Global Journal of Medical and Clinical Case Reports
Synovial Sarcoma of Thyroid Gland: Case Report and Review of Literature
Ashraf H Hassouna1* and Asmaa I Salama2
2Department of Pathology, National Cancer Institute, Cairo University, Egypt
Cite this as
Hassouna AH, Salama AI (2017) Synovial Sarcoma of Thyroid Gland: Case Report and Review of Literature. Glob J Medical Clin Case Rep 4(2): 051-052. DOI: 10.17352/2455-5282.000046Background
Synovial sarcoma accounts for 5 to 10% of soft tissue sarcoma. It occurs mainly in young adults, where 90% of cases occur before 50 years. Over 80% of cases arise in deep soft tissue of extremities, especially around the joints [1]. Unusual sites (about 15%) have been reported, including head, neck and trunk where differential diagnosis is difficult and surgical treatment is complex due to proximity to vital structures. Here, we report a case of synovial sarcoma in an unusual site which is thyroid gland with special clinical, histopathological, immunohistochemical and molecular features.
Case Report
A 52 year-old male presented to the Surgical Oncology Clinic, National Cancer Institute, Cairo University in December 2013. He complained of recurrent neck mass. He underwent right hemithyroidectomy 5 years ago and was diagnosed as medullary thyroid carcinoma then completion to total thyroidectomy 1 month later which revealed no residual tumor.
Computed tomography on the neck (2 November 2013) revealed right side cystic lesion 2x1.4 cm with no wall enhancement or wall calcification. Multiple small lymph nodes are detected. Isotopic bone scan done on 16 November 2013 revealed active disease locally. Hence, wide local excision was performed on 1 December 2013 and gross examination revealed fibromuscular tissue 4x3 cm entangling multiple nodules ranging in size from 0.2x0.3 cm to 2x1.5 cm, the largest showed soft yellowish cut section.
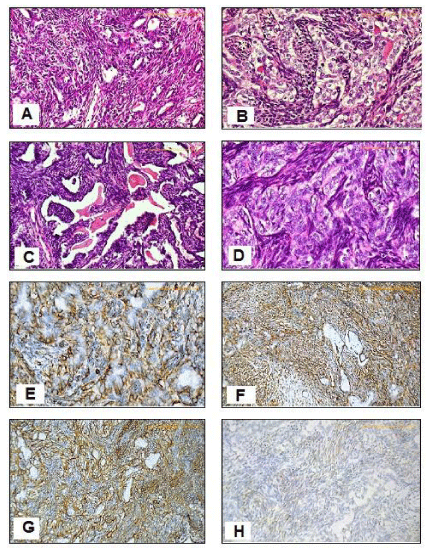
Microscopic examination revealed soft tissue infiltration by biphasic neoplastic growth composed predominantly of central necrosis and hyaline degeneration marginated by viable interlacing bundles of proliferating spindle cells having plumpy ovoid and spindle overlapping hyperchromatic nuclei with modest cytoplasm admixed with tubular structures lined by epithelioid cells having ovoid nuclei with more ample eosinophilic cytoplasm. There was intraluminal homogenous pink material. Rest of nodules were seven lymph nodes showing reactive lymphoid hyperplasia. Tumor cells reached the least circumferential margin. Differential diagnosis was papillary thyroid carcinoma with spindle cell nodules versus synovial sarcoma. In addition, medullary thyroid carcinoma should be excluded, hence, immunohistochemistry was done. Tumor cells were positive for vimentin and CD99 in both the spindle and Epithelioid cell components. CK and EMA were positive in the epithelioid cell component. Bcl2 is positive in the spindle cell component. Tumor cells were negative to TTF1, thyroglobulin, calcitonin, actin, CEA, and synaptophysin, hence, excluding both papillary and medullary thyroid carcinomas (Figure 1).
Correlation with the previous pathology was asked and paraffin blocks of previous thyroidectomy were submitted from which Hx&E sections were prepared, examined and revealed the same morphology encountered in the current pathology with additional evident thyroid infiltration. Immunostaining for TTF1, thyroglobulin, calcitonin, CEA, synaptophysin, CD99 and Bcl2 revealed the same results obtained upon the current pathology, emcee, the diagnosis of synovial sarcoma (Biphasic) was settled.
The recurrent nature of the mass was an indication for postoperative adjuvant radiotherapy. Patient received 60Gy/30 fractions/7 weeks localized to the thyroid bed using two anterior oblique wedged fields of 6 MV photons from linear accelerator and he tolerated the treatment well.
Discussion
Primary thyroid sarcomas are very rare with dilemma in their recognition and differential diagnosis. This finding suggest that synovial sarcoma is not related to normal synovium but could arise from pleuripotent mesenchymal cells located in the thyroid capsule or thyroid stroma, capable of epithelioid differentiation [2].
To our knowledge, only five cases were reported [3-6]. The median age was 55 years and our case aged at initial diagnosis 47 years old which is younger than the median age reported in other locations.
Synovial sarcoma typically affects adolescents and young adults and usually located periarticular. Due to unusual location, diagnosis is usually settled based on combine histopathological, immunohistochemical and molecular analyses. The presence of biphasic growth detected in our case, with mixture of spindle and epithelioid cell components, and evident nuclear overlap, lack of typical nuclear morphology of papillary thyroid carcinoma, favour the diagnosis of synovial sarcoma rather than papillary thyroid carcinoma with spindle cell nodules. Positive immunostaining for vimentin, CD99, bcl2, EMA and CK combined with negative staining of TTF1, thyroglobulin, calcitonin and synaptophysin help to establish such diagnosis. Molecular analysis (detecting the SYT-SSX gene fusion) by FISH came to confirm the diagnosis.
Data about the outcome of thyroid synovial sarcoma are hindered by the paucity of reported cases which precludes any prognosis analysis. However, unusual location may result in error in diagnosis, like to what happens in our cases, which resulted in delay of proper diagnosis, beside to proximity to vital structures created difficulty in complete excision with adequate safety margin.
In conclusion, we report here the sixth case of primary thyroid synovial sarcoma which could attract more attention to its diagnosis and management. Complete surgical excision with safety margin is the best choice for treatment, but multimodality treatment could achieve high disease free survival especially when adequate safety margin could not be obtained.
- Fisher C, de Bruilin DRH, Geurts van Kessel (2002) Synovial Sarcoma In: WHO Classification of Tumors, Pathology and Genetics of Tumors of Soft Tissue and Bone, Fletcher CDM, Unni KK and Mertens F (eds), IARC Press, Lyon.
- Dickersin GR (1991) Synovial sarcoma: a review and update, with emphasis on the ultrastructural characterization of the nonglandular component. Ultrastruct Pathol 15: 379. Link: https://goo.gl/eI8b7J
- Ryu CH, Cho KJ, Choi SH (2011) Synovial sarcoma of the thyroid gland. Clin Exp Otorhinolaryngol 4: 204-206. Link: https://goo.gl/Zg6Wwq
- Kikuchi I, Anbo J, Nakamura S, Sugai T, Sasou S, et al. (2003) Synovial sarcoma of the thyroid. Report of a case with aspiration cytology findings and gene analysis. Acta Cytol 47: 495-500. Link: https://goo.gl/MiVRqz
- Jang KS, Min KW, Jang SH, Paik SS, Tae K, et al. (2007) Primary synovial sarcoma of the thyroid gland. J Korean Med Sci 22: 154-158. Link: https://goo.gl/scQsW1
- Ghafouri A, Anbara T, Mir A, Lashkari M, Nazari M (2013) Thyroid synovial sarcoma: a case report. Acta Med Iran 51: 69-72. Link: https://goo.gl/4qjd7j

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
