Archives of Clinical Gastroenterology
Dietary molybdenum may stimulate the growth of colonic sulfur reducing bacteria, increasing hydrogen sulfide levels in the human colon and the possible health effects of an excess of colonic sulfides
Brian James Grech*
Cite this as
Grech BJ (2022) Dietary molybdenum may stimulate the growth of colonic sulfur reducing bacteria, increasing hydrogen sulfide levels in the human colon and the possible health effects of an excess of colonic sulfides. Arch Clin Gastroenterol 8(2): 029-035. DOI: 10.17352/2455-2283.000109Copyright License
© 2022 Grech BJ. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Molybdenum is a trace mineral needed in small quantities by most life forms. In living organisms, a molybdenum atom is found within molybdenum-dependent enzymes or molybdoenzymes. Molybdoenzymes catalyze reactions in carbon, sulfur, and nitrogen metabolism. Only four molbdoenzymes have been identified in humans. Most of the known molybdoenzymes are found in bacteria. Dietary molybdenum can be administrated to humans, to treat Wilson disease and tungsten poisoning; and it may be useful in arthritis. Sulfur-reducing bacteria are the bacterial group that reduces certain sulfur molecules to hydrogen sulfide. These bacteria can inhabit anaerobic parts of the gastrointestinal tract of mammals and are the predominant producer of hydrogen sulfide in the human colon. Hydrogen sulfide plays a major role in the malodor of human flatus. Some individuals have reported an increase in foul odoriferous gases from the colon after molybdenum supplementation. The underlying mechanism as to how this occurs is currently not known. Possible bacteria that are involved could be sulfur-reducing bacteria and methionine dissimilating bacteria. Supplementing sheep with molybdenum and with sulfur exclusively in the form of methionine can stimulate the growth of sulfur-reducing bacteria and increase the level of sulfides in the rumen. The molybdoenzyme, thiosulfate reductase, is found in sulfur-reducing bacteria and catalyzes the reduction of thiosulfate to hydrogen sulfide. The source of thiosulfate could be from ruminal epithelial cells detoxifying methanethiol, produced by methionine dissimilating bacteria, degrading the dietary methionine to methanethiol. Therefore, the molybdenum could be activating thiosulfate reductases of sulfur-reducing bacteria in the rumen of these animals. The human colon can also harbor sulfur-reducing bacteria, and dietary molybdenum and methionine can reach this organ. Therefore, dietary molybdenum may be stimulating the growth of sulfur-reducing bacteria in some individuals. Sulfides in the human colon could have beneficial and detrimental effects on health. Such effects could include the already mentioned malodor of flatus, the stabilizing of the microbiome-mucosa interface in an intestinal dysbiosis, the treatment of hypertension and the promotion of inflammation in ulcerative colitis.
Abbreviations
MDB: Methionine Dissimilating Bacteria; SCE: Cystathionine γ-lyase; SRB: Sulfur-Reducing Bacteria; UC: Ulcerative Colitis
Introduction
Molybdenum is a trace mineral needed in small quantities by most life forms. In living organisms, a molybdenum atom is found within molybdenum-dependent enzymes or molybdoenzymes. With the exception of the iron-molybdenum cofactor of the bacterial molybdoenzyme, nitrogenase, the active site of all the other molybdoenzymes has a molybdenum atom bounded to a molybdopterin backbone molecule. This complex is called the molybdenum cofactor. Molybdoenzymes are involved in carbon, sulfur, and nitrogen metabolism. More than 50 molybdoenzymes have been biochemically characterized. In addition to these enzymes, numerous genes that could code for molybdenum-containing proteins with unknown functions have been annotated in the bacterial genomes that have been sequenced. Only four of these molybdoenzymes have been identified in humans. Most of these molybdoenzymes are found in bacteria [1-3].
Dietary molybdenum can be administrated in certain human diseases [4]. Molybdenum has an antagonistic property on copper; and molybdenum maybe used to treat copper metabolism disorders, e.g. Wilson Disease [5]. Tungsten can alter the metabolism of molybdenum and molybdenum can be administered to treat tungsten poisoning [6]. There seems to be a considerable excess of molybdenum-dependent apoenzymes within cells and molybdenum supplementation may increase the activity of mammalian molybdoenzymes, in individuals that are not suffering from a molybdenum deficiency. For example, molybdenum may treat arthritic joint pain by increasing the activity of mammalian xanthine oxidoreductases in cells [7].
Sulfur-Reducing Bacteria (SRB) are a bacterial group that inhabit anaerobic environments, this includes anaerobic parts of the gastrointestinal tract of mammals. SRB are a diverse group and consists of about 74 genera within the bacteria domain [8]. Species from the Desulfovibrio genera account for 66% of the SRB in the human colon [9]. These sulfidogenic bacteria can perform anaerobic respiration, i.e. they use sulfur molecules as the terminal electron acceptor and reduce it to hydrogen sulfide. In contrast, in aerobic respiration, molecular oxygen is utilized as the terminal electron acceptor and is reduced to water. SRB can synthesize and excrete a large amount of hydrogen sulfide [8].
Hydrogen sulfide is a colorless noxious gas with the characteristic malodor of rotten eggs. Hydrogen sulfide inhibits cytochrome c oxidase activity in the mitochondrial electron transport chain, reducing adenosine triphosphate levels and metabolic activity in cells. This gas plays a major role in the malodor of human flatus and SRB are the predominant producers of hydrogen sulfide in the colon [10,11]. Hydrogen sulfide is also the third intercellular gaseous mediator, in mammals. Nitric oxide and carbon monoxide are the other two gaseous mediators. Small amounts of hydrogen sulfide is synthesized in the cytoplasm and mitochondria of mammalian cells, through the reverse trans-sulfuration pathway. Hydrogen sulfide acts as a messenger molecule and participates in the regulation of homeostasis of a myriad of mammalian systems [12].
Individuals have reported an increase in foul odoriferous gases from the colon after molybdenum supplementation [13]. The underlying mechanism as to how this occurs is currently not known. However, it is possible that SRB are involved. This mini-review discusses this possibility; and possible beneficial and detrimental health effects of sulfides in the human colon.
Dietary molybdate may activate thiosulfate reductases of SRB in the rumen of sheep, increasing the level of hydrogen sulfide in this organ
Supplementing sheep with molybdate can increase the level of SRB and hydrogen sulfide in their rumen [14,15]. Molybdenum is transported and stored in its oxyanion form, i.e. as molybdate, in organisms [1]. Mills [14] measured hydrogen sulfide levels in the rumen of sheep supplemented with and without molybdate; and recorded higher levels in the sheep that had been supplemented with molybdate. Huisingh and colleagues [15] fed sheep diets with and without molybdate; and with sulfur exclusively in the form of DL-methionine and found that there was about a six-fold increase in ruminal SRB when their diet was supplemented with molybdate. The group also cultured ruminal fluid specimens from these sheep, with and without molybdate; and with sulfur in the form of methionine. This resulted in an almost two-fold increase in hydrogen sulfide when molybdate was added to the culture media. Both Mill and Huisingh et al. hypothesized that the sulfide came from sulfur- containing amino acids and Huisingh and colleagues hypothesized that molybdenum could be activating a bacterial enzyme.
The dietary molybdate fed to the sheep in Mill and Huisingh et al. studies, or added to cultures of ruminal fluid in Huisingh and colleagues experiments, could be increasing the activity of thiosulfate reductases of SRB; and the increased activity could be increasing the level of hydrogen sulfide gas in the rumen of the sheep and in the cultures. Thiosulfate reductase belongs to the sulfurtransferase enzyme family and enzymes from this family, transfer sulfur-containing groups. The accepted names for this enzyme is thiosulfate-thiol sulfurtransferase or thiosulfate-dithiol sulfurtransferase [16]. Thiosulfate reductase catalyzes the reduction of thiosulfate to sulfite and hydrogen sulfide [17]. This enzyme is found in prokaryotes and eukaryotes [16] and the prokaryotic thiosulfate reductase is predicted to be a molybdoenzyme [18].
Desulfovibrio desulfuricans is probably one of the predominate SRB species in the gut of sheep [19-21] and this species probably contains functional thiosulfate reductases. The annotated thiosulfate reductase gene in the genome sequence of the D. desulfuricans strain G11 [22], is homologous to the equivalent genes in the sequenced genomes of Desulfovibrio valgaris (E - value = 0.0, Percentage Identity = 59.29%; unpublished data, Grech BJ). The D. desulfuricans strain G11 was isolated from the rumen of a steer [19]. Thiosulfate reductase activity has been observed in D. valgaris subspecies vulgaris Marburg [17].
The production of thiosulfate in the rumen could be from the epithelial cells lining this organ. Thiosulfate production in the mammalian gut is better understood in the mammalian colon and this review will attempt to explain the phenomena observed in the rumen of sheep, using our knowledge of the colon. Methionine dissimilating bacteria (MDB) degrades methionine in the colon to methanethiol gas [23,24]. Methionine g-lyase is the enzyme that catalyzes this reaction [25]. In the colon this gas can either be absorbed by fecal matter, passed through the rectum, or cross the plasma membrane of colonic epithelia. Methanethiol gas that passes into the epithelia is quickly oxidized to thiosulfate, by a specialized biochemical pathway within these cells [26,27].
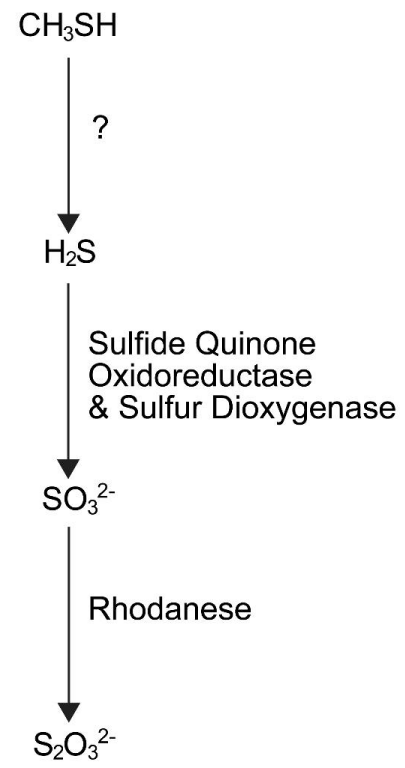
The biochemical pathway in colonic epithelial cells that oxidized methanethiol has not been completely elucidated; however, a tentative pathway has been proposed. The process probably begins with the metabolism of methanethiol to hydrogen sulfide. It is not known how this occurs. The hydrogen sulfide product is probably then oxidized to sulfite by sulfide quinone oxidoreductase and sulfur dioxygenase. Rhodanese with sulfur transferase activity probably converts the sulfite to thiosulfate (Figure 1). Thiosulfate produced by these cells either enters the colon venous blood [26,27], or the lumen of the colon. The key enzyme in this tentative pathway is probably rhodanese [28].
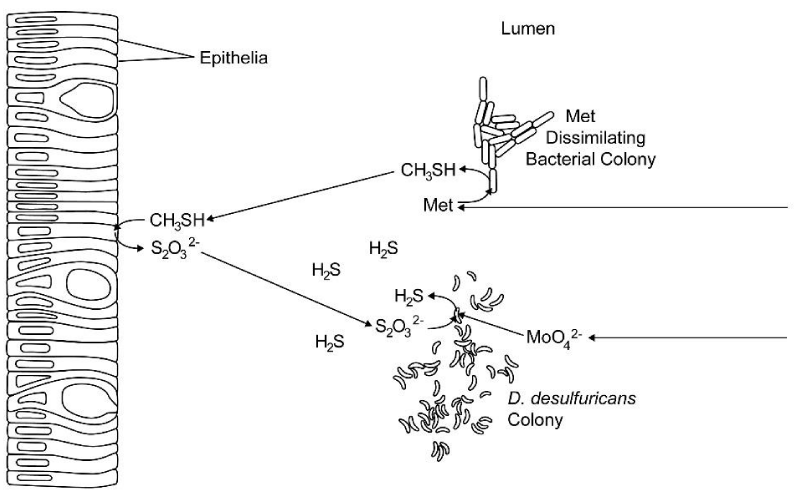
Rhodanese activity has been observed in the rumen of sheep and, therefore, the ruminal epithelial cells can probably oxidize methanethiol and hydrogen sulfide to thiosulfate [29-31]. When the sheep in the studies performed by Mills and Huisingh et al. were supplemented with molybdate, ruminal MDB probably converted methionine to methanethiol. The rumen epithelia may have then converted the methanethiol to thiosulfate and some of the thiosulfate may have entered the lumen. The molybdate probably increased the activity of thiosulfate reductases in SRB and the increased activity of this enzyme probably reduced the thiosulfate in the lumen to hydrogen sulfide (Figure 2).
Huisingh and colleagues [15] also found that supplementing the sheep with molybdate and sulfur exclusively in the form of sulfate, did not change the ruminal SRB level and there was over a two-fold decrease in hydrogen sulfide from the SRB cultures. Molybdate has been proposed as a structural analog of sulfate and it is thought that molybdate competes with the sulfate substrates, in the first step of dissimilative and assimilative sulfate reduction, in SRB. The enzyme that catalyzes this step is adenosine 5’-triphosphate sulfurylase [32]. Therefore, it is unlikely that sulfate was a source of sulfur when the sheep were fed molybdate and sulfate.
Humans harbor SRB in their Colon; and dietary molybdenum and methionine could reach this organ
The ingestion of a molybdenum supplement with a high protein-content food could result in molybdenum escaping absorption and proteins escaping digestion and absorption; in the proximal proportion of the small intestine. This could result in the molybdenum and methionine reaching the colon. About 50% of humans in western countries harbor SRB in there colon [33-35]. Dietary molybdenum has been detected in the feces of humans and generally increases as intake increases [36]; and there is a direct relationship between the level of meat intake and the level of fecal excretion of sulfide from the human bowel [37]. Given that humans can have SRB, molybdenum, and methionine in the lumen of their colon; and the reports of the worsening of the malodor of flatus after molybdenum supplementation; molybdenum maybe stimulating the growth of SRB in some individuals.
Possible health effects of luminal sulfides in the human colon
Luminal sulfides that are tentatively produced by the presents of molybdenum, methionine, MDB, and SRB in the human colon; could produce the already mention malodor of flatus and stools; and it could have other effects on health. The organ that is most likely to be effective by these sulfides is the colon. Healthy colonic epithelial cells seem to be well adapted to dealing with high levels of sulfides. The metabolism of sulfides, that diffuse across the plasma membrane of these cells generates adenosine triphosphate molecules. Consequently, intestinal epithelia can accept sulfides as an energy source and sulfides have been shown to stimulate the proliferation of these cells [38]. In addition to this, the single layer of epithelia functions as a physical barrier preventing sulfides in the lumen of the intestine, from entering other tissues throughout the body [26,28,39]. Other effects of colonic sulfides may include the stabilization of the microbiome-mucosa interface in an animal model of an intestinal dysbiosis, the positive influence on hydrogen sulfide homeostasis in mammalian cells and the promotion of inflammation in the colon in ulcerative colitis (UC) [39-42]. Interestingly, some of these effects could be beneficial to health.
In certain human diseases there maybe a higher than normal likelihood of dietary molybdenum and methionine entering the colon. The diarrhea-predominant subtype of irritable bowel syndrome maybe one of these diseases. This is because, the transit of food through the small intestine can be shorter in this subtype of this syndrome [43,44].
Colonic luminal sulfides may stabilize the microbiome-mucosa interface in an intestinal dysbiosis
Intestinal microbes living in biofilms may promote homeostasis to the microbiota [39]. Biofilms are slimy extracellular matrices constructed from polymeric substances in which microbes are embedded within. A single biofilm can comprise of a syntrophic consortium of microorganisms. Biofilms adhere bacterial cells to each other and to surfaces; trap nutrients for cell growth; and protect cells by reducing their exposure to harmful substances [45,46]. In the colon, disrupted biofilms can increase the cytotoxicity of bile and the number of planktonic bacteria (i.e. free-floating or free-swimming bacterial cells) [46].
In the colon, mucus is organized as a mucus bilayer composed of an adherent inner layer and a loose detached outer layer. These layers protect the epithelia lining of the intestine from hazardous luminal contents [47]. In mice and rats, a degraded and compromised colonic inner mucus layer in conjunction with disrupted biofilms, can lead to bacteria in contact with epithelia; and an increased infiltration of dietary toxins and bioactive components from the lumen into the epithelia. These changes can result in the recruitment of inflammatory cells, changes to the metabolism of serotonin, and an increase production of mucus from goblet cells [39,41].
Hydrogen sulfide appears to favorably modulate bacterial biofilms and inhibit planktonic bacteria. The administration of hydrogen sulfide donors (i.e. sodium hydrosulfide or diallyl disulfide) at a low dose to human-derived intestinal biofilms, results in a higher metabolic activity of the bacteria and an increased biofilm mass. This is likely due to an increased number of bacteria, proteins, and polysaccharides within the biofilms. When higher doses of diallyl disulfide were administered to these biofilms, it was shown to inhibit the metabolic activity of human strains of planktonic bacteria [39,41].
Hydrogen sulfide may also restore a degraded and compromised inner colonic mucus layer and prevent certain nonsteroidal anti-inflammatory drugs inducing a dysbiosis. Experiments with cystathionine γ-lyase (CSE) deficient rats, results in a thinner than normal inner mucus layer and bacteria in contact with the intestinal epithelia. CSE plays a role in the regulation of mucus production from intestinal goblet cells. A similar effect is seen in rats treated with a CSE inhibitor i.e. b-cyanoalanie. Intracolonic administration of diallyl disulfide to rats that are treated with b-cyanoalanie seems to restore the mucus layer. After the treatment a void is observed like that of healthy mice and rats, that separates the epithelia from the microbiota. A mucus layer secreted from intestinal goblet cells probably fills this void [39,41]. The administered hydrogen sulfide has probably diffused into intestinal goblet cells, triggering these cells to secrete mucus into the lumen of the colon. Finally, an intestinal dysbiosis plays a key role in the pathogenesis of irritable bowel syndrome [48] and in inflammatory bowel disease [49] and a hydrogen sulfite donor may treat these diseases.
Sulfides in the lumen of the colon could treat hypertension
The absence of a microflora in germ free mice has been shown to be associated with reduced activity of CSE; an increase in cysteine levels in gastrointestinal and extra-intestinal tissues; reduced free levels of hydrogen sulfide in the inferior vena cava blood plasma and gastrointestinal tissues; and reduced bound sulfane sulfur levels in blood plasma, adipose tissue, and lung tissue [41]. CSE is also a key enzyme in the synthesis of endogenous hydrogen sulfide in mammalian cells [12] and reduced activity of this enzyme leads to reduced levels of endogenous hydrogen sulfide. Cysteine is a substrate for CSE and increases in tissue cysteine levels indicates less utilization of this substrate by CSE [41].
Luminal hydrogen sulfide may regulate hydrogen sulfide homeostasis in mammalian cells and this may treat hypertension. The intracolonic administration of sodium hydrosulfide to hypertensive and normotensive rats, has been shown to lower arterial blood pressure. Interestingly, the hypertensive rat showed a more pronounced decrease in blood pressure than the normotensive rats. Also the intracolonic administration of sodium hydrosulfide to rats, has been shown to be as potent as intravenous administration of a hydrogen sulfide donor and the therapeutic effect from intracolonic administration lasts longer. The underlying mechanism by which hydrogen sulfide lowers blood pressure in rats is not known, however it is thought that it induces peripheral vasodilation [50].
It is currently not known how colonic bacteria and the exogenous hydrogen sulfide derived from these microbes regulates endogenous hydrogen sulfide synthesis of its host, however some ideas have been put forward. The regulation of hydrogen sulfide synthesis in mammals could be either by some liver-dependent mechanism, that responds to the presents of thiosulfate or sulfane sulfur in the portal blood. This idea is supported by an increase in portal but not peripheral blood levels of thiosulfate and sulfane sulfur products, after the administration of sodium hydrosulfide to the intestinal lumen or rats. It is also possible that endogenous hydrogen sulfide synthesis could be regulated by the enteric nervous system. The enteric nervous system could respond to signals from the Entrochromaffin cells, that are produced when luminal sulfides enters these cells [51].
Colonic Luminal Sulfides May Promote Inflammation in UC
Luminal sulfides maybe implicated in the etiology of UC and it may increase the risk of a relapse of this disease. UC is the most common form of inflammatory bowel disease and effects the colon and rectum. The disease is characterized by broad epithelial cell damage, crypt abscesses, and an accumulation of neutrophils in colonic tissue. The etiology of this disease is poorly understood. Possible factors that could be involve are: genetics, an intestinal dysbiosis, environmental components, and an intestinal immune system dysfunction [52].
The tentative role of sulfides in the etiology of UC and in relapses in UC, originates from several lines of evidence. When fecal sulfide and SRB levels in the colon are measured in UC and healthy individuals, UC levels can be higher. In addition to this, in an animal model of colitis, it is possible to induce a pathological state like that observed in UC using dextran sulfate sodium. It is thought that SRB metabolize sulfate from dextran sulfate sodium to hydrogen sulfide, in this model. Furthermore, the consumption of foods high in sulfur is associated with an increased likelihood of relapse in UC sufferers in remission [28]. Finally, there can be a reduction in the expression of rhodanese messenger RNA and lower rhodanese enzymatic activity in UC. Lower rhodanese activity probably reduces the capacity of colonic epithelia to detoxify sulfides and the high level of sulfides may damage colonic tissue [42].
Conclusion
This review hypothesized that dietary molybdenum could activate thiosulfate reductase of SRB and that the dietary methionine could be a source of sulfur in the rumen of sheep in Mill and Huisingh et al. studies. This mechanism has the potential to increase the ruminal sulfide concentration to a level that could not be produced from MDB degrading methionine and the mechanism agrees with Mill and Huisingh et al. hypotheses [14,15]. It is also possible that ruminal sulfide levels in sheep and other ruminal animals, could be increased by feeding dietary molybdenum with other sulfur-containing amino acids, i.e. cysteine, homocysteine, and taurine. Consequently, dietary molybdenum maybe useful in animal studies to increase sulfide levels in the rumen, when sulfur-containing amino acids are the sources of sulfur. An assay of thiosulfate reductase activity in cultures of SRB that contain the thiosulfate reductase gene, with sulfur exclusively in the form of thiosulfate and with other nutrients at levels that meet the requirement for normal growth, with and without the addition of molybdenum, should confirm this hypothesis.
The review provides evidence to support the reports by individuals, that dietary molybdenum can worsen the odor of flatus and stools; and suggests that molybdenum and methionine in the human colon could have beneficial or detrimental effects on health. Molybdenum and methionine supplementation could treat an intestinal dysbiosis. It could also regulate hydrogen sulfide homeostasis. Therefore, the presents of molybdenum and methionine in the colon could treat diseases that seem to be unrelated to the colon.
Hydrogen sulfide-based therapies have considerable promise for the treatment of many diseases [53] and this suggests a novel use for dietary molybdenum and methionine, in humans. However, given that it could produce a malodor of flatus and stools, it is unlikely that this tentative hydrogen sulfide-based therapy would be used on humans in clinics. In addition to this, practitioners may need to exercise precautions when prescribing dietary molybdenum to patients with UC. This is because sulfides could have detrimental effects in this disease. Although, if we assume that the underlying mechanism put forward in this review is correct and that this also occurs in humans; and that a low rhodanese activity is a cause of high colonic sulfide levels in UC, molybdenum in the colon would not increase the level of sulfides in this organ.
Molybdenum and methionine supplementation maybe useful in humans in short-term studies of certain diseases. A good candidate maybe the diarrheal type of irritable bowel syndrome. This is because, there is a higher than normal likelihood of dietary molybdenum and methionine entering the colon, there is an intestinal dysbiosis; and in an animal model that mimics the visceral hypersensitivity seen in the colon, hydrogen sulfide has been shown to treat symptoms [54]. These possibilities justify a need for more work in this area.
I thank the University of Queensland, Brisbane, Australia, for their assistance with obtaining copies of research papers and review papers.
- Mendel RR, Kruse T. Cell biology of molybdenum in plants and humans. Biochim Biophys Acta. 2012 Sep;1823(9):1568-79. doi: 10.1016/j.bbamcr.2012.02.007. Epub 2012 Feb 17. PMID: 22370186.
- Hille R, Hall J, Basu P. The mononuclear molybdenum enzymes. Chem Rev. 2014 Apr 9;114(7):3963-4038. doi: 10.1021/cr400443z. Epub 2014 Jan 28. PMID: 24467397; PMCID: PMC4080432.
- Peng T, Xu Y, Zhang Y. Comparative genomics of molybdenum utilization in prokaryotes and eukaryotes. BMC Genomics. 2018 Sep 19;19(1):691. doi: 10.1186/s12864-018-5068-0. PMID: 30231876; PMCID: PMC6147048.
- National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. Canberra, Australia. 2017;189- 192.
- Li WJ, Chen C, You ZF, Yang RM, Wang XP. Current Drug Managements of Wilson's Disease: From West to East. Curr Neuropharmacol. 2016;14(4):322-5. doi: 10.2174/1570159x14666151130222427. PMID: 26639459; PMCID: PMC4876588.
- Sardesai VM. Molybdenum: an essential trace element. Nutr Clin Pract. 1993 Dec;8(6):277-81. doi: 10.1177/0115426593008006277. PMID: 8302261.
- Grech BJ. Mechanistic insights into the treatment of iron-deficiency anemia and arthritis in humans with dietary molybdenum. Eur J Clin Nutr. 2021 Aug;75(8):1170-1175. doi: 10.1038/s41430-020-00845-7. Epub 2021 Jan 29. PMID: 33514867.
- Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA. Metabolic Diversity of Microorganisms. In: Brock Biology of Microorganisms, 14th Ed. England: Pearson Education Limited. 2014;437-439.
- Gibson GR, Macfarlane GT, Cummings JH. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut. 1993 Apr;34(4):437-9. doi: 10.1136/gut.34.4.437. PMID: 8491386; PMCID: PMC1374298.
- Tangerman A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J Chromatogr B Analyt Technol Biomed Life Sci. 2009 Oct 15;877(28):3366-77. doi: 10.1016/j.jchromb.2009.05.026. Epub 2009 May 21. PMID: 19505855.
- Suarez FL, Springfield J, Levitt MD. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut. 1998 Jul;43(1):100-4. doi: 10.1136/gut.43.1.100. PMID: 9771412; PMCID: PMC1727181.
- Huang CW, Moore PK. H2S Synthesizing Enzymes: Biochemistry and Molecular Aspects. Handb Exp Pharmacol. 2015;230:3-25. doi: 10.1007/978-3-319-18144-8_1. PMID: 26162827.
- Grech BJ. Molybdenum Supplementation May Increase Sulfides in the Human Colon. Arch Clin Gastroenterol. 2022 .
- MILLS CF. Comparative studies of copper, molybdenum and sulphur metabolism in the ruminant and the rat. Proc Nutr Soc. 1960;19:162-9. doi: 10.1079/pns19600042. PMID: 13770944.
- Huisingh J, Milholland DC, Matrone G. Effect of molybdate on sulfide production from methionine and sulfate by ruminal microorganisms of sheep. J Nutr. 1975 Sep;105(9):1199-205. doi: 10.1093/jn/105.9.1199. PMID: 1159535.
- Moss G P. International Union of Biochemistry and Molecular Biology. School of Physical and Chemical Sciences, Queen Mary, University of London, Mile End Road, London 2021;https://www.qmul.ac.uk/sbcs/iubmb/.
- Barrett EL, Clark MA. Tetrathionate reduction and production of hydrogen sulfide from thiosulfate. Microbiol Rev. 1987 Jun;51(2):192-205. doi: 10.1128/mr.51.2.192-205.1987. PMID: 3299028; PMCID: PMC373103.
- Leimkühler S, Iobbi-Nivol C. Bacterial molybdoenzymes: old enzymes for new purposes. FEMS Microbiol Rev. 2016 Jan;40(1):1-18. doi: 10.1093/femsre/fuv043. Epub 2015 Oct 13. PMID: 26468212.
- McInerney MJ, Bryant MP, Pfennig N. Anaerobic Bacterium that Degrades Fatty Acids in Syntrophic Association with Methanogens. Arch Microbiol. 1979;122:129-135
- Forsberg CW. Sulfide production by some rumen bacteria. Ann Rech Vet. 1979;10(2-3):347-9. PMID: 533168.
- Howard BH, Hungate RE. Desulfovibrio of the sheep rumen. Appl Environ Microbiol. 1976 Oct;32(4):598-602. doi: 10.1128/aem.32.4.598-602.1976. PMID: 984832; PMCID: PMC170313.
- Sheik CS, Sieber JR, Badalamenti JP, Carden K, Olson A. Complete Genome Sequence of Desulfovibrio desulfuricans Strain G11, a Model Sulfate-Reducing, Hydrogenotrophic, and Syntrophic Partner Organism. Genome Announc. 2017 Oct 26;5(43):e01207-17. doi: 10.1128/genomeA.01207-17. PMID: 29074670; PMCID: PMC5658508.
- Claesson R, Edlund MB, Persson S, Carlsson J. Production of volatile sulfur compounds by various Fusobacterium species. Oral Microbiol Immunol. 1990 Jun;5(3):137-42. doi: 10.1111/j.1399-302x.1990.tb00411.x. PMID: 2080068.
- Macfarlane GT, Cummings JH, Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986 Jun;132(6):1647-56. doi: 10.1099/00221287-132-6-1647. PMID: 3543210.
- Sato D, Nozaki T. Methionine gamma-lyase: the unique reaction mechanism, physiological roles, and therapeutic applications against infectious diseases and cancers. IUBMB Life. 2009 Nov;61(11):1019-28. doi: 10.1002/iub.255. PMID: 19859976.
- Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest. 1999 Oct;104(8):1107-14. doi: 10.1172/JCI7712. PMID: 10525049; PMCID: PMC408582.
- Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol. 2001 Jul 15;62(2):255-9. doi: 10.1016/s0006-2952(01)00657-8. PMID: 11389886.
- Buonvino S, Arciero I, Melino S. Thiosulfate-Cyanide Sulfurtransferase a Mitochondrial Essential Enzyme: From Cell Metabolism to the Biotechnological Applications. Int J Mol Sci. 2022 Jul 30;23(15):8452. doi: 10.3390/ijms23158452. PMID: 35955583; PMCID: PMC9369223.
- Aminlari M, Gilanpour H, Taghavianpour H, Veseghi T. Comparative studies on the distribution of rhodanese and beta-mercaptopyruvate sulfurtransferase in different organs of sheep (Ovis aries) and cattle (Bos taurus). Comp Biochem Physiol C Comp Pharmacol Toxicol. 1989;92(2):259-62. doi: 10.1016/0742-8413(89)90050-9. PMID: 2565183.
- Aminlari M, Gilanpour H. Comparative studies on the distribution of rhodanese in different tissues of domestic animals. Comp Biochem Physiol B. 1991;99(3):673-7. doi: 10.1016/0305-0491(91)90353-f. PMID: 1769215.
- Al-Qarawi AA, Mousa HM, Ali BH. Tissue and intracellular distribution of rhodanese and mercaptopyruvate sulphurtransferase in ruminants and birds. Vet Res. 2001 Jan-Feb;32(1):63-70. doi: 10.1051/vetres:2001110. PMID: 11254178.
- Taylort BF, Oremland RS. Depletion of Adenosine Triphosphate in desulfovibrio by Oxyanions of Group VI Elements. Curr Microbiol. 1979;3:101-103.
- Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol Ecol. 1991;86:103-112.
- Zinkevich V V, Beech IB. Screening of sulfate-reducing bacteria in colonoscopy samples from healthy and colitic human gut mucosa. FEMS Microbiol Ecol. 2000 Dec 1;34(2):147-155. doi: 10.1111/j.1574-6941.2000.tb00764.x. PMID: 11102692.
- Stewart JA, Chadwick VS, Murray A. Carriage, quantification, and predominance of methanogens and sulfate-reducing bacteria in faecal samples. Lett Appl Microbiol. 2006 Jul;43(1):58-63. doi: 10.1111/j.1472-765X.2006.01906.x. PMID: 16834722.
- Turnlund JR, Keyes WR, Peiffer GL. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men at five intakes of dietary molybdenum. Am J Clin Nutr. 1995 Oct;62(4):790-6. doi: 10.1093/ajcn/62.4.790. PMID: 7572711.
- Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000 Dec;72(6):1488-94. doi: 10.1093/ajcn/72.6.1488. PMID: 11101476.
- Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015 May;14(5):329-45. doi: 10.1038/nrd4433. Epub 2015 Apr 7. PMID: 25849904.
- Wallace JL, Motta JP, Buret AG. Hydrogen sulfide: an agent of stability at the microbiome-mucosa interface. Am J Physiol Gastrointest Liver Physiol. 2018 Feb 1;314(2):G143-G149. doi: 10.1152/ajpgi.00249.2017. Epub 2017 Oct 12. PMID: 29025733; PMCID: PMC5866422.
- Blachier F, Davila AM, Mimoun S, Benetti PH, Atanasiu C, Andriamihaja M, Benamouzig R, Bouillaud F, Tomé D. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids. 2010 Jul;39(2):335-47. doi: 10.1007/s00726-009-0445-2. Epub 2009 Dec 18. PMID: 20020161.
- Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med. 2013 Jul;60:195-200. doi: 10.1016/j.freeradbiomed.2013.02.024. Epub 2013 Mar 1. PMID: 23466556; PMCID: PMC4077044.
- De Preter V, Arijs I, Windey K, Vanhove W, Vermeire S, Schuit F, Rutgeerts P, Verbeke K. Decreased mucosal sulfide detoxification is related to an impaired butyrate oxidation in ulcerative colitis. Inflamm Bowel Dis. 2012 Dec;18(12):2371-80. doi: 10.1002/ibd.22949. Epub 2012 Mar 20. PMID: 22434643.
- Cann PA, Read NW, Brown C, Hobson N, Holdsworth CD. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983 May;24(5):405-11. doi: 10.1136/gut.24.5.405. PMID: 6840614; PMCID: PMC1419989.
- Lu CL, Chen CY, Chang FY, Lee SD. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: an Oriental study. Clin Sci (Lond). 1998 Aug;95(2):165-9. PMID: 9680498.
- Madigan MT, Martinko JM, Bender KS, Buckley D, Stahl D. Microbial Ecosystems. In: Brock Biology of Microorganisms, 14th Ed. England: Pearson Education Limited. 2014:626- 29.
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 2013 Apr 1;3(4):a010306. doi: 10.1101/cshperspect.a010306. PMID: 23545571; PMCID: PMC3683961.
- France MM, Turner JR. The mucosal barrier at a glance. J Cell Sci. 2017 Jan 15;130(2):307-314. doi: 10.1242/jcs.193482. Epub 2017 Jan 6. PMID: 28062847; PMCID: PMC5278669.
- Hong SN, Rhee PL. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J Gastroenterol. 2014 Mar 14;20(10):2470-81. doi: 10.3748/wjg.v20.i10.2470. PMID: 24627584; PMCID: PMC3949257.
- Sugihara K, Kamada N. Diet-Microbiota Interactions in Inflammatory Bowel Disease. Nutrients. 2021 May 1;13(5):1533. doi: 10.3390/nu13051533. PMID: 34062869; PMCID: PMC8147260.
- Tomasova L, Dobrowolski L, Jurkowska H, Wróbel M, Huc T, Ondrias K, Ostaszewski R, Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide. 2016 Nov 30;60:50-58. doi: 10.1016/j.niox.2016.09.007. Epub 2016 Sep 22. PMID: 27667183.
- Tomasova L, Konopelski P, Ufnal M. Gut Bacteria and Hydrogen Sulfide: The New Old Players in Circulatory System Homeostasis. Molecules. 2016 Nov 17;21(11):1558. doi: 10.3390/molecules21111558. PMID: 27869680; PMCID: PMC6273628.
- Ulcerative Colitis. National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health. 2014 ;https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis.
- Wallace JL, Vaughan D, Dicay M, MacNaughton WK, de Nucci G. Hydrogen Sulfide-Releasing Therapeutics: Translation to the Clinic. Antioxid Redox Signal. 2018 Jun 1;28(16):1533-1540. doi: 10.1089/ars.2017.7068. Epub 2017 May 15. PMID: 28388861.
- Distrutti E, Sediari L, Mencarelli A, et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H- [1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther. 2006;319:447-458.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
