Archives of Clinical Nephrology
An End-Stage Renal Disease Patient with Invasive Fungal Rhinosinusitis
Fatemeh Yassari1, Nooshin Dalili1*, Farin Rashid farokhi2 and Mihan Pourabdollah3
2Nephrology, Masih Daneshvari Hospital, Shahid Beheshti Medical University, Iran
3Clinical pathology, Masih Daneshvari Hospital, Shahid Beheshti Medical University, Iran
Cite this as
Yassari F, Dalili N, farokhi FR, Pourabdollah M (2017) An End-Stage Renal Disease Patient with Invasive Fungal Rhinosinusitis. Arch Clin Nephrol 3(1): 004-006. DOI: 10.17352/acn.000017Introduction
Mucormycosis is one of the invasive fungal infections particularly in immunocompromised patients with impaired host defense.
It is characterized by fungal rhino sinusitis with invasion of adjustment structures including brain, pulmonary, or gastrointestinal systems or can be presented as a disseminated disease.
Predisposing factors are diabetic ketoacidosis, neutropenia, corticosteroid or deferoxamine use, iron overload, malnutrition and skin macerations.
We present a known case of end-stage renal disease patient under hemodialysis without history of diabetes or other risk factors listed above, who admitted because of fever and diagnosed with invasive rhino -sinusitis mucormycosis.
Case History
A 46 –year- old woman presented by fever and malaise. Her major complaints were loss of appetite and weakness since 2 weeks ago also mentioned a history of tonsillitis from 2days ago which was using oral amoxicillin for it. Physical examination revealed fever (oral temperature= 38c) and pallor in sclera but there were no lymphadenopathy, peripheral or facial edema or nasal discharge .In oral cavity examination postnasal discharge and exudate plus erythematous tonsils were obvious. Lung, heart, abdomen and skin examination revealed no abnormality.
In past medical history the patient had history of hemodialysis 3 times a week since 18months ago via a permanent catheter placed in right internal jugular vein due to end-stage renal disease with unknown origin. She was hypertensive with no history of diabetes mellitus, corticosteroid usage or ischemic heart disease.
Her drug history included Amlodipine table l 5mg two times daily, Folic acid 5mg, Caplet Ca-Co3 500mg three times daily and ASA tablet 80mg once daily. We couldn’t find any positive family history of diabetes or chronic kidney disease. The patient had a history of recurrent fever and chills and therefore multiple admissions to hospital since 2 months ago, each time treated empirically for catheter infection with intravenous Vancomycin plus Ceftazidim. In recent admission a transthoracic echocardiography and then trans esophageal echocardiography were done because of high suspicious of infectious endocarditis due to catheter infection. Because of tonsillitis and odynophagia, ear-nose-throat specialists visited the patient and Nystatin drop plus oral Fluconazole for treatment of candida infection in oral cavity were added to other antibiotics.
Laboratory data
Routine lab results are shown in Table 1.
Chest X-Ray revealed no abnormality. Trans-esophageal echocardiography showed no vegetation or any signs of infective endocarditis.
Clinical course
After 72 hours the patient showed no progress in clinical findings and high fever went on. She started complaining of numbness in left side of her face and in the oral cavity we found a necrotic and bad smelling sore located on upper palate.
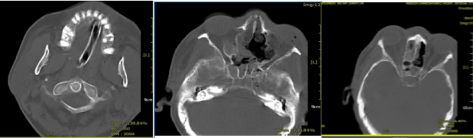
Paranasal sinuses and facial CT scan immediately ordered which showed necrotic mass in Maxillary and Ethmoid sinuses with invasion to ophthalmic fossa and oral cavity Figure 1.
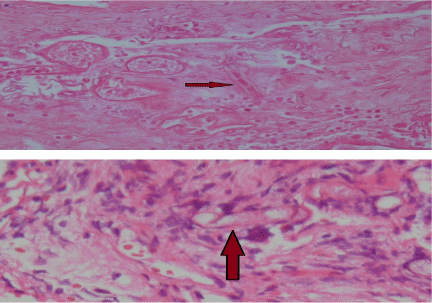
Regarding to high clinical suspicion of invasive fungal infection (mucormycosis) prompt systemic antifungal therapy and urgent functional endoscopic sinus surgery (FESS) and taking biopsy were done. The biopsy specimen was sent for histopathology and culture. The culture yielded the growth of mucor species after 5 days incubation at 280 C Figure 2.
Aggressive surgical debridement of involved tissue for orbital decompression was performed immediately and Liposomal Amphotericin B infusion at 5mg/kg daily was started along with broad spectrum antibiotics. Serum urea and electrolytes were monitored daily. At the end of first week, new areas of necrosis were noted in the palate and maxilla and despite the antifungal therapy, the disease progressed aggressively and the patient died.
Pathologic findings
Section of biopsy specimen showed extensive areas of necrosis and few fungal hyphae that were wide and pauci-septated in favor of fungal infection morphologically mucorals Figure 2.
Discussion
The present report describes a woman with history of end-stage renal disease infected with aggressive mucormycosis infection of sinus and orbit.
Due to clinical evidences phagocytosis is the major host defense mechanism against mucormycosis, and other evidences illustrate that mucormycosis infection seriously threaten neutropenic patients and patients with dysfunction of phagocytes [1].
The ability of phagocytes is ruined by hyperglycemia and acidosis in order to move toward and destroy the organisms by either oxidative or non-oxidative mechanisms. An elevation in available serum iron during diabetic ketoacidosis, which correlate with fungal growth directly, is the main reason for the increased susceptibility to mucor mycosis of patients with diabetic keto-acidosis. One of the most common underlying conditions is hyperglycemia itself usually with an associated metabolic acidosis. According to findings a rate of about 70% of rhinocerebral cases (occasionally referred to as craniofacial) are among diabetic patients in ketoacidosis .According to a report of 179 rhino-orbital-cerebral mucormycosis, 70 percent (126 out of 179) of the patients had diabetes mellitus and that most had ketoacidosis at the time of presentation while in the absence of any apparent risk factors it is difficult to find reports of rhino-orbital-cerebral mucormycosis. [2,3].
Although in comparison to general population, end –stage renal disease patients are not excessively susceptible to sinusitis, rhinitis, or pharyngitis [4-6], but the risk of mucormycosis will be increased in those hemodialysis patients that aluminum chelation therapy by high dose deferoxamine has been administered. The capacity of transferrin for binding iron and increasing the iron load could be disrupted by acidosis [7-9]. Mucor mycosis may affect as any of the following seven systems: rhino-cerebral, pulmonary, gastrointestinal, central nervous system, cutaneous, disseminated, and miscellaneous (bones, joints, heart, kidney, mediastinum) but rhinocerebral mucormycosis is the most common form of the disease, in which first the para nasal sinuses of a susceptible host will be inhaled by spores. Acute sinusitis with fever, nasal congestion, purulent nasal discharge, headache, and sinus pain are usually the symptoms of the infection. Not only all of the involved sinuses progresses but also those, which spread to contiguous structures, such as the palate, orbit, and brain, usually progresses rapidly. However, there are some reports that state an indolent course can cause rhino-orbital-cerebral mucormycosis [10].
Tissue necrosis of the palate resulting in palatal scar, destruction of the turbinates, perinasal swelling, and erythema and cyanosis of the facial skin overlying the involved sinuses are the symptoms of extension beyond the sinuses. It is probable that a black scar, be visible in the nasal mucosa or the palate. If fungus invade vascular, the necrosis of tissues will result in black eschar. [11].
Periorbital edema, proptosis, and blindness are among the signs of orbital involvement. Infarction of sensory branches of the fifth cranial nerve causes facial numbness which is frequent. Spread of the infection from the ethmoid sinus to the frontal lobe causes obtundation. Cranial nerve palsies, thrombosis of the sinus, and involvement of the carotid artery are result of sphenoid sinuses spread to the adjacent cavernous sinus [12].
Rhinocerebral mucormycosis has also occurred rarely in patients who received a solid organ trans- plant or those with prolonged neutropenia [13,16]. Recently, for those patients undergo hematopoietic stem cell trans- plantation, rhinocerebral disease is diagnosed as an increasing problem .These cases have largely been associated with steroid use for graft-versus-host disease [14-17].
We present a konwn case of end-stage renal disease patient under hemodialysis without history of diabetes or other known risk factors for fungal infections who admitted because of fever and diagnosed with invasive rhino -sinusitis mucormycosis. Although this invasive fungal infection is primarily known to be a disease of subjects with altered host defenses associated with underlying conditions and predisposing factors such as diabetes mellitus, hematologic malignancies, chemotherapy, radiotherapy, neutropenic states, persistent acidosis, iron or aluminum overload, protein energy malnutrition, diarrhea, dehydration, metabolic acidosis in small children, corticosteroid therapy, organ transplantation, and less frequently, AIDS, but rarely mucor mycosis can be a threatening complication for the hemodialysis patients as well . The mechanism by which uremic patients are predisposed to mucor mycosis is not clear. Probably, the altered immunologic state, acidosis and malnutrition, all play a role in the pathogenesis. The clues to a successful outcome seem to be early diagnosis and prompt institution of antifungal and surgical therapy. Newer techniques such as those directed to detect mucor antigens may become helpful in the future.
- Chinn RY, Diamond RD (1982) Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun 38: 1123–1129. Link: https://goo.gl/9T7xWq
- Ibrahim AS, Edwards JEJ, Filler SG (2003) Zygomycosis, 241–251. In. Dismukes WE, Pappas PG, Sobel JD (ed.), Clinical mycology. Oxford University Press, New York.
- Ribes JA, Vanover-Sams CL, Baker DJ (2000) Zygomycetes in human disease. Clin Microbiol Rev 13:236–301. Link: https://goo.gl/160hG6
- Naqvi SB, Collins AJ (2006) Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis13: 199-204. Link: https://goo.gl/ZWQoZR
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, et al. (2005) Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41: 634-653. Link: https://goo.gl/JRtQTJ
- McNulty JS (1982) Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 92: 1140-1143. Link: https://goo.gl/8nUs8G
- Boelaert JR, Fenves AZ, Coburn JW (1991) Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry. Am J Kidney Dis 1991: 660-66. Link: https://goo.gl/mevMCC
- Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, et al. (1993) Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest 91:1979-1986. Link: https://goo.gl/LnGH0W
- de Locht M, Boelaert JR, Schneider YJ (1994) Iron uptake from ferrioxamine and from ferri rhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol 47:1843-1850. Link: https://goo.gl/2MjtCJ
- Harri l WC, Stewart MG, Lee AG, Cernoch P (1996) Chronic rhinocerebral mucormycosis. Laryngoscope 106: 1292-1297. Link: https://goo.gl/TaIuOP
- Rajagopalan S (2005) Serious infections in elderly patients with diabetes mellitus. Clin Infect Dis 40: 990-996. Link: https://goo.gl/EfP7aR
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, et al. (2012) Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 54 Suppl 1: S23-34. Link: https://goo.gl/etCs8n
- Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E (2004) Improved outcome of zygomycosis in patients with hematological diseases? Leukemia Lymphoma 45: 1351–1360. Link: https://goo.gl/DJg6PN
- Marr KA, Carter RA, Crippa F, Wald A, Corey L (2002) Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 34: 909–917. Link: https://goo.gl/iWnJ4e
- Morrison VA, McGlave PB (1993) Mucormycosis in the BMT population. Bone Marrow Transplant 11: 383–388. Link: https://goo.gl/xXemaq
- Petrikkos G, Skiada A, Sambatakou H, Toskas A, Vaiopoulos G, et al. (2003) Mucormycosis: ten-year experience at a tertiary-care center in Greece. Eur J Clin Microbiol Infect Dis 22: 753–756. Link: https://goo.gl/0yqEHr
- Pillsbury HC, Fischer ND (1997) Rhinocerebral mucormycosis. Arch Otolaryngol 103: 600–604. Link: https://goo.gl/k33CGl

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
