Archives of Clinical Nephrology
Encapsulating Peritoneal Sclerosis: Case report and Current Status
Jacques Rottembourg* and Belkacem Issad
Cite this as
Rottembourg J, Issad B (2017) Encapsulating Peritoneal Sclerosis: Case report and Current Status. Arch Clin Nephrol 3(1): 039-046. DOI: 10.17352/acn.000025Encapsulating peritoneal sclerosis (EPS), is a rare but devastating complication of long-term peritoneal dialysis (PD) with a high mortality rate. The incidence is between 0.5 and 3.3%, decreasing with time. EPS is defined as a clinical syndrome with major signs of gastrointestinal obstruction, inflammatory parameters, radiological and macroscopic changes. Duration of treatment and cessation of PD are the main risk for development of EPS: about 75% of EPS occurred in patients transferred on hemodialysis or in the two years after kidney transplantation. Morphological alterations are disappearance of mesothelial layer, sub-mesothelial fibrosis, interstitial sclerosis and vasculopathy, ultrafiltration failure, fast transport status of the peritoneal membrane, and loss of sodium sieving are the most predicting functional abnormalities. Some biomarkers could be found in the peritoneal effluent. The pathophysiology is probably a consequence of a multiple-hit process in which expression of growth factors and cytokines play a role. Medical strategies (corticosteroids, immunosuppressive drugs, tamoxifen) in association with parenteral nutrition and/or surgery (enterolysis) are discussed. Prevention is the use of physiological peritoneal solutions, icodextrine instead of high glucose concentration solution, and peritoneal lavage after peritoneal dialysis cessation for any reason.
Introduction
Encapsulating peritoneal sclerosis (EPS) is a rare cause of intestinal obstruction which is characterized by fibrotic encapsulation of the bowel due to a progressive intraabdominal inflammatory process. Firstly described by Owtschinnikow as “peritonitis chronica fibrosa incapsulata” in 1907 [1].With the development of continuous ambulatory peritoneal dialysis (CAPD) procedure in the late seventieth, this devastating complication was described by Gandhi [2], our team [3] and others [4,5]. In 2000 the International Society of Peritoneal Dialysis (ISPD) has defined EPS as a clinical syndrome with signs of intermittent and persistent or recurrent complaints of gastrointestinal obstruction with macroscopic and or radiological confirmation of sclerosis, calcified peritoneal thickening or encapsulation of the intestines. The presence of disturbed intestinal function, such as the clinical symptoms of partial or total obstruction is essential for the diagnosis [6]. These symptoms are caused by the development of a fibrosis cocoon that slowly covers the intestines and leads to obstruction, malnutrition, weight loss cachexia and death. Since then, others have attempted to modify this definition in order to provide a more specific framework for diagnosis [7].
Reported mortality rates for EPS are high (25-55%) especially during the first years following the development of PD as an alternative for Hemodialysis (HD) for the treatment of end stage renal disease (ESRD).The mortality increases also with the duration of treatment [8-12]. An overall increase in EPS incidence was observed (from 0.3% in 1996 to 3.5% in 2005) despite a decrease in the number of patients receiving PD treatment during the same period [10-12]. Several registries and study groups were set up to investigate this complication [13-17]. The overall incidence of EPS is around 2.5% [18], but some registries reported higher incidence: The Scotland Registry reported a cumulative incidence of 8.1% at eight years [12], and an Australian registry study, a cumulative incidence of 19.4% after eight years of PD treatment [8]. Similar findings were reported in a prospective study in which the incidence of EPS was 0.7% after five years, 2.1% after eight years, 5.9% after ten years and 17.2% after fifteen years of PD treatments [19].
This review describes a recent case of EPS in our team, and provides an overview of current knowledge about EPS, focusing on the considerable progress that has been made with regard to understanding the pathophysiology of peritoneal fibrosis and EPS. The emerging roles of different growth factors in these processes are highlighted and an update on the diagnosis and therapeutic options for EPS is provided.
Presentation Case
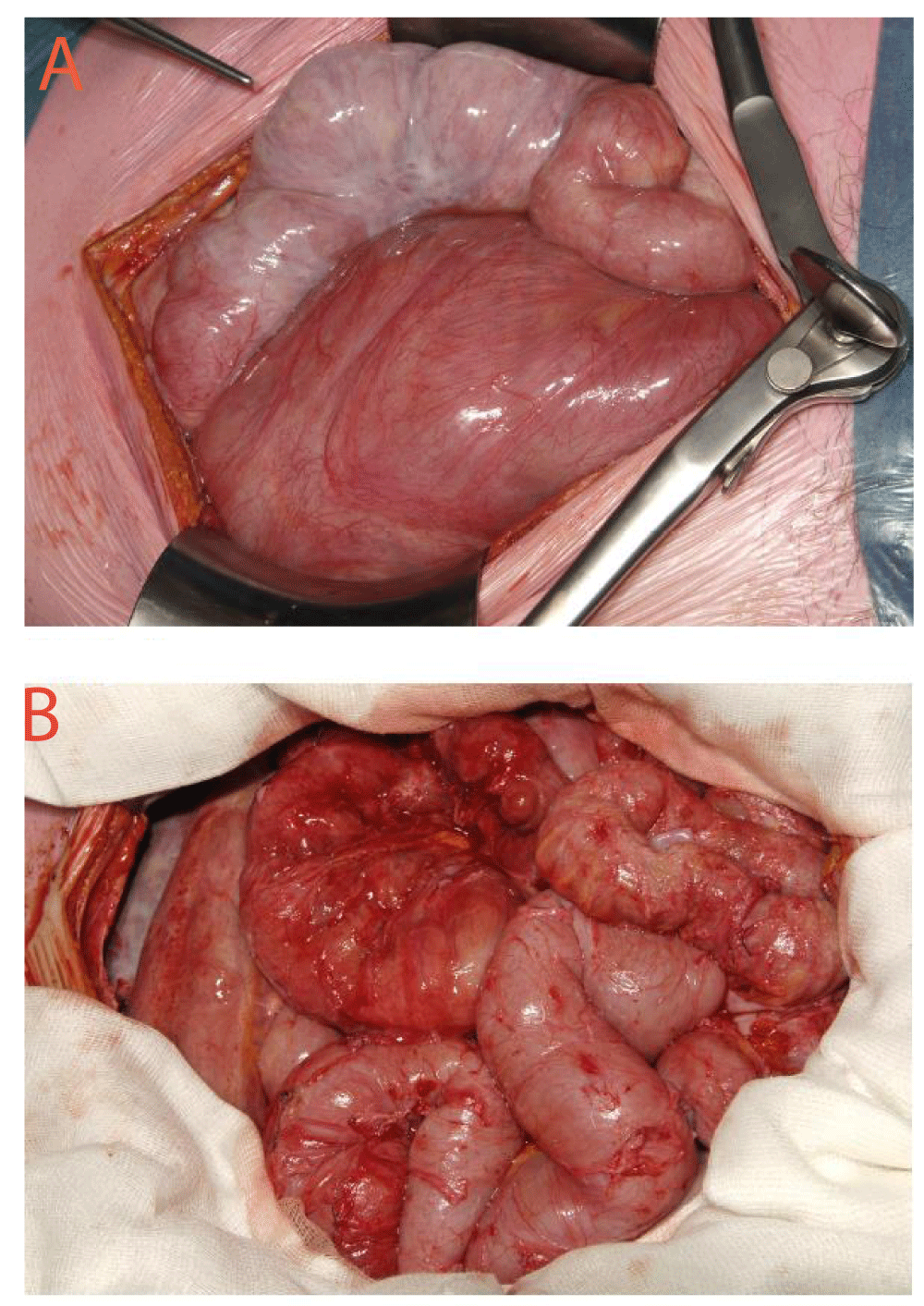
A 26 years old man suffering from interstitial nephropathy in relation with sarcoidosis received peritoneal dialysis (CAPD), then automated peritoneal dialysis (APD) from 2002 until 2009. The patient had during the PD period 3 histories of peritonitis and one catheter exit-site infection. The transfer from CAPD to APD was in relation with an ultrafiltration failure. Thereafter, as the patient moved in another city, a transfer from PD to HD was decided. At that time the patient asks to be on a kidney transplantation waiting list. With the transfer on HD, the patient loosed about 6 kg. Abdominal ultrasound, performed at that time, showed a normal abdominal cavity. Six months later, an inflammation status was present with non-specific symptoms such as abdominal pain, anorexia, nausea, diarrhea, defecation problems and weight loss. Biologically, signs of systemic inflammation were progressively present such as fever, leukocytosis, elevated C reactive protein, low serum albumin. The abdominal CT-scan revealed diffuse mild thickening and contrast enhancement of the peritoneum along with massive ascites in the peritoneal cavity. Peritoneal calcifications were also present. Jejunal and ileal loop walls appeared as thickened and edematous. After 6 days of parenteral nutrition and corticosteroids, it was decided to operate the patient because of the unresolved intestinal obstruction symptoms. At the explorative laparotomy performed end 2012, a classical EPS was found with ascites and a thick dense whitish membrane encasing the right colon and the intestine (Figure 1A). The membranous sac was excised from the surrounding intestine along with adhesiolysis (Figure 1B). No ileal injury was found despite a prolong dissection. Operative duration time was over five hours. The patient showed a significant recovery postoperatively and was discharged from the hospital uneventfully one week after. The histopathological evaluation of the membrane revealed fibrosis tissue with the absence of mesothelium and accompanied by massive collagen deposition, scattered mononuclear inflammatory cells and fibroblast proliferation. Three years after, the patient in good clinical conditions, could receive a kidney transplant without any clinical abdominal symptom.
Pathophysiology of EPS
Morphological alterations of the peritoneum: The healthy peritoneum consists of three parts: a monolayer mesothelial cells layer attached to a basement membrane. In between there is a sub-mesothelial contact zone which contains fibroblasts, macrophages, and blood vessels [20]. Alterations of the peritoneal structures are present at the uremic phase of ESRD, and continue during PD treatment [21]. During a PD treatment, even without any complication, the mesothelial layer gradually disappears, the peritoneal vasculature shows progressive medial fibrosis, and hyalinization conducting to deposition of collagen appears [22], the sub-mesothelial zone thickens as the result of interstitial fibrosis and sclerosis, composed of myofibroblasts and various types of collagen [23], new blood vessels are formed with the extension of the vasculopathy, in the same way than during diabetic retinopathy [24].
Moreover advance glycation end products (AGEs) accumulate in the mesothelial layer, sub-mesothelial layer and vascular wall in PD patients [25]. Deposition of AGEs is associated with the presence of peritoneal fibrosis and functional problems such as loss of ultrafiltration [26]. Whether AGEs cause peritoneal damage or are innocent bystanders is not yet really known. One hypothesis is that AGEs accumulation (in relation too with the use of high glucose concentration solutions) influences the permeability of blood vessel walls, which would explain the association between AGE accumulation and fast transport status or loss of ultrafiltration [27].
Simple sclerosis, which is characterized by thickening of the parietal peritoneum and vascular alterations in the absence of encapsulation, develops in the majority of patients treated by PD for a quite long period [28]. Once bowel encapsulation is present, the patient manifests the clinical symptoms of partial or total bowel obstruction that defines EPS.
Functional alterations of the peritoneum: Early studies described a decrease in ultrafiltration and increased peritoneal permeability in patients with EPS [29]. Ultrafiltration failure seemed to be the predominant early change in patients who are progressively developing EPS. Ultrafiltration capacities were lower in patients with EPS than in patients with a similar duration of PD who do not develop EPS [30]. Small solute transport increases in patients undergoing long-term PD treatment; free water transport also decreases in patients on long-term PD treatment in the years before onset of EPS [31]. These modifications indicate that a decrease in osmotic conductance might accompany the early stages of EPS, and could identify patients at risk of development of severe EPS, and might indicate that a switch to HD should be considered. But such a strategy was also considered as non-efficient [32].
Pathogenesis of EPS
Biomarkers in the effluent dialysate: CA125, Interleukine (IL)-6 are both biomarkers of the mesothelial cellular mass. Vascular endothelial growth factor (VEGF) has an important role in the proliferation and migration of endothelial cells during angiogenesis [33]. Human peritoneal mesothelial cells can produce VEGF in response to various stimuli present in the PD fluid [33]. Level of VEGF expression in peritoneal effluent showed a linear relationship with the duration of PD treatment [34]. This finding suggests that VEGF is important in the development of neo-angiogenesis and eventually in the development of EPS. In an animal model, exposure to high level of TGF-β led to simple sclerosis, whereas prolonged exposure led to neo-angiogenesis, extensive adhesions and cocooning or encapsulation of the intestines which seems to be similar to the features of EPS [35].
Epithelial-to-mesenchymal transition (EMT): This physiological process, under normal conditions, repairs damaged tissue throughout the body and also in the peritoneum. However, if uncontrolled, EMT can lead to initiation of fibrotic processes [36]. EMT is present in fibrotic peritoneal tissue from patients under PD predominantly in the sub-mesothelial layer [37]. In the peritoneal effluent some factors in favor with the development of EPS through EMT could be identified: high glucose concentration solutions, AGEs products, acid pH of some solutions, and some other growth factors. Although EMT is generally accepted to have a role in the development of EPS, its exact contribution to the processes underlying EPS remains unclear [38].
Theories on the pathogenesis of EPS:
• The two-hit theory: The hypothesis of a two-hit theory, now generally accepted, proposes that two causative factors are required for the onset of EPS [39].The first hit occurs as a consequence of PD treatment, which causes disruption of normal peritoneal layer and mesothelial physiology. This damage occurs over several years of PD treatment. Some patients exposed to a second hit that triggers the development of EPS [40]. The second hit might be an infection such as a peritonitis, discontinuation of PD for any reason, or use of inadequate PD solutions [29].
• Genetic predisposition: Genetic predisposition may induce the development of EPS in patients who have already peritoneal changes after several years of treatment: in the UK, a large database of DNA samples has been set up to study the genetic basis of this association [17]. In some patients, an association between the occurrence of EPS and Alport syndrome was observed, suggesting that Alport syndrome might predispose patients to EPS [41].
Diagnosis of EPS
Clinical diagnosis: The chronic and insidious development of this clinical syndrome is specific for EPS. As a consequence, EPS is often not recognized in the early stages. The first signs of EPS, as abdominal complaints (nausea, vomiting, diarrhea alterations in defecation, sub-occlusive periods) may be accompanied by increased levels of inflammatory markers or short period of blood-stained ascites (easily shown during the drain period of the peritoneal fluid). The gastro-intestinal symptoms might gradually progress to severe abdominal pain, anorexia, malnutrition, weight loss and the development of an abdominal mass with sub-occlusive periods. Peritoneal functions often show decreased net ultrafiltration and fast small solute transport status [42]
Radiological findings: The diagnosis of EPS can be confirmed by various imaging studies. Abdominal ultrasound might reveal a thick-walled mass containing bowel loops, loculated ascites and some fibrous adhesions. Plain abdominal radiography might show gas, some fluid levels, and dilatation of the bowel lumen indicating obstruction. In some patients calcifications are visible. At present CT-scan is the best-studied imaging technique when combined with a bowel opacification showing peritoneal enhancement, thickening and calcifications, adhesions of the bowel loops, signs of obstruction, fluid loculation, and ascites. MRI might revealed dilatation of the intestines, focal thickening of the bowel wall, massive ascites and compression of the bowel.
A classification in four progressive stages has been proposed by Nakamoto [43], (Table 1):
• The pre-EPS stage.
• The inflammatory stage
• The progressive stage
• The fibrotic stage
Risk factors
EPS is probably a multifactorial disease and, therefore, several risk factors that might cause peritoneal changes should be considered in its development.
Uremia: As chronic renal failure could also induce signs of vasculopathy and thickening of the sub-mesothelial zone, even before the initiation of PD [21], we should not be surprised that factors other than those related to PD itself are associated with EPS development [44].
The composition of dialysis solutions: The majority of the PD solutions, essentially those used at the beginning of the PD era, were non bio-compatible: acid pH, or lactate buffer could not be involved in the EPS procedure, but in our team the role of acetate buffer, used at the beginning of the CAPD, in June 1978, might be involved in the EPS development [3]. All PD solutions contain glucose, which is a major cause of peritoneal membrane injury. Heat sterilization of the PD fluids leads also to the formation of glucose degradation products, which accelerate the formation of AGEs [45]. High cumulative glucose load was associated with increased sub-mesothelial fibrous tissue [46]. Moreover, patients who developed EPS had a higher cumulative glucose exposure than those who did not, despite a similar duration of PD treatment [47]. These observations led to the development of new bio-compatible PD fluids, essentially bicarbonate buffered solutions; in clinical studies, patients had reduced levels of markers associated with peritoneal damage, suggesting an improvement in preservation of the peritoneal membrane [48,49].
Various dialysis solutions that do not contain glucose and are based instead on, icodextrin and amino-acids have also been introduced [50, 51]. Some studies have described an association between used of icodextrin-based solutions and increased expression of local inflammatory markers [52], other data suggest that the use of these solutions preserves both peritoneal membrane function and mesothelial cell mass [53]. A case-control study showed no difference in prescriptions of icodextrin-based dialysis solutions between patients with and those without EPS [54].
Duration of PD: The duration of PD is the most important risk factor for both peritoneal alterations and development of EPS [14]. Surprisingly, however, most cases of EPS are diagnosed only after discontinuation of the PD treatment for kidney transplantation or transfer to HD: 69% of the cases in a Japanese study [10, 19]. Only one study shows that peritoneal lavage after the discontinuation of PD has a positive effect in preventing EPS [55].
Peritonitis: Peritonitis episodes have long been considered to be a risk factor for EPS development [8, 56, 57]. In particular peritonitis caused by pseudomonas sp, staphylococcus aureus, haemophilus, or fungal organisms has been implicated in EPS [58, 59]. A japanese prospective study showed that 25% of EPS cases were associated with bacterial peritonitis [60], as an another study showed no relationship between bacterial peritonitis and EPS [12]. Some observations indicate that the cause of peritonitis (but not the incidence) might be relevant to the development of EPS [61].
Young age of patients: Although observational studies (and our presentation case) consistently shows that patients with EPS are often young (median age ranging from 34.7 to 52 years), this finding is difficult to interpret as older patients are less likely to remain on PD for more than 4 years [61].
Ultrafiltration failure: Patients with EPS almost always have fast transport failure and ultrafiltration failure: this was first described by our team [29]. In one study, half of the patients on PD for > 2 years, who developed ultrafiltration failure and continued PD developed EPS [62].
Kidney transplantation and discontinuation of PD: In some studies, a high incidence of post-transplantation EPS has been reported [63]. Discontinuation of PD, especially for kidney transplantation, but also for any other reason could favors the development of inflammatory biomarkers related previously: various causes of this phenomenon have been proposed with the use of immunosuppressive drugs, and tacrolimus [64]. Nevertheless, whether post-transplantation EPS is caused by discontinuation of PD, or results from the transplantation process itself and the use of some immunosuppressive drugs, currently remains unclear.
Exposure to Chlorhexidine and Practolol: A strong association has been reported between the use of chlorhexidine, a chemical antiseptic used at the early stage of CAPD, to sterilize dialysis tubing connections and development of EPS [65,66]. In 1974, a study reported an association between the use of practolol and onset of EPS in three patients not on PD treatment [67].
Therapeutic strategies
An early diagnosis supports a medical approach as a real bowel obstruction is more in favor with a surgical approach.
Cessation of PD treatment: At a asymptomatic, or inflammatory phase of EPS (Table 1), cessation of PD and transfer to HD seems to be the best strategy. The maintenance of the peritoneal catheter with regular peritoneal lavage in order to evacuate fibrin, growth factors and cytokines was recommended [45,68].
Immunosuppressive therapy: The rationale for the use of immunosuppressive agents in the treatment of patients with early EPS is to damp down the inflammation that precedes the encapsulating phase. Successful treatment has been described with corticosteroids [6,69,70]. The recommended dosage is about 0.5 to 1 mg/kg/day during one month, preceded by 3 bolus of methylprednisolone; then the dosage is decreased for a one year treatment.
Some successful treatments were reported with azathioprine, mycophenolate mofetil, sirolimus [71-75], alone or in association with corticosteroids.
Tamoxifen: Tamoxifen is a non-steroidal anti-estrogenic drug that is effective in the treatment of several fibrotic diseases such as retroperitoneal fibrosis. Several small case-series described positive effects [76,77]. A large clinical trial in England could not confirm this effect, however, perhaps because only one third of the participants displayed clinical symptoms of severe EPS [14]. By contrast a retrospective analysis of data from the Dutch EPS study showed a decrease in mortality of patients with EPS after treatment with tamoxifen [78].
Parenteral nutrition: While nutritional support is crucial at all stages of EPS, it is of paramount importance during the late stages of EPS, when gastro-intestinal symptoms become permanent and malnutrition may set in. At this point enhancement of nutritional support using total parenteral nutrition is usually necessary [14], it was realized before surgery in our presentation case.
Angiotensin II inhibitors: Angiotensin II inhibitors may be important in the prevention and treatment of EPS, as angiotensin II has pro-inflammatory and pro-fibrotic effects that act through TGF-β. The anti-fibrotic properties of angiotensin inhibitors are shown in renal fibrosis. In patients on PD, ACE inhibitors seem to have a positive effect on peritoneal function [79], however a protective effect of ACE inhibitors has not been found on EPS [80].
Surgery: Accepted indications for surgery in EPS include persistent bowel obstruction, failing nutritional status. It is important that surgical intervention be sought prior to the appearance of malnutrition, which may increase postoperative morbidity and mortality [81]. Surgical procedure is always a very long procedure including Noble plication during which the small bowel walls are stitched together, to prevent the formation of postoperative adhesions, in combination with enterolysis, have yielded dramatic reduction in recurrent symptoms [82]. The intervention frees the bowel from the cocoon and thereby may restore bowel function.
Summary statements
Two recent papers summarize the actual status and recommendations for preventing PD patients of the development of this rare disease [83,84].
1. EPS is recognized as a potential and rare complication of long-term PD treatment, occurring in patients on PD for more than 5 years. Although the incidence of EPS then increases with further time on PD, the condition remains infrequent and the majority of long-term patients are not affected.
2. The decision about when to discuss EPS as a potential complication of long-term PD treatment should be undertaken at some point with the patient, not necessarily at the start of PD but more reasonably at the 3-4 year point of therapy.
3. Encapsulating peritoneal sclerosis is associated with considerable morbidity and mortality. It is therefore important to develop strategies to reduce the risk to an individual patient.
4. No single strategy to reduce the risk of development of EPS has been proven in clinical trial but there is some evidence to support the followings:
- minimizing dialysate glucose exposure, although it is important to ensure that fluid volume status is not compromised as a result.
- preventing acute PD-related peritonitis using interventions recommended by the ISPD peritonitis guidelines.
- use of neutral-pH solutions, low glucose degradation product dialysis solutions.
1. The more severe clinical features of EPS with bowel obstruction, poor nutritional status, and ascites may develop even if PD is discontinued (patient transferred to HD or transplanted)
2. There are no specific predictors for the development of EPS:
- although many patients have ultrafiltration failure and fast small solute transport status, this not true for all patients and this is a common finding in patients on long-term PD.
- there is no evidence that CT scanning has any value in predicting EPS.
- progressive loss of osmotic conductance to glucose may reflect the development of peritoneal interstitial fibrosis, and may help identifying patients at risk of EPS. However, this needs to be confirmed in prospective studies.
3. Although changes in peritoneal membrane function, loss of ultrafiltration and frequent peritonitis are poor predictors of EPS, they are factors suggesting that transfer to HD should be considered and discussed with the patient, if appropriate, to optimize dialysis delivery. Such patients should be monitored closely for possible development of EPS if changing dialysis modality to HD.
4. Older patients and those with numerous comorbidities have a limited life expectancy when starting dialysis. Few will therefore survive long enough on PD to be at risk of developing EPS. Such patients are unlikely to be candidates for kidney transplantation so their quality of life on dialysis is very important. In considering the risk of development of EPS with such patient, it is therefore important to consider realistically their life expectancy, the feasibility of HD for that patient, and how this would affect their quality of life. Discussions with the patient should be part of a shared decision-making process about overall prognosis and goals of care.
- Owtschinnikow PJ (1907) Peritonitis chronica fibrosa incapsulata. Chirurgie 83: 623-634. Link: https://goo.gl/haCQ5P
- Gandhi VC, Humayun HM, Ing TS, Daugirdas JT, Jablokow VR, et al. (1980) Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med 140: 1201-1203. Link: https://goo.gl/DjnmF6
- Rottembourg J, Gahl GM, Poignet JL, Mertani E, Strippoli P, et al. (1983) Severe abdominal complications in patients undergoing continuous peritoneal dialysis. Proc Eur Dial Transplant Assoc 20: 236-242. Link: https://goo.gl/vryyTR
- Denis J, Paineau J, Potel G, Fontenaille C, Guenel J (1980) Continuous ambulatory peritoneal dialysis. Ann Intern Med 93: 508-513. Link:
- Slingeneyer A, Mion C, Canaud B, Faller B, Beraud JJ (1983) Progressive sclerosing peritonitis: a late and severe complication of maintenance peritoneal dialysis. Trans Am Soc Artif Intern Organs 29: 633-640. Link: https://goo.gl/87XPKf
- Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG (2000) Encapsulating peritoneal sclerosis: definition, etiology, diagnosis and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee. Perit Dial Int 20: 543-555. Link: https://goo.gl/NJb5Rk
- Korte MR, Boeschoten EW, Betjes MG (2009) The Dutch EPS Registry: increasing the knowledge of encapsulating peritoneal sclerosis. Neth J Med 67: 359-362. Link: https://goo.gl/VZJh6r
- Rigby RJ, Hawley CM (1998) Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant 13: 154-159. Link: https://goo.gl/XfML6q
- Nomoto Y, Kawajuchi Y, Kuso H, Hirano H, Sakai S, et al. (1996) Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Study Group. Am J Kidney Dis 28: 420-428. Link: https://goo.gl/N3uj1p
- Kawanishi H (2001) Encapsulating peritoneal sclerosis in Japan: prospective multicenter controlled study. Perit Dial Int 21 (Suppl 3): S67-S71. Link: https://goo.gl/9VsFjD
- Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PEC, et al. (2005) Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end stage renal failure. Kidney Int 68: 2381-2388. Link: https://goo.gl/hjmsNB
- Brown MC, Simpson K, Kerssens JJ, Mactier R (2009) Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 4: 1222-1229. Link: https://goo.gl/9FXRAx
- Brown EA, Van Biesen W, Finkelstein FO, Hurst H, Johnson DW, et al. () Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int 29: 595-600. Link: https://goo.gl/dFrkbb
- Balasubramaniam G, Brown EA, Davenport A, Cairns H, Cooper B, et al. (2009) The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 24: 3209-3215. Link: https://goo.gl/2bPbTF
- Johnson DW, Cho Y, Livingston BER, Hawley CM, McDonald SP, et al. (2010) Encapsulating peritoneal sclerosis: incidence, predictors and outcomes. Kidney Int 77: 904-912. Link: https://goo.gl/zTVpPC
- Korte MR, Boeschoten EW, Betjes MG (2009) The Dutch EPS Registry: increasing the knowledge of encapsulating peritoneal sclerosis. Neth J Med 67: 359-362. Link: https://goo.gl/L2LZd9
- Summers AM, Brenchley PE (2006) An international encapsulating peritoneal sclerosis registry and DNA bank: why we need one now. Perit Dial Int 26: 559-563. Link: https://goo.gl/PgkJer
- Kawanishi H, Shintaku S, Banshodani M, Hashimoto S (2015) Past and present perspectives on encapsulating peritoneal sclerosis. Contrib Nephrol 185: 87-97. Link: https://goo.gl/emYEys
- Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, et al. (2001) Encapsulating peritoneal sclerosis in Japan: prospective multicenter controlled study. Perit Dial Int 21(Suppl3): S67-S71. Link: https://goo.gl/CFNXnV
- Flessner MF (2003) The transport barrier in intraperitoneal therapy. Am J Physiol Renal Physiol 288: F433-F442. Link: https://goo.gl/kbpAaS
- Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, et al. (2002) morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470-479. Link: https://goo.gl/WkLSy5
- Honda K, Nitta K, Horita S, Yumura W, Nihei H (1996) Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 72: 171-176. Link: https://goo.gl/GcbmR7
- Mateijsen MA, Van der Wal AC, Hendricks PM, Zweers MM, Mulder J, et al. (1999) Vascular and interstitial changes in the peritoneum of CAPD patients with peritoneal sclerosis. Perit Dial Int 19: 517-525. Link: https://goo.gl/qeghQe
- Korte MR, Sampimon DE, Betjes MGH, Krediet RT (2011) Encapsulating peritoneal sclerosis: The state of affairs. Nat Rev Nephrol 7: 528-538. Link: https://goo.gl/rCmtfD
- Nakayama M, Kawaguchi Y, Yamada K, Hasegawa T, Takazoe K, et al. (1999) Immuno-histochemical detection of advanced glycosylation end-products and its possible pathophysiological role in CAPD. Kidney Int 51: 182-186. Link: https://goo.gl/cS8sBk
- Honda K, Nitta K, Horita S, Yumura W, Nihei H, et al. (1907) Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultrafiltration. Nephrol Dial Transplant 14: 1541-1549. Link: https://goo.gl/VsjaTt
- Park MS, Lee HA, Chu WS, Yang DH, Hwang SD (2000) Peritoneal accumulation of AGE and peritoneal membrane permeability. Perit Dial Int 20: 452-460. Link: https://goo.gl/pNkDRZ
- Garosi G (2009) Different aspects of peritoneal damage: fibrosis and sclerosis. Contr Nephrol 163: 45-53. Link: https://goo.gl/sxuCoH
- Rottembourg J, Issad B, Langlois P, Tranbaloc R, Adamou A, et al. (1985) Loss of Ultrafiltration and sclerosing encapsulating peritonitis during CAPD: evaluation of the potential risk factors. Adv Perit Dial 1: 109-117. Link: https://goo.gl/FP3rWq
- Verger C, Celicout B (1985) Peritoneal permeability and encapsulating peritonitis. Lancet 1: 986-987. Link: https://goo.gl/yNcJRt
- Sampimon DE, Coester A, Struijk DG Krediet RT (2007) Time course of peritoneal transport parameters in peritoneal dialysis patients who develop peritoneal sclerosis Adv Perit Dial 23: 107-111. Link: https://goo.gl/zUrhxc
- Brown EA, Van Biesen W, Finkelstein O, Hurst H, Johnson DW, et al. (2009) Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: position paper for ISPD. Perit Dial Int 29: 595-600. Link: https://goo.gl/Fw6oYa
- Mandl-Weber S, Cohen CD, Haslinger B, Kretzler M, Sitter T (2002) Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int 61: 570-578. Link: https://goo.gl/kuarZh
- Zweers MM, Struijk DG, Smit W, Krediet RT (2001) Vascular endothelial growth factor in peritoneal dialysis: a longitudinal follow-up. J Lab Clin Med 137: 125-132. Link: https://goo.gl/44F1j2
- Margetts PJ, Kolb M, Galt T, Hoff CM, Shokley TR, et al. (2001) Gene transfer of transforming growth factor-β1 to the rat peritoneum: effects on membrane function. J Am Soc Nephrol 12: 2029-2039. Link: https://goo.gl/ZBYpLB
- Liu Y (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1-12. Link: https://goo.gl/RmuTFW
- Jimenez-Heffernan JA, Aguilera A, Aroeira LS, Bajo MA, del Peso G, et al. (2004) Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch 444: 247-256. Link: https://goo.gl/FEf4Uh
- Williams JD, Craig KJ, Topley N, Williams GT (2003) Peritoneal dialysis: changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney Int Suppl 84: S158-S161. Link: https://goo.gl/sKm83d
- Kawanishi H (2002) Surgical treatment for encapsulating peritoneal sclerosis. Adv Perit Dial 18: 139-143. Link: https://goo.gl/ETrXnA
- Honda K, Oda H (2005) Pathology of encapsulating peritoneal sclerosis. Perit Dial Int 25 (Suppl.4): S19-S29. Link: https://goo.gl/JGHkeJ
- Sampimon DE, Vlijm A, Phoa SS, Krediet RT, Struijk DG (2010) Encapsulating peritoneal sclerosis in a peritoneal dialysis patient using biocompatible fluids only: is Alport syndrome a risk factor? Perit Dial Int 30: 240-242. Link: https://goo.gl/ieAJmB
- Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ (2010) The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis. Kidney Int 78: 611-618. Link: https://goo.gl/4aGBce
- Nakamoto H (2005) Encapsulating peritoneal sclerosis- a clinician approach to diagnosis and medical treatment. Perit Dial Int 25 (Suppl 4): S30-S38. Link: https://goo.gl/SnN1Pp
- Habib AM, Preston E, Davenport A (2010) Risk factors for developing encapsulating peritoneal sclerosis in the icodextrin era of peritoneal dialysis prescription. Nephrol Dial Transpl 25: 1633-1638. Link: https://goo.gl/wHbQKY
- Moriishi M, Kawanishi H, Kawai T, Takahashi S, Hirai T, et al. (2002) Preservation of peritoneal catheter from prevention of encapsulating peritoneal sclerosis. Adv Perit Dial 18: 149-153. Link: https://goo.gl/AsNS9c
- Plum J, Hermann S, Fussholler A, Schoenike G, Donner A, et al. (2001) Peritoneal sclerosis in peritoneal dialysis patients related dialysis settings and peritoneal transport properties. Kidney Int Suppl 78: S42-S47. Link: https://goo.gl/UEs3E3
- Hendricks PM, Ho-Dac-Pannekeet MM, Van Gulik TM, Struijk DG, Phoa SS, et al. (1997) Peritoneal sclerosis in chronic peritoneal dialysis patients: analysis of clinical presentation, risks factors, and peritoneal transport kinetics. Perit Dial Int 17: 136-143. Link: https://goo.gl/MHEB1p
- Hekking LHP, Zareie M, Driesprong BAJ, Faict D, Welten AGA, et al. (2001) Better preservation of peritoneal morphologic features and defense in rats after long-term exposure to a bicarbonate/ lactate –buffered solution. J Am Soc Nephrol 12: 2775-2786. Link: https://goo.gl/qyaCrF
- Krediet RT (2005) On behalf of the European Best Practice Guideline working group on Peritoneal Dialysis. Europrean Best practice guidelines for peritoneal dialysis. Peritoneal dialysis solutions. Nephrol Dial Transpl 20 (Suppl 9): ix 16-ix20.
- Mistry CD, Mallick NP, Gokal R (1987) Ultrafiltration with an isosmotic solution during long-peritoneal dialysis exchanges. Lancet 2: 178-182. Link: https://goo.gl/9FscLq
- Goodship THJ, Lloyd S, McKenzie PW, Earnshaw M, Smeaton I, et al. (1987) Short-term studies on the use of amino acids as an osmotic agent in continuous ambulatory peritoneal dialysis. Clin Sci 73: 471-478. Link: https://goo.gl/VC7iVF
- Parikova A, Zweers MM; Struijk DG, Krediet RT (2003) Peritoneal effluent markers of inflammation in patients treated with icodextrin-based and glucose-based dialysis solutions. Adv Perit Dial 19: 186-190. Link: https://goo.gl/1FAoXS
- Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, et al. (2003) Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338-2344. Link: https://goo.gl/XG1QYj
- Habib AM, Preston E, Davenport A (2010) Risk factors for developing encapsulating peritoneal sclerosis in the icodextrin era of peritoneal dialysis prescription. Nephrol Dial Transplant 5: 1633-1638. Link: https://goo.gl/nRKVJZ
- Yamamoto T, Nagasue K, Okumo S, Yamakawa T (2010) The role of peritoneal lavage and the prognostic significance of mesothelial cell area in preventing peritoneal encapsulating sclerosis. Perit Dial Int 30: 343-352. Link: https://goo.gl/992a9X
- Bradley JA, McWhinnie DL, Hamilton DNH, Starnes F, Macpherson SG, et al. (1983) Sclerosing obstructive peritonitis after continuous ambulatory peritoneal dialysis. Lancet 2: 113-114. Link: https://goo.gl/NyKcm8
- Yamamoto R, Otsuka Y, Nakayama M, Maruyama Y, Katoh N, et al. (2005) Risk factors for encapsulating peritoneal sclerosis in patients who have experienced peritoneal dialysis treatment. Clin Exp Nephrol 9: 148-152. Link: https://goo.gl/FBYrQk
- Flanigan M, Anderson D, Freeman RM (1984) Peritoneal dialysis complicated by fungal peritonitis and peritoneal fibrosis. Am J Med 76: A113-A125. Link: https://goo.gl/hYJ8SW
- Chew CG, Clarkson AR, Faull RJ (1987) Relapsing CAPD peritonitis with rapid peritoneal sclerosis due to Haemophilus influenza. Nephrol Dial Transplant 12: 821-822. Link: https://goo.gl/NFHxYp
- Kawanishi H, Kawaguchi Y, Fukui S, Hara S, Imada A, et al. (2004) Encapsulating peritoneal sclerosis in Japan: a prospective controlled multicenter study. Am J Kidney Dis 44: 729-737. Link: https://goo.gl/TvtSVi
- Korte MR, Sampimon DE, Lingsma HF, Fieren MW, Looman CWN, et al. (2011) Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit Dial Int 31: 269-278. Link: https://goo.gl/vVDp7E
- Smit W, Parikova A, Struijk DG, Krediet RT (2005) The difference in cause of early and late ultrafiltration failure in peritoneal dialysis. Perit Dial Int 25 (Suppl 3): S41-S45. Link: https://goo.gl/Dp6Xtp
- Fieren MW, Betjes MGH, Korte MR, Boer WH (2007) Posttransplant encapsulating peritoneal sclerosis: a worrying new trend. Perit Dial Int 27: 619-624. Link: https://goo.gl/hgLNnF
- Mallucio M, Sharma V, Lagman M, Vyas S, Yang H, et al. (2003) Tacrolimus enhances transforming growth factor-β1 expression and promotes tumor progression. Transplantation 76: 597-602. Link: https://goo.gl/j3DJ7j
- Junor BJR, Briggs D, Forwell MA, Dobbie JW, Henderson I (1985) Sclerosing peritonitis- the contribution of chlorhexidine in alcohol. Perit Dial Int 5: 101-104. Link: https://goo.gl/rS8Mna
- Oules R, Challah S, Brunner FP (1988) Case-control study to determine the cause of sclerosing peritoneal disease. Nephrol Dial Transplant 3: 66-69. Link: https://goo.gl/4nEaxb
- Brown P, Baddeley H, Read AE, Davies JD, McGarry J (1974) Sclerosing peritonitis, an unusual reaction to a β-adrenergic –blocking drug (practolol). Lancet 2: 1477-1481. Link: https://goo.gl/vVaXuB
- Otsuka Y, Nakayama M, Ikeda M, Sherif AM, Yokohama K, et al. (2005) Restoration of peritoneal integrity after withdrawal of peritoneal dialysis: characteristic features of patients at risk of encapsulating peritoneal sclerosis. Clin Exp Nephrol 9: 315-319. Link: https://goo.gl/1SktmT
- Kuriyama S, Tomonari H (2001) Corticostroid therapy in encapsulating peritoneal sclerosis. Nephrol Dial Transplant 16: 1304-1305. Link: https://goo.gl/C3dcJe
- Bandhari S, Wilkinson A, Sellars L (1994) Sclerosing peritonitis: value of immunosuppression prior to surgery. Nephrol Dial Transplant 9: 436-437. Link: https://goo.gl/cAA6F3
- Wong CF, Beshir S, Khalil A, Pai P, Ahmad R (2005) Successful treatment of encapsulating peritoneal sclerosis with azathioprine and prednisolone. Perit Dial Int 25: 285-287. Link: https://goo.gl/nVaEv4
- Rajani R, Smyth J, Koffman CG, Abbs I, Goldsmith DJ (2002) Differential effect of sirolimus vs prednisolone in the treatment of sclerosing encapsulating peritonitis. Nephrol Dial Transplant 17: 2278-2280. Link: https://goo.gl/9ECGwa
- Lafrance JP, Letourneau I, Ouimet D, Bonnardeaux A, Leblanc M, et al. (2008) Successful treatment of encapsulating peritoneal sclerosis with immunosuppressive therapy. Am J Kidney Dis 51: e7-e10. Link: https://goo.gl/226bgT
- Junor BJ, McMillan MA (1993) Immunosuppression in sclerosing peritonitis. Adv Perit Dial 9: 187-189. Link: https://goo.gl/MvM1WY
- Fagugli RM, Selvi A, Quintaliani G, Bianchi M, Buonocristiani U (1999) Immunosuppressive treatment for sclerosing peritonitis. Nephrol Dial Transplant 14: 1343-1345. Link: https://goo.gl/ug5YYF
- Allaria PM, Giangrande A, Gandini E, Pisoni IB (1999) Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent. J Nephrol 12: 395-397. Link: https://goo.gl/k28MNM
- Eltoum MA, Wright S, Atchley J, Mason JC (2006) Four consecutive cases of peritoneal dialysis-related encapsulating peritoneal sclerosis treated successfully with Tamoxifen. Perit Dial Int 26: 203-206. Link: https://goo.gl/C66a6i
- Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, et al. (2010) Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol Dial Transplant 26: 691-697. Link: https://goo.gl/c8gx1B
- Kolesnyk I, Deker FW, Nordzij M, Le Cessie S, Struijk DG, et al. (2007) Impact of ACE inhibitors and A II receptor blockers on peritoneal membrane transport characteristics in long-term peritoneal dialysis patients. Perit Dial Int 27: 446-453. Link: https://goo.gl/r8wSL1
- Sampimon DE, Kolesnyk I Korte MR, Fieren MW, Struijk DG, et al. (2010) Use of angiotensin II inhibitors in patients that develop encapsulating peritoneal sclerosis. Perit Dial Int 30: 656-659. Link: https://goo.gl/QfVF76
- Kawanishi H, Moriishi M, Ide K, Dohi K (2008) Recommandation of the surgical option for the treatment of encapsulating peritoneal sclerosis. Perit Dial Int 28: (Suppl 3): S205-S210. Link: https://goo.gl/Q3Lkue
- Kawanishi H, Moriishi M, Tsuchiya S (2006) Experience of 100 surgical cases of encapsulating peritoneal sclerosis: investigation of recurrent cases after surgery. Adv Perit Dial 22: 60-64. Link: https://goo.gl/KMefkd
- Ryckelynck JP, Bechade C, Bouvier N, Ficheux M, Huraulult de Ligny B, et al. (2017) Encapsulating peritoneal sclerosis Nephrol Therap 2017. 01. 020. Link: https://goo.gl/FWg5BU
- Brown EA, Bargman J, Van Biesen W, Chang MY, Finkelstein FO, et al. (2017) Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis. Position paper for ISPD: 2017 Update. Perit Dial Int 37: 362-374. Link: https://goo.gl/7G4LDR

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
