Journal of Cardiovascular Medicine and Cardiology
The pathophysiology of chronic cardiorenal disease based on central hemodynamics
Michiya Ohno*
Cite this as
Ohno M (2018) The pathophysiology of chronic cardiorenal disease based on central hemodynamics. J Cardiovasc Med Cardiol 5(3): 027-035. DOI: 10.17352/2455-2976.000067As patients with chronic kidney disease (CKD) and chronic heart failure (CHF) increase, there is concern about a future heart failure pandemic. Deterioration of renal function is an independent prognostic factor for CHF after decongestion. Interactions between renal disease and cardiac disease are increasing, including nephrosclerosis and heart failure with preserved ejection fraction (HFpEF), which are both derived from augmentation of central pulse pressure by age-related arterial stiffening. Thus, it is necessary to treat multiple underlying diseases of cardiorenal syndrome simultaneously. However, an effective therapeutic strategy for HFpEF has not been established. This review reconsiders the pathophysiology of chronic cardiorenal disease related to arterial stiffening from the viewpoint of central hemodynamics and explores treatment options.
Introduction
Compared with the peripheral blood pressure, the central blood pressure is more closely related to organ changes that are representative of arteriosclerosis and renal dysfunction, including left ventricular (LV) mass and common carotid artery intima-media thickness (IMT) [1-4]. In addition, central blood pressure has been shown to be a predictor of cardiovascular events [1,5-7]. It has been suggested that elevation of central blood pressure has a key role in the linkage of heart and kidney disease, and central blood pressure may be a predictor of the progression of hypertension [8]. Accordingly, antihypertensive agents that more effectively reduce central blood pressure could be useful for prevention or improvement of organ damage.
The prevalence of heart failure is increasing worldwide and an accelerated increase in people with heart failure has been predicted in the future [9,10]. In particular, heart failure with preserved ejection fraction (HFpEF) is common among elderly persons [11-14].
Chronic kidney disease (CKD) is a prognostic factor for heart failure [15-17] and it is also increasing worldwide, along with underlying diseases such as hypertension and age-related nephrosclerosis [18,19]. There has also been an increase of diabetic kidney disease (DKD), which is defined as atypical diabetic nephropathy characterized by mild proteinuria with deterioration of renal function and nephrosclerosis [20-23].
Renal insufficiency (i.e., an estimated glomerular filtration rate (GFR) < 60.0 mL/min/1.73 m2] is common in patients with chronic heart failure (CHF), and approximately 36% of CHF patients also have renal insufficiency [16]. Moreover, renal dysfunction (assessed by GFR) is independently associated with an elevated risk of all cause death, cardiovascular death, and hospitalization for heart failure in patients with CHF and a preserved or reduced EF [16].
Cardiorenal syndrome (CRS) is the term for the bidirectional pathophysiological interaction between cardiac and renal dysfunction, and it is classified into 5 types in consideration of whether the condition is acute, chronic, or secondary [24]. Worsening renal function (WRF) occurs in approximately one third of patients admitted to hospital with acute decompensated heart failure (ADHF), and is associated with an increased length of stay, a higher readmission rate, and worse short-term and long-term survival [25-28]. Therefore, a treatment strategy is needed for ADHF and acute CRS (type 1). With aging of the population, it is predicted that there will also be an increase of chronic CRS (type 2), chronic renocardiac syndrome (type 4), and secondary CRS (type 5) derived from arteriosclerosis.
Thus, ventricular-arterial-renal interactions are becoming an issue of increasing interest with large clinical implications. This article reviews the pathophysiology of renal and cardiac damage (HFpEF) related to arterial stiffening from the perspective of central hemodynamics, and also discusses potential therapeutic options for reducing central pulse pressure [PP].
Central blood pressure
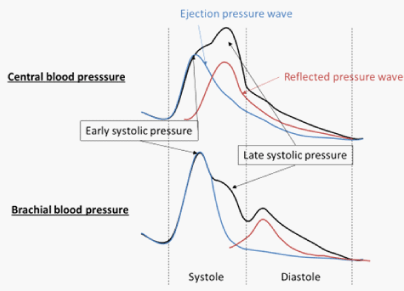
Components of central blood pressure: Central blood pressure has been noted as an index of the overall health of the arterial tree [29]. It is constituted by the ejection pressure wave that spreads from the heart to periphery and the reflected pressure wave that spreads in reverse from the peripheral arteries to the central region. When the reflected pressure wave is added to the ejection pressure wave during late systole, and the central pulse pressure wave is amplified by the augmentation pressure [AP] [30].
Young persons with normal blood pressure have distensible arteries, but elderly persons with hypertension do not. Loss of aortic distensibility due to arterial stiffening results in an increase of the central PP due to elevation of the central systolic blood pressure and a decline of the central diastolic blood pressure with loss of the cushion and Windkessel effects, because the reflected pressure wave moves faster and the reflected wave thus shifts from diastole to late systole. In elderly hypertensive persons, the augmentation pressure [AP] and the central pulse pressure wave are amplified as a consequence of the reflected pressure wave moving to late systole. In contrast, the reflected pressure wave is slower and appears during diastole in young normotensive persons because of the flexibility and distensibility of their arteries (Figure 1) [29,31]. The interaction between the ejection pressure and the reflected pressure is assessed by pulse wave analysis and is expressed as the augmentation index [AI]. In elderly persons with increased arterial stiffness, the central systolic blood pressure is generally determined by the reflected pressure wave, whereas the brachial systolic blood pressure measures the ejection pressure wave (Figure 2).
Clinical implications: The AI of central blood pressure is determined by the pulse wave velocity, which reflects segmental arterial elasticity and peripheral reflectance. Elevation of the central PP and AI lead increased LV afterload and decreased coronary perfusion due to a decline of diastolic pressure, and thus are more closely related to cardiovascular target organ damage and cardiovascular events compared with the brachial pressure. [1-4]. The central blood pressure reflects LV afterload, and thus is also more closely related to production of brain natriuretic peptide (BNP), a marker of cardiac workload and hypertrophy, relative to the brachial blood pressure [2,32]. Therefore, central PP is considered to be a risk indicator that is more pathophysiologically relevant to the pathogenesis of cardiovascular disease than the peripheral pressure [1,5,7,33-35]. For example, central blood pressure is superior for prediction of new-onset hypertension during follow-up of persons with high normal blood pressure [8].
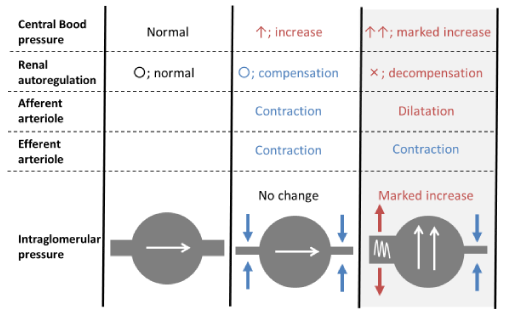
Relationship among central blood pressure, renal hemodynamics, and organ damage: Elevation of the central PP is considered to exacerbate renal dysfunction, including an increase of albuminuria, as it has a direct influence on renal hemodynamics [1,3,4,32,36-38]. The parameter used most widely for noninvasive evaluation of renal hemodynamics as a significant predictor of progressive renal dysfunction is the resistive index (RI), which is derived from the pulsatile flow-velocity waveform [4,39-43]. An increase of the RI is related to elevation of the central PP and the AI [4], and is also associated with renal microvascular damage [4,36,44-46]. In persons with normal flexible and distensible arteries, the PP from the central aorta is gradually absorbed by renal arterioles including the afferent arterioles, finally resulting in non-pulsatile flow in the efferent arterioles to maintain a constant intraglomerular pressure. Autoregulation to constantly maintain the intraglomerular pressure at approximately 60 mmHg depends on adjustment of pressure in the afferent and efferent arterioles by vasoconstriction or vasodilation. It involves secretion of renin, tubular glomerular feedback, myogenic responses, and sympathetic nervous system activity. This mechanism keeps the intraglomerular pressure around 60 mmHg, even if the systolic blood pressure varies widely from 90 to 180 mmHg [47]. One of the key reasons for progression of renal damage due to elevation of central blood pressure is considered to be afferent arteriole dysfunction, although the details are still unclear. All blood entering the glomerulus passes through the afferent arteriole and it acts as a gatekeeper that regulates renal blood flow by active contraction or dilatation. However, when the PP cannot be absorbed by the afferent arterioles, it reaches the efferent arterioles (Figure 3) [29]. As a result, the glomerular capillary walls are affected and hyperfiltration due to elevation of the intraglomerular pressure becomes persistent, leading to albuminuria. Thus, abnormal central hemodynamics lead to abnormal renal hemodynamics and hypertensive renal damage seems to occur due to elevation of the central PP.
Differing effect of antihypertensive agents on central blood pressure and brachial blood pressure: The effects of various antihypertensive agents differ between the aorta and the brachial artery. Vasodilators, including Ca channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), α-blockers, and nitrates, reduce the central blood pressure more effectively than the brachial blood pressure [48,49]. On the other hand, β-blockers have a weak effect on the central blood pressure [50].
The most pivotal study examining the relative importance of central and brachial blood pressures has been the Conduit Artery Function Evaluation (CAFÉ) study of the Angio-Scandinavian Cardiac Outcomes Trial (ASCOT) [5]. Although brachial blood pressure was similarly reduced in both the amlodipine ±perinopril and atenolol ±thiazide arms of the CAFÉ study, there was significantly greater reduction of central systolic pressure and PP (due to reduction of reflected pressure wave) with amlodipine-based treatment. In addition, changes in the indicators of cardiovascular target organ damage, including left ventricular mass and IMT, are more dependent on reduction of central blood pressure than brachial blood pressure, and improvement due to antihypertensive therapy depends on the extent of central blood pressure reduction rather than a decrease of brachial pressure [1,2,51]. These reports suggest that measuring central blood pressure to monitor treatment would be useful for prevention of cardiovascular disease.
Currently, a cut-off value for normal/abnormal central blood pressure has not been established, although it was recently proposed that 130/90 mmHg could be a cut-off value for central blood pressure by investigation of a Taiwanese database [52].
It has been suggested that measurement of central pressure could be medically cost effective because guiding management of hypertension based on central blood pressure results in a significantly different therapeutic pathway compared with conventional cuff blood pressure measurement, using less medication to achieve blood pressure control and avoiding adverse effects on LV mass, aortic stiffness, or quality of life [53]. Accordingly, it is necessary for the central blood pressure level that prevents cardiovascular target organ damage to be determined by large-scale controlled studies in the future.
Relationship between central blood pressure and HFpEF
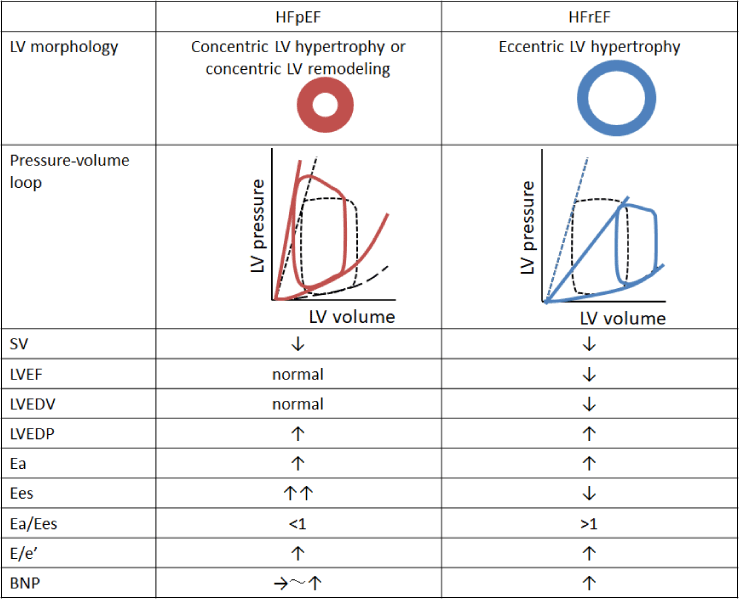
Clinical features of HFpEF: Among all patients with heart failure, those with HFpEF are increasing due to aging of the population, and the combination of HFpEF with CHF is common. These patients do not have a good prognosis, like patients who have heart failure with reduced EF (HFrEF), and effective treatment has not yet been established [14,54]. Compared to HFrEF patients, HFpEF patients are more likely to be elderly women and to have hypertensive heart disease as the underlying disorder [55-58]. HFrEF is associated with deterioration of systolic function on top of diastolic dysfunction, so that LV end-diastolic volume increases to maintain stroke volume, resulting in elevation of LV end-diastolic pressure [59-61]. Conversely, HFpEF is related to diastolic dysfunction and elevation of LV end-diastolic pressure [EDP] occurs without an increase of LV end-diastolic volume (EDV) (Figure 4,5). Diagnosis of HFpEF is difficult clinically, but no increase of LV EDV and LVEF ≥50% are requirements [62]. HFpEF can cause pulmonary congestion due to elevation of LV EDP derived from increased LV chamber stiffness, in the setting of excessive LV preload or afterload and increased heart rate during exercise [63,64]. BNP may be normal in up to 30% of patients with HFpEF [65,66], especially those who are obese [67] or only have exertional symptoms [68].
Is arterial afterload due to augmentation of central PP the main pathophysiology of HFpEF?
Epidemiologic studies have shown that 40% to 50% of patients with heart failure have HFpEF, and that they are older than those with HFrEF and are more often female [54,58,69]. Moreover, a community study has demonstrated that advancing age and female gender are associated with increased vascular and ventricular systolic diastolic stiffness, even in the absence of cardiovascular disease, suggesting the combination of ventricular-vascular stiffness may contribute to the increased prevalence of HFpEF, particularly in elderly women [58]. Patients with HFpEF are assumed to have vascular disease with arterial stiffening as well as cardiac disease, because this condition is often accepted to arise from hypertension. HFpEF derived from arterial stiffness is pathophysiologically and clinically characterized by the failure of compensation due to ventricular-arterial coupling (i.e., ventricular-arterial coupling disease) [57,70], indicating that this is a key determinant of cardiovascular health. It has been observed that age-related vascular stiffening, which can have a marked effect on ventricular-arterial coupling, is accompanied by changes of LV stiffness and diastolic compliance [71]. Accordingly, HFpEF should be considered to have a different pathogenesis from HFrEF [54,56,57,72].
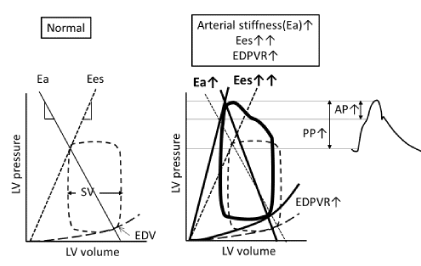
In HFpEF, ventricular-arterial disease leads to afterload mismatch, which results in decompensation of LV end-systolic elastance (Ees) for arterial stiffness and augmentation of central PP [73-75].
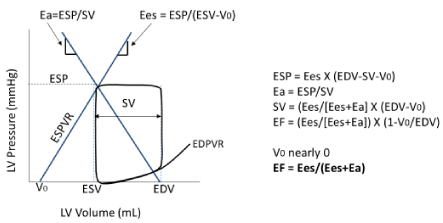
Effective arterial elastance (Ea) is an indicator of arterial stiffness that is defined by the negative slope between the end-systolic pressure-volume point and EDV [i.e., the ratio of end-systolic pressure to stroke volume (SV) shown in Figure 4]. Ees is an index of LV contractility that is also described by the slope of the end-systolic pressure-volume relationship (ESPVR) (Figure 4). In elderly patients with hypertension, central PP is boosted by an increase of the reflected pressure wave during late systole [29]. Elevation of Ea derived from this increase of central PP causes a change in the LV loading sequence with an increase of the late-systolic load, resulting in abnormal ventricular-arterial coupling and an eventual increase of Ees with LV hypertrophy. Furthermore, an increase of late systolic loading may incrementally impair early diastolic relaxation [76,77]. Thus, an increase of the cardiac arterial afterload with elevation of central PP causes secondary cardiac dysfunction [57], and is considered to underlie the pathophysiology of HFpEF in the elderly [76].
Treatments that can reduce late systolic vascular loading and arterial stiffness may be useful for patients with diastolic dysfunctionby changing the loading conditions, which could explain why nitroglycerin improves LV systolic diastolic function in HFpEF patients [48,77].
EF can be expressed as a function of Ees and Ea in the following formula (Figure 4) [78]: EF = Ees/(Ees+Ea)
If Ees = Ea, the value of EF becomes 0.5. In patients with HFrEF, EF decreases below 0.5 and Ees is lower than Ea. On the other hand, in patients with HFpEF, Ees is ≥Ea and EF is maintained at ≥0.5.
Under physiological conditions, the Ea/Ees ratio is maintained at about 1 to preserve SV. Even if Ea increases substantially with advancing age, Ees also increases to keep the Ea/Ees ratio around 1, and this coupling is compensated [57,70,71]. On the other hand, the Ea/Ees ratio is <1 in HFpEF because the increase of Ees exceeds that of Ea [Figures 5, 6] [56,79]. Although Ees is elevated in hypertensive heart disease depending on increase of Ea, the heart still has the reserve force to augment Ees for maintenance of the LV ejection fraction. In contrast, the excessive rise of Ees over Ea in HFpEF reflects impaired myocardial contractility and an increase of passive myocardial stiffness, which is followed by structural changes such as hypertrophy and fibrosis with the gradual progression of HFpEF. This process suggests that HFpEF will have a poor prognosis [80]. It has not been identified whether HFpEF with a normal EF shows myocardial contractility. In heart failure with a reduced or preserved EF, a substantial increase of Ees irrespective of the increase/decrease of Ea causes failure of ventricular-arterial coupling or afterload mismatch, resulting in a decrease of SV. In addition, excessive augmentation of Ees is followed by myocardial structural changes (hypertrophy and fibrosis), leading to more marked elevation of diastolic elastance in comparison with HFrEF, which can easily precipitate pulmonary congestion due to an abrupt increase of LV end-diastolic pressure during exercise [64,81]. Furthermore, arterial stiffening and LV remodeling by activation of the sympathetic nervous system and renin-angiotensin-aldosterone [RRA] system lead to progression of ventricular-arterial coupling disease [82].
Potential therapeutic strategies targeting abnormal ventricular-arterial stiffening in HFpEF
At present, the use of ARBs, ACEIs, mineralocorticoid receptor antagonists (MRAs), and β-blockers has been established for HFrEF, but not for HFpEF [82-85]. Because the RAA system is activated, elevation of central blood pressure followed by increased arterial stiffness causes ventricular remodeling due to excessive afterload, resulting in progression of heart failure. Therefore, vasodilators (ARBs, ACEIs, and CCBs) are considered to be more effective for reducing the central PP in HFpEF with increased arterial stiffness to manage CHF after decongestion.
Treatment of chronic CRS for preservation of cardiac and renal function
It is important to use diuretics for decongestion when ADHF or acute exacerbation of CHF occurs regardless of whether a patient has HFpEF and HFrEF. At the same time, renal congestion due to volume overload (i.e., excessive renal afterload) causes WRF [86-88] complicated by elevation of central and renal venous pressures, which should also be treated with diuretics.
Repeated exacerbation and improvement of heart failure leads to residual damage, and cardiac function cannot recover fully to the previous state despite treatment for heart failure. As a result of recurrent episodes, the prognosis of heart failure deteriorates in an additive fashion [89]. Thus, the objective of treating CHF is to maintain cardiac and renal function or delay progression of dysfunction without acute exacerbations.
The therapeutic strategy for CHF, especially HFpEF, involves reducing afterload (i.e., afterload mismatch) to maintain ventricular performance. CCBs and/or ARBs are useful antihypertensive agents that lower central PP by vasodilatation. The increase of proteinuria mediated by elevation of intraglomerular pressure can be treated by using ARBs that dilate the renal efferent arterioles. When high-dose loop diuretics are used continuously to treat CHF without congestion, the RAA system is activated, leading to renal impairment along with exacerbation of CHF by progression of LV remodeling [82]. Reducing the daily dosage/number of doses of loop diuretics by using long-acting agents is important for minimizing RAA system activity. In addition, use of a selective vasopressin-2 receptor antagonist may be favorable for renal protection because it induces water diuresis that does not activate the RAA system [90] while maintaining renal hemodynamics [91]. In the future, investigations should be performed to determine the optimum diuretic dosages and combinations adjusted by the pathophysiologic state of heart failure and renal function. Improving vascular distensibility including vasodilatation by nitric oxygen (NO) is also attractive [57]. However, it is necessary to consider hemodynamic treatment that not only reduces afterload on the heart but also renal afterload during heart failure.
WRF is frequent among patients hospitalized for heart failure [28,92,93]. The prevalence of WRF is comparable in HFpEF and HFrEF, being associated with baseline CKD, the history of hypertension and diabetes, age, and diuretic use [94], which are also underlying lifestyle-related diseases for HFpEF. It seems that HFpEF may arise secondary to the aging process and lifestyle-related diseases associated with arterial stiffening and LV remodeling. Therefore, a strategy for preventing HFpEF (for which there is no established treatment) may involve early prevention of various lifestyle-related diseases associated with aging.
Conclusion
Clarifying the pathophysiology of ventricular-arterial-renal interactions with advancing age is very important in relation to developing therapeutic strategies for chronic CRS. Elevation of the central systolic blood pressure with an increase of PP promotes LV remodeling, LV hypertrophy, and renal impairment, leading to a worse cardiovascular prognosis compared with elevation of the peripheral systolic blood pressure. Measurement of the central pressure is required clinically to better identify the mechanisms of ventricular-arterial-renal interactions.
The therapeutic strategy for CHF, especially HFpEF, should involve reducing afterload (i.e., afterload mismatch) to maintain ventricular performance. CCBs and/or ARBs are useful antihypertensive agents that lower central PP by vasodilatation. Moreover, regular exercise, proper caloric intake without excessive amounts of salt and management of body weight without obesity from adolescence may be needed to slow the rate of progression of arterial stiffness at an accelerating pace as aging. To establish an effective therapeutic strategy for cardiorenal disease based on central hemodynamics, it is necessary for the central blood pressure level that prevents cardiovascular target organ damage to be determined by large-scale controlled studies in the future.
- Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, et al. [2009] Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens 27: 461-467. Link: https://tinyurl.com/ycb8bm29
- Hashimoto J, Imai Y, O'Rourke MF [2007] Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens 20: 378-384. Link: https://tinyurl.com/ycycsew8
- Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, et al. [2010] Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J, Go AS, Rafey M, Rahman M, Sheridan A, Gadegbeku CA, Robinson NA, Joffe M. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens 23: 282-289. Link: https://tinyurl.com/ydcvyf6t
- Hashimoto J, Ito S [2011] Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58: 839-846. Link: https://tinyurl.com/y9dkl7pc
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, et al. [2006] Collier D, Hughes AD, Thurston H, O'Rourke M; CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation [CAFE] study. Circulation 113: 1213-1225. Link: https://tinyurl.com/yd24nlmm
- Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, et al. [2008] Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol 51: 2432-2439. Link: https://tinyurl.com/y785vly2
- Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, et al. [2007] Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 50: 197-203. Link: https://tinyurl.com/y7nl4ekz
- Tomiyama H, O'rourke MF, Hashimoto H, Matsumoto C, Odaira M, et al. [2013] Yoshida M, Shiina K, Nagata M, Yamashina A. Central blood pressure: a powerful predictor of the development of hypertension. Hypertens Res 36: 19-24. Link: https://tinyurl.com/yaw2tcpt
- Bristow MR, Lowes BD [2005] Management of heart failure. In: Zipes DP eds. Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 7th ed. Philadelphia: Elsevier Sunders 603-624.
- Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, et al. [2008] Ito M, Suzuki K, Tanabe N, Kodama M, Aizawa Y. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J 72: 489-491. Link: https://tinyurl.com/yazte4hf
- Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, et al. [1999] Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J 20: 447-455. Link:
- Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, et al. [2003] Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289: 194-202. Link: https://tinyurl.com/y7vzj23r
- Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, et al. [2004] Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 25: 1614-1619. Link: https://tinyurl.com/ycpoa9o3
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, et al. [2006] Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251-259. Link: https://tinyurl.com/y9ugklqh
- Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, et al. [2000] Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102: 203-210. Link: https://tinyurl.com/y8ns589q
- Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, et al. [2006] Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity [CHARM] Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113: 671-678. Link: https://tinyurl.com/y9e4vm6l
- van Kimmenade RR, Januzzi JL Jr, Baggish AL, Lainchbury JG, Bayes-Genis A, et al. [2006] Richards AM, Pinto YM. Amino-terminal pro-brain natriuretic Peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol 48:1621-1627. Link: https://tinyurl.com/ydb4ed3h
- Foley RN, Collins AJ [2013] The USRDS: what you need to know about what it can and can't tell us about ESRD. Clin J Am Soc Nephrol 8: 845-851. Link: https://tinyurl.com/y9wjyvno
- Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, et al. [2015] Hamano T, Wakai K, Wada A, Nitta K. An Overview of Regular Dialysis Treatment in Japan [As of 31 December 2013]. Ther Apher Dial 19: 540-574. Link: https://tinyurl.com/y9b97ak9
- KDOQI [2007] KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 49: S12-154. Link: https://tinyurl.com/ybyucwab
- de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, et al. [2013] BEACON Trial Investigators. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492-2503. Link: https://tinyurl.com/y7e5dg3h
- Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, et al. [2014] Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis 64: 510-533. Link: https://tinyurl.com/y8y6pp46
- Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, et al. [2016] Weiss NS, de Boer IH. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988-2014. JAMA 316: 602-610. Link: https://tinyurl.com/y73ddets
- Ronco C, House AA, Haapio M [2008] Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med 34: 957-962. Link: https://tinyurl.com/ycgj89vc
- Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, et al. [2004] Massie BM, O'Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB, Krumholz HM. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 147: 331-338. Link: https://tinyurl.com/yb328blj
- Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B [2006] POSH Investigators. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure [POSH]. Eur Heart J 27: 1216-1222. Link: https://tinyurl.com/yda8kr9k
- Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, et al. [2007] Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 13: 599-608. Link: https://tinyurl.com/yaa38dgf
- Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, et al. [2004] Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61-67. Link: https://tinyurl.com/ycdaz778
- O'Rourke MF, Hashimoto J [2007] Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50: 1-13. Link: https://tinyurl.com/y8yussjp
- Torjesen AA, Wang N, Larson MG, Hamburg NM, Vita JA, et al. [2014] Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham Heart Study. Hypertension. 64:259-65. Link: https://tinyurl.com/ybkxpfu6
- Tomiyama H, Yamashina A [2010] Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J 74: 24-33. Link: https://tinyurl.com/y8q89yup
- Hashimoto J, Ito S [2014] Central pulse pressure links microalbuminuria with plasma B-type natriuretic peptide elevation: causal implication for cardiorenal syndrome in hypertension. J Hypertens 32: 1665-1671. Link: https://tinyurl.com/y9pxuc8n
- Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, et al. [2009] Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol 54: 1730-1734. Link: https://tinyurl.com/y8t23p9v
- Agabiti-Rosei E, Mancia G, O'Rourke MF, Roman MJ, Safar ME, et al. [2007] Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension 50: 154-160. Link: https://tinyurl.com/yd7axlbd
- McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, et al. [2008] Rowe CV, Cockcroft JR, Wilkinson IB; Anglo-Cardiff Collaborative Trial Investigators. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension 51: 1476-1482. Link: https://tinyurl.com/y82qlhan
- O'Rourke MF, Safar ME [2005] Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200-204. Link: https://tinyurl.com/yab8qmxu
- Munakata M, Nunokawa T, Yoshinaga K, Toyota T [2006] J-TOPP Study Group. Brachial-ankle pulse wave velocity is an independent risk factor for microalbuminuria in patients with essential hypertension--a Japanese trial on the prognostic implication of pulse wave velocity [J-TOPP]. Hypertens Res 29: 515-521. Link: https://tinyurl.com/y7e4pw2n
- Taal MW, Sigrist MK, Fakis A, Fluck RJ, McIntyre CW [2007] Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin Pract 107: 177-181. Link: https://tinyurl.com/y79fuwxe
- Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, et al. [2001] Gebel MJ, Galanski M, Koch KM, Haller H. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med 344: 410-417. Link: https://tinyurl.com/yaa27v5a
- Radermacher J, Ellis S, Haller H [2002] Renal resistance index and progression of renal disease. Hypertension 39: 699-703. Link: https://tinyurl.com/y7bcgaor
- Radermacher J, Mengel M, Ellis S, Stuht S, Hiss M, et al. [2003] Schwarz A, Eisenberger U, Burg M, Luft FC, Gwinner W, Haller H. The renal arterial resistance index and renal allograft survival. N Engl J Med 349: 115-124. Link: https://tinyurl.com/y8fwx9wb
- Nosadini R, Velussi M, Brocco E, Abaterusso C, Carraro A, et al. [2006] Piarulli F, Morgia G, Satta A, Faedda R, Abhyankar A, Luthman H, Tonolo G. Increased renal arterial resistance predicts the course of renal function in type 2 diabetes with microalbuminuria. Diabetes 55: 234-239. Link: https://tinyurl.com/y8j7tyay
- Okura T, Kurata M, Irita J, Enomoto D, Jotoku M, et al. [2010] Nagao T, Koresawa M, Kojima S, Hamano Y, Mashiba S, Miyoshi K, Higaki J. Renal resistance index is a marker of future renal dysfunction in patients with essential hypertension. J Nephrol 23: 175-180. Link: https://tinyurl.com/y9zfwwyx
- Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, et al. [2005] Moriya H, Suzuki S, Miura S. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 46: 603-609. Link: https://tinyurl.com/y73x64rs
- Pontremoli R, Viazzi F, Martinoli C, Ravera M, Nicolella C, et al. [1999] Berruti V, Leoncini G, Ruello N, Zagami P, Bezante GP, Derchi LE, Deferrari G. Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant 14: 360-365. Link: https://tinyurl.com/y96nrorn
- Hamano K, Nitta A, Ohtake T, Kobayashi S [2008] Associations of renal vascular resistance with albuminuria and other macroangiopathy in type 2 diabetic patients. Diabetes Care 31: 1853-1857. Link: https://tinyurl.com/y9wbfxua
- Dworkin LD, Brenner BM [2004] The renal circulation. In: Brenner BM, Editor. Brenner & Rector's The Kidney, 7th ed. Philadelphia: W. B. Saunders 309-352.
- Kelly RP, Gibbs HH, O'Rourke MF, Daley JE, Mang K, et al. [1990] Morgan JJ, Avolio AP. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J. 11:138-44. Link: https://tinyurl.com/ycv3djhc
- Matsui Y, Eguchi K, O'Rourke MF, Ishikawa J, Miyashita H, et al. [2009] Shimada K, Kario K. Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension 54: 716-723. Link: https://tinyurl.com/ybhgqonm
- Miyashita H, Aizawa A, Hashimoto J, Hirooka Y, Imai Y, et al [2010] Kawano Y, Kohara K, Sunagawa K, Suzuki H, Tabara Y, Takazawa K, Takenaka T, Yasuda H, Shimada K. Cross-sectional characterization of all classes of antihypertensives in terms of central blood pressure in Japanese hypertensive patients. Am J Hypertens. 23: 260-268. Link: https://tinyurl.com/y8l6hmt4
- Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, et al. [2000] Laloux B, Brunner H, Laurent S. Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 101: 2601-2606. Link: https://tinyurl.com/ybvwurua
- Cheng HM, Chuang SY, Sung SH, Yu WC, Pearson A, et al. [2013] Lakatta EG, Pan WH, Chen CH. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol 62: 1780-1787. Link: https://tinyurl.com/ydhdmp2f
- Sharman JE, Marwick TH, Gilroy D, Otahal P, Abhayaratna WP, et al. [2013] Stowasser M; Value of Central Blood Pressure for GUIDing ManagEment of Hypertension Study Investigators. Randomized trial of guiding hypertension management using central aortic blood pressure compared with best-practice care: principal findings of the BP GUIDE study. Hypertension 62: 1138-1145. Link: https://tinyurl.com/y94tcmvx
- Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, et al. [2016] Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail 9: 003116. Link: https://tinyurl.com/y7ffel8r
- Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S, Goto D, Takeshita A [2006] JCARE-CARD Investigators. Clinical characteristics and outcome of hospitalized patients with heart failure in Japan. Circ J 70: 1617-1623. Link: https://tinyurl.com/ycws3fe3
- Kawaguchi M, Hay I, Fetics B, Kass DA [2003] Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107: 714-720. Link: https://tinyurl.com/y9yq8kbx
- Kass DA [2005] Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 46: 85-193. Link: https://tinyurl.com/y7vbupfk
- Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA [2005] Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112: 2254-2262. Link: https://tinyurl.com/ybmzexbv
- Cheng CP, Noda T, Nozawa T, Little WC [1993] Effect of heart failure on the mechanism of exercise-induced augmentation of mitral valve flow. Circ Res 72: 795-806. Link: https://tinyurl.com/yan8waae
- Ohno M, Cheng CP, Little WC [1994] Mechanism of altered patterns of left ventricular filling during the development of congestive heart failure. Circulation 89: 2241-2250. Link: https://tinyurl.com/y8b9wrc8
- Maeder MT, Kaye DM [2009] Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 53: 905-918. Link: https://tinyurl.com/y6u9mnuj
- Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, et al. [2007] How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539-2550. Link: https://tinyurl.com/y7n2946o
- Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D [2005] Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail 11: 177-187. Link: https://tinyurl.com/yb7lcbqj
- Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, et al. [2008] Aaron MF, Aranda JM Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 118: 1433-1441. Link: https://tinyurl.com/y86cokv6
- Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, et al. [2012] Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol 110: 870-876. Link: https://tinyurl.com/ybnfnbwo
- Redfield MM [2016] Heart Failure with Preserved Ejection Fraction. N Engl J Med 375: 1868-1877. Link: https://tinyurl.com/yd83r3lx
- Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, et al. [2012] Semigran MJ, Borlaug BA, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J 164: 763-770. Link: https://tinyurl.com/ybelqozp
- Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM [2010] Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3: 588-55. Link: https://tinyurl.com/yaflldp2
- Hogg K, Swedberg K, McMurray J [2004] Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 43: 317-327. Link: https://tinyurl.com/y8j3vzhb
- Antonini-Canterin F, Carerj S, Di Bello V, Di Salvo G, La Carrubba S, et al. [2009] Vriz O, Pavan D, Balbarini A, Nicolosi GL; Research Group of the Italian Society of Cardiovascular Echography [SIEC]. Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologist's point of view. Eur J Echocardiogr 10: 36-43. Link: https://tinyurl.com/yayoov6b
- Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, et al. [1998] Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 32: 1221-1227. Link: https://tinyurl.com/ydxzy93v
- Burkhoff D, Maurer MS, Packer M [2003] Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation 107: 656-658. Link: https://tinyurl.com/yab4z85j
- Quinones MA, Gaasch WH, Alexander JK [1976] Influence of acute changes in preload, afterload, contractile state and heart rate on ejection and isovolumic indices of myocardial contractility in man. Circulation 53: 293-302. Link: https://tinyurl.com/ybsbh4oc
- Ross J Jr [1976] Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog Cardiovasc Dis 18: 255-264. Link: https://tinyurl.com/yb3qjuuk
- Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, et al. [2009] Augeul L, Ovize M, Derumeaux G. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two-dimensional strain imaging study Eur J Echocardiogr 10: 914-921. Link: https://tinyurl.com/y9sdhlpq
- Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, et al. [2007] Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol 50: 1570-1577. Link: https://tinyurl.com/ycrabn7h
- Fujimoto N, Onishi K, Dohi K, Tanabe M, Kurita T, Takamura T, et al. [2008] Yamada N, Nobori T, Ito M. Hemodynamic characteristics of patients with diastolic heart failure and hypertension. Hypertens Res 31: 1727-1735. Link: https://tinyurl.com/yc4hbmy8
- Robotham JL, Takata M, Berman M, Harasawa Y [1991] Ejection fraction revisited. Anesthesiology 74: 172-183. Link: https://tinyurl.com/yd3rmhvq
- Borlaug BA, Kass DA [2008] Ventricular-vascular interaction in heart failure. Heart Fail Clin 4: 23-36. Link: https://tinyurl.com/yccqznsk
- Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM [2009] Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 54: 410-418. Link: https://tinyurl.com/yavg32a3
- Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ [1991] Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol 17: 1065-1072. Link: https://tinyurl.com/y7qllbnu
- Metra M, Teerlink JR [2017] Heart failure. Lancet 390: 1981-1995. Link: https://tinyurl.com/y8tmswum
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. [2013] 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: 1810-1852. Link: https://tinyurl.com/yd2thxew
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. [2016] Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology [ESC]. Developed with the special contribution of the Heart Failure Association [HFA] of the ESC. Eur J Heart Fail 18: 891-975. Link: https://tinyurl.com/y7txtkj3
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, et al. [2016] 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 134: 282-293. Link: https://tinyurl.com/yahka2b5
- Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, et al [2009] Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53: 589-596. Link: https://tinyurl.com/yaewba4q
- Dupont M, Mullens W, Tang WH [2011] Impact of systemic venous congestion in heart failure. Curr Heart Fail Rep 8: 233-241. Link: https://tinyurl.com/y8xgtvmy
- Ross EA [2012] Congestive renal failure: the pathophysiology and treatment of renal venous hypertension. J Card Fail 18: 930-938. Link: https://tinyurl.com/y7cwkfqr
- Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, et al. [2005] Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 96: 11-17. Link: https://tinyurl.com/y84mpunr
- Ambrosy A, Goldsmith SR, Gheorghiade M [2011] Tolvaptan for the treatment of heart failure: a review of the literature. Expert Opin Pharmacother 12: 961-976. Link: https://tinyurl.com/ycsz25fr
- Costello-Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, et al. [2006] Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol 290: 273-278. Link: https://tinyurl.com/ycsz25fr
- Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, et al. [2003] Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail 9: 13-25. Link: https://tinyurl.com/ya56h2ob
- de Silva R, Nikitin NP, Witte KK, Rigby AS, Goode K, et al. [2006] Bhandari S, Clark AL, Cleland JG. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J 27: 569-581. Link: https://tinyurl.com/yc267vuj
- Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, et al . [2014] Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 35: 455-469. Link: https://tinyurl.com/y9cozo3h

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
