunities and Challenges. In: Al-Khayri J, Jain S, Johnson D(eds). Advances in Plant Breeding Strategies: Industrial and Food Crops. Springer, Cham. 2019. https://doi.org/10.1007/978-3-030-23265-8_17
- Djordjević P, Jelena B, Kostić AŽ, Kiralan M. Saffron. Antioxidant activities of bioactive compounds and various extracts obtained from saffron. 2021; 41–97. https://doi.org/10.1016/B978-0-12-821219-6.00002-6.
- Bukhari SI, Manzoor M, Dhar MK. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed Pharmacother. 2018 Feb;98:733-745. doi: 10.1016/j.biopha.2017.12.090. Epub 2018 Jan 4. PMID: 29306211.
- Jadouali SM, Atifi H, Mamouni R, Majourhat KH, Bouzoubaâ Z, Laknifli A, Faouzi A. Chemical characterization and antioxidant compounds of flower parts of Moroccan crocus sativus L. Journal of the Saudi Society of Agricultural Sciences. 2019; 18(4):476-480. doi: 10.1016/j.jssas.2018.03.007.
- Hashemi M, Manholm L, Johansson MN, Coldrey M. Simulation performance of NLOS wireless backhaul using automatically aligned antennas with limited scan range. 10th European Conference on Antennas and Propagation (EuCAP). Davos, Switzerland. 2016; 1–5.
- Karimi E, Oskoueian E, Hendra R, Jaafar HZ. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010 Sep 6;15(9):6244-56. doi: 10.3390/molecules15096244. PMID: 20877220; PMCID: PMC6257777.
- Ochiai T, Shimeno H, Mishima K, Iwasaki K, Fujiwara M, Tanaka H, Shoyama Y, Toda A, Eyanagi R, Soeda S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007 Apr;1770(4):578-84. doi: 10.1016/j.bbagen.2006.11.012. Epub 2006 Dec 5. PMID: 17215084.
- Gout B, Bourges C, Paineau-Dubreuil S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr Res. 2010 May;30(5):305-13. doi: 10.1016/j.nutres.2010.04.008. PMID: 20579522.
- Sadeghnia HR, Cortez MA, Liu D, Hosseinzadeh H, Snead OC 3rd. Antiabsence effects of safranal in acute experimental seizure models: EEG and autoradiography. J Pharm Pharm Sci. 2008;11(3):1-14. doi: 10.18433/j38g6j. PMID: 18801302.
- Kalantari S, Shokrpour M, Abedi Z, Salami SA. Grouping of some Iranian saffron accessions using morphological attributes at reproductive and vegetative stages. Paper presented at fourth international saffron symposium, India, Kashmir. 2012; 22-25.
- Ghalamkari E, Shokrpour M, Kalantari S, Vahedi M. Qualitative evaluation of some saffron (Crocus sativus L). Paper presented at 4th National Congress on medicinal plants. Mashhad, Iran. 2015; 12–13.
- Mustafa A, Hussain A, Naveed M, Ditta A, Nazli ZEH, Sattar A. Response of okra (Abelmoschus esculentus L) to soil and foliar applied L-tryptophan. Soil & Environment. 2016; 35:76–84.
- Zaheer IE, Ali S, Saleem MH, Imran M, Alnusairi GSH, Alharbi BM, Riaz M, Abbas Z, Rizwan M, Soliman MH. Role of iron-lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol Biochem. 2020 Oct;155:70-84. doi: 10.1016/j.plaphy.2020.07.034. Epub 2020 Jul 25. PMID: 32745932.
- Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003 Aug;81(4):247-65. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. PMID: 12848846.
- Wahba H, Motawe H, Ibrahim A. Growth and chemical composition of Urtica pilulifera L. plant as influenced by foliar application of some amino acids. Journal of Materials and Environment Science. 2015; 6(2):499-50 .
- Sanikhani M, Akbari A, Kheiry A. Effect of phenylalanine and tryptophan on morphological and physiological characteristics in colocynth (Citrullus colocynthis L.). Plant Process and Function: 2020; 9(35):317-328.
- Noroozlo YA, Souri MK, Delshed M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agriculture. 2019; 4:164–172. https://doi.org/10.1515/opag-2019-0016.
- Guo D, Ali A, Ren C, Du J, Li R, Lahori AH, Xiao R, Zhang Z, Zhang Z. EDTA and organic acids assisted phytoextraction of Cd and Zn from a smelter contaminated soil by potherb mustard (Brassica juncea, Coss) and evaluation of its bioindicators. Ecotoxicol Environ Saf. 2019 Jan 15;167:396-403. doi: 10.1016/j.ecoenv.2018.10.038. Epub 2018 Oct 23. PMID: 30366273.
- Farid M, Abubakar M, Asam ZUZ, Sarfraz W, Abbas M, Shakoor MB, Ali S, Ahmad SR, Jilani A, Iqbal J, Al-Sehemi AG, Al-Hartomy OA. Microwave Irradiation and Glutamic Acid-Assisted Phytotreatment of Tannery and Surgical Industrial Wastewater by Sorghum. Molecules. 2022 Jun 22;27(13):4004. doi: 10.3390/molecules27134004. PMID: 35807251; PMCID: PMC9268057.
- Faten SA, Shaheen AM, Ahmed AA, Mahmoud AR. Effect of foliar application of amino acids as antioxidants on growth, yield, and characteristics of Squash. Research Journal of Agriculture and Biological Science 6: 2010; 583-588.
- Ahmad R, Azeem K, Muhammad A, Zahir Z, Tariq M. Effect of compost enriched with N and Tryptophan Tryptophan on soil and maize. Agronomy for Sustainable Development. 2008; 28.299-305.
- Nahed G, Abdel Aziz AA, Mazher M, Farahat MM. Response of vegetative growth and chemical constituents of Thuja orientalis L. plant to foliar application of different amino acids at Nubaria. Journal of American Science 6: 2010; 295-301.
- Hanan Z. Effect of tryptophan and paclobutrazol on Caraway (Carum carvil L.) and Coriander (Coriandrum sativum L) plants. MSC. Thesis. Fac. of Agric., Cairo. 2000.
- Ghazal GM. Growth and oil yield of thymus vulgaris plant as influenced by some amino acids and ascorbic acid. World Journal of Pharmaceutical Sciences 3: 2015; 2321-3086.
- Al-Said MA, Kamal AM. Effect of folairfoliar spray with folic acid and some amino acids and some amino acids on flowering yield and quality of sweet pepper. Journal of Agricultural Science. 2008; 33(10):7403-7412.
- Arnon AN. Method of extraction of chlorophyll in the plants. Agronomy journal. 1967; 23:112-121.
- Caballero-Ortega H, Pereda-Miranda R, Abdullaev FI. HPLC quantification of major active components from 11 different saffron (Crocus sativus L) sources. Food Chemistry. 2007; 100 (3): 1126-1131. https://doi.org/10.1016/j.foodchem.2005.11.020.
- Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant bio stimulants. Plant and Soil. 2014; 383:3-41. https://doi.org/10.1007/s11104-014-2131-8.
- Ferrara L, Naviglio D, Gallo M. Extraction of Bioactive Compounds of Saffron (Crocus sativus L.) by Ultrasound Assisted Extraction (UAE) and by Rapid Solid-Liquid Dynamic Extraction (RSLDE). European Scientific Journal. 2014; 10(3):1-13. DOI: https://doi.org/10.19044/esj.2014.v10n3p%25p.
- Gresta F, Avola G, Lombardo GM, Siracusa L, Ruberto G. Analysis of flowering, stigmas yield and qualitative traits of saffron (Crocus sativus L.) as affected by environmental conditions. Scientia Horticultural. 2009; 119(3):320-324. https://doi.org/10.1016/j.scienta.2008.08.008.
- ISO/TS 3632-1/2.Technical Specification. Crocus sativus L Saffron. Ed. ISO, Geneva, Switzerland. 2003.
- Koocheki AR, Tabrizi L, Mohammad Abadi M. Evaluation of effect of high corm density and three methods of cultivation on some agronomical traits of saffron and corm behavior. Horticulture Journal of Iran. 2011; 3(1):36- 49.
- Liu XQ, Ko KY, Kim SH, Lee KS. Effect of amino acid fertilization on nitrate assimilation of leafy radish and soil chemical properties in high nitrate soil. Communications in Soil Science and Plant Analysis. 2008; 39:269-281. https://doi.org/10.1080/00103620701759301.
- Maghsoudi MS. Agricultural saffron, industry, nutrition and treatment. Tehran, Iranian Agricultural Science Publishing. 2010; 9.
- Ramaih S, Guedira M, Paulsen GM. Relationship of indoleacetic acid and tryptophan to dormancy and preharvest sprouting of wheat. Funct Plant Biol. 2003 Oct;30(9):939-945. doi: 10.1071/FP03113. PMID: 32689078.
- Moghaddam RP. Medicinal and industrial use of saffron. Ferdowsi University of Mashhad. Faculty of Agriculture. 2015.
- Tzin V, Galili G. The Biosynthetic Pathways for Shikimate and Aromatic Amino Acids in Arabidopsis thaliana. Arabidopsis Book. 2010;8:e0132. doi: 10.1199/tab.0132. Epub 2010 May 17. PMID: 22303258; PMCID: PMC3244902.
- Qartavol VS. Comparison of effective compounds and antioxidant activity of saffron produced in Kashmar and Marand. Scientific research of agriculture and saffron technology. 2016; 4 (3):224 215. https://doi.org/10.22048/jsat.2016.38671.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
