International Journal of Aquaculture and Fishery Sciences
Dietary inclusion effect of ginger (Zingiber officinale) and garlic (Allium sativum) blend on growth, feed nutrients utilization and retention in African Catfish (Clarias gariepinus) fry in intensive system
Paulin Nyadjeu*, Noël Arlette Tamko Ndjuissi, Doriane Divine Mane Yemdjie, Nesrine Yolande Chamsy Dedou, Georges Fonkwa, Ghislain Nguimdop Nguenang and Minette Eyango Tabi-Tomedi
Cite this as
Nyadjeu P, Tamko Ndjuissi NA, Mane Yemdjie DD, Chamsy Dedou NY, Fonkwa G, et al. (2021) Dietary inclusion effect of ginger (Zingiber officinale) and garlic (Allium sativum) blend on growth, feed nutrients utilization and retention in African Catfish (Clarias gariepinus) fry in intensive system. Int J Aquac Fish Sci 7(2): 014-023. DOI: 10.17352/2455-8400.000068This study was conducted to evaluate the dietary inclusion effect of ginger-garlic mixture on growth, feed nutrient utilization and whole-body composition of Clarias gariepinus fry. Fry weighing 1.2±0.01g were divided into four triplicate treatments before being fed with diets containing 0mg (D0 or control), 50mg (D1), 100mg (D2) and 200mg (D3) of ginger-garlic mixture /kg diet (1:1 proportion) for 56 days. Fish were fed at the rate of 10% of their body weight and every 14 days, intermediate sampling was done during which fry per treatment were counted, measurements taken on a representative sample and the feeding rate adjusted. Main water parameters were recorded daily before feeding. The results obtained indicate that, adding ginger-garlic blend in the diet enhanced significantly growth, feed nutrients utilization and retention as well as whole-body composition of C. gariepinus fry depending to the inclusion level. Fish fed with the highest supplemented level (200mg/kg diet) have recorded a final weight of 25.5±0.16g, with a weight gain (WG) of 24.34±0.16g, feed conversion ratio (FCR) of 1.46±0.01 and protein efficiency ratio (PER) of 1.53±0.02. These parameters were significantly different to those recorded in fish fed with control diet (final weight=17.02±0.27g, WG=15.23±0.28g, FCR=2.03±0.01, and PER=1.10±0.01). Similar trend to growth parameters was observed with macro-nutrient retention (protein=36.2±0.33%, lipid=29.1±0.33%, ash=23.13±0.68% and energy=24.99±0.25%) as compared to control (protein=24.94±0.01%, lipid=21.91±0.01%, ash=13.40±0.01% and energy=18.00±0.01%) Thus, the improvement in growth induced by the feed addition of ginger-garlic mixture could be largely attributed to the synergistic actions of the bioactive molecules contained in each phyto-additive on the functioning of the digestive system as well as to their hypoglycaemic and antioxidant properties.
Introduction
African catfish, Clarias gariepinus (Burchell, 1822) is an air-breathing species when very active or under low dissolved oxygen conditions. It is indigenous to the inland waters of much of Africa, more precisely sub-Saharan Africa. Clarias gariepinus is cultured in several countries throughout Africa as well as in Europe, Asia and South America [1]; though its contribution to world aquaculture production (89.1%) is very low and represent only 0.33% [2,3]. In Africa on the other hand, out of the contribution of 16% to 18% to total fish production that represent about 2.7% of global production, most of that production (99%) is dominated by tilapia and African catfish [4,5]. In sub-Saharan Africa countries, particularly in Cameroon, although the statistics are currently unavailable, African catfish is the most cultured species mostly in small-scale fish farms [6,7]. Moreover, interest in growing C. gariepinus is increasing in all parts of the country and the success of its farming is linked to improvement of its artificial reproduction technic and its acceptability by consumers as well as by fish farmers due to beneficial characteristics including fast growth and high market demand. Nevertheless, some challenges such as the unavailability of high quality feed at low cost is a major constraint which hinders its production. The only way to overcome this challenge is to encourage local production of quality feeds with a high protein content, which should contain not only the necessary nutrients but also complementary additives to improve the quality of the feed, maintain the fish healthy and promote their growth. Feed additives are edible substances that are supplemented to feeds in small amounts (
Garlic (
Ginger (
Garlic is generally paired with ginger to make stews and soups in most homes and restaurants all over the world. They are often used in a small quantity as food additives to flavour or preserve food and to stimulate appetite by increasing the flow of gastric juice as well as enhancing food taste [26]. Owing to physiological and pharmacological properties of different bioactive ingredients contained in ginger and garlic, they are considered as safe herbal medicines with very little and insignificant adverse effects in human being, livestock, including fish [27,28]. Previous studies revealed that dietary supplementation of the combination of ginger and garlic in the diet of commercial broilers did not improved growth and feed utilization compared to control diet [29]. On the other hand [30] had previously indicated that feeding Rainbow Trout (Oncorhychus mykiss) with a combination of garlic and ginger have improved weight gain, specific growth rate and feed conversion ratio compared to control diet. Moreover [17] have shown that dietary inclusion of the mixture of garlic and ginger enhance growth, feed utilization parameters and survival in Clarias gariepinus post-larvae. Despite these few studies, in our knowledge, research regarding the combined effects of garlic and ginger as phytogenic feed additive in the diet of African catfish during the nursery phase are almost non-existent. Thus, the present study was aimed to evaluate the effect of different inclusion levels of the mixture of garlic and ginger powders as a feed additive on growth, feed nutrient utilization and whole body composition of African catfish fry in intensive system.
Materials and methods
Study location and experimental system
The study was carried out in the hatchery unit of a small-scaled private farm called “Massoma fish farm” at Bojongo-Bonaberi, Wouri Division, Littoral Region of Cameroon from April to May 2020. The experiment was conducted in twelve (12) circular plastic tanks each (10 litres) and each tank was two third filled (6.5 litres) with water. All the tanks were mounted in an open circuit connected by a pressurized piping system of diameter 25 mm and supplied with borehole water by means of a pump.
Experimental fish and housing
A total of 444 apparently healthy Clarias gariepinus fry weighing 1.2±0.01g were obtained from an artificial reproduction carried out in farm hatchery. They were randomly stocked into 12 circular plastic tanks of 37 fry each and allowed to acclimatize for 7 days before the beginning of the feeding trial. Commercial feed (Coppens, 0.2mm, Coppens International B.V, Netherlands) was used to feed the fish during the period of acclimatization. The breeding tanks were divided into four treatments as listed below.
- Treatment 1 (D0: 0mg/kg diet: Z. officinale (0mg/kg diet) + A. sativum (0mg/kg diet)
- Treatment 2 (D1 :50mg/kg diet: Z. officinale (25mg/kg diet) + A. sativum (25mg/kg diet)
- Treatment 3 (D2 100mg/kg diet: Z. officinale (50mg/kg diet) + A. sativum (50mg/kg diet)
- Treatment 4 (D3: 200mg/kg diet: Z. officinale (100mg/kg diet) + A. sativum (100mg/kg diet)
Each treatment was made up of three replicates. Fish faeces and residual feed in each of the rearing tanks were siphoned off daily in the morning by the use of a rubber tube and the water renewed by half. Prior to the beginning of the experiment, fish were starved for 24 hours after which, twenty four fish (2 per tank) were randomly pick up for initial determination of the carcass composition. The remaining fry (420) were hand-fed four times daily (08:00 a.m, 11:00 p.m, 14:00 p.m and 17:00 p.m respectively) with different diets at a rate of 10% of their body weight (BW) during the first 28 days of feeding and 5% for the rest of the feeding period, with feeding ration being adjusted in accordance to weight gain of fish after random sampling at 14 days interval. Each day before feeding, the physico-chemical parameters such as temperature (T°C) was measured using a Maximum-minimum thermometer, dissolved oxygen (D.O), using JBL Test Kits, pH, nitrite (NO2-) and nitrate (NO3-), using Test strips (JBL Easy Test 6in1) (Table 1). During intermediate samplings, fifteen fish from each tank were randomly harvested to record growth in terms of total body length (TBL) and body weight (BW). At the end of the feeding trial, fish were harvested and then weighed as total fish weight of each replicate within dietary treatment and counted for the calculation of both the growth and feed efficiency parameters. Fifteen fish (five fish per tank) from each treatment were selected to analyse the final proximal composition of the carcass according to the method of Association of Official Analytical Chemistry [31].
Collection and Processing of the feed additives
Fresh ginger (Zingiber officinale, cameroonian yellow ginger) and fresh garlic bulbs (Allium sativum, cameroonian white garlic) were procured from a local market. The dry skin of both fresh garlic and ginger were removed before use; they were peeled and cut into small pieces then dried in oven at 60°C for 24 hours in the department of Processing, Quality Control of Aquatic Products of the Institute of Fisheries and Aquatic Sciences. The dried ginger and garlic were crushed into powdered form mechanically and sieved with a hand sieve to obtain 500g of each products which were then stored in airtight containers till formulation and preparation of experimental diets.
Formulation and preparation of experimental diets
Four isonitrogenous (45% CP) diets were formulated using the Pearson’s square method (Pearson, 1976) and distributed to C gariepinus fry divided into four treatments. Dietary treatments included D0: 0mg/kg diet in which fry fed basal diet or control diet, D1: 50mg/kg diet, D2: 100mg/kg diet and D3: 200mg/kg diet in which fry were fed with basal diet containing the mixture of ginger and garlic powders at equal proportion (1:1). Feed ingredients used for the experimental diets include: ginger powder, garlic powder, fish meal, soya bean cake, peanut cake, maize meal, Wheat bran, vitamin/mineral premix and bone meal. The formulation was based on the percentage composition of the ingredient (Table 2). To prepare the diets, the dried and grinded ingredients of each diet were weighed and mixed thoroughly in a bowl, palm oil and warm water were then added slowly along to the mixture and mixed manually for about 30 minutes to achieve a proper consistency. The resulting mixture was pelletized (2 mm) using an electric pelleting machine and allowed to dry for 24h by air circulation before being packed into airtight containers and stored at room temperature to be crumbled before use. Formulated diet samples (10g) were analysed following the procedures of [31]. Moisture was analysed by drying the sample in an air convection oven at 105 °C overnight. Crude protein was analysed by the Kjeldahl method after acid digestion (% crude protein = % nitrogen × 6.25) while crude lipid was determined by extraction with petroleum ether using the Soxhlet method. The ash content in the diet was analysed by combustion of samples in a muffle furnace at 550 °C for 12 h (Table 2)
Growth, survival rate and feed utilization parameters
Growth performances, survival rate, feed utilization and nutrients retention were assessed for each treatment by determination of weight gain (WG), length gain (LG), mortality rate (MR), condition factor (K), Feed intake (FI), feed conversion ratio (FCR), feed efficiency ratio (FER), protein efficiency ratio (PER) and nutrients retention (NR). Calculations were carried out using the following formulae:
Where: Wf = final weight; Wi = initial weight; Lf= final length; Li= initial length; T= number of days in the experimental period;
Where,
Statistical analysis
All results were expressed as mean ± SD (Standard Deviation). The data collected during every fish sampling were analysed by one-way analysis of variance (ANOVA-1) repeated measure followed by Tukey’s multiple comparisons test with n=3 replications containing 35 fish each. Differences were regarded as significant when p<0.05; Regression analysis was established between fish growth, feed intake as well as macro-nutrient retention and dietary supplementation level of the ginger-garlic mixture. All statistical analyses were conducted using GraphPad Prism version 6.0.
Results
Growth performance
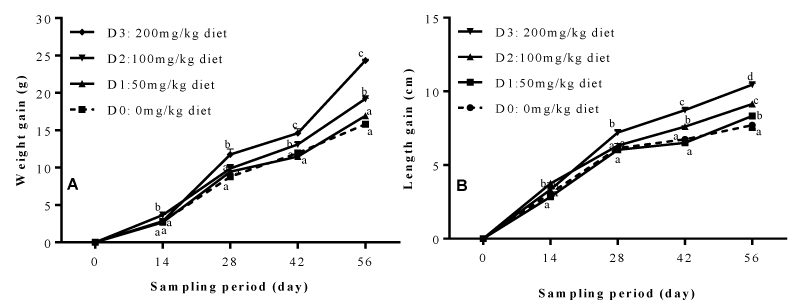
Figure 1 below illustrates the growth performance in terms of weight gain (A) and length gain (B) of Clarias gariepinus fry during 56 days of feeding. The general observation is that, besides the fact that the two curves (A and B) show the same trend of evolution; it also emerges that, the dietary inclusion of the garlic and ginger mixture induced a significant increase in both weight gain and length gain depending on dose and feeding time. At the end of the feeding trial, fry in treatment D3 registered the highest value of weight gain (24.34 ± 0.16g), significantly high (P<0.05) by 37.48% compared to fish weight gain in treatment D0 (15.23 ± 0.28g).
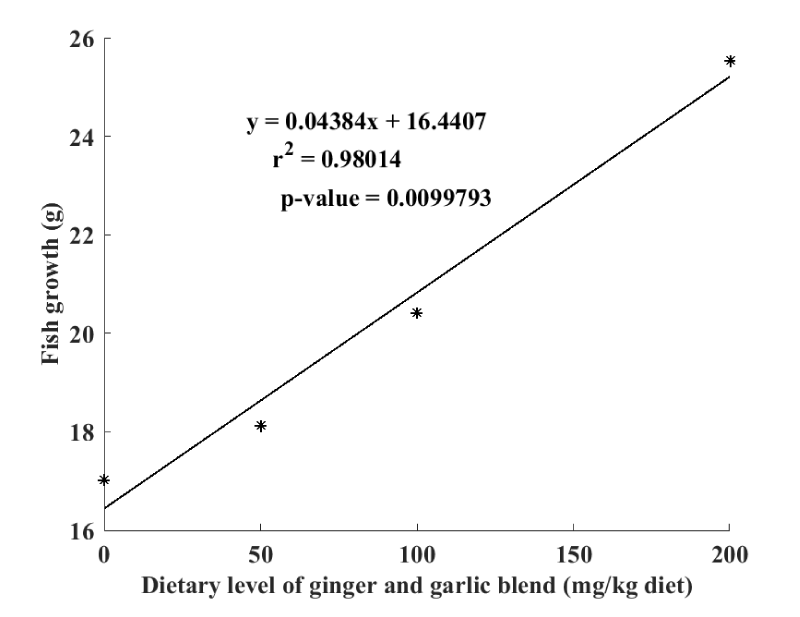
The relationship between fish growth and the level of dietary inclusion of garlic and gingerblend powders is illustrated by the linear regression curve represented in Figure 2. It is clearly confirmed that Clarias gariepinus fry growth has increased with the increase level of garlic and ginger mixture in the diet (R2 = 0.98).
Feed utilization and mortality
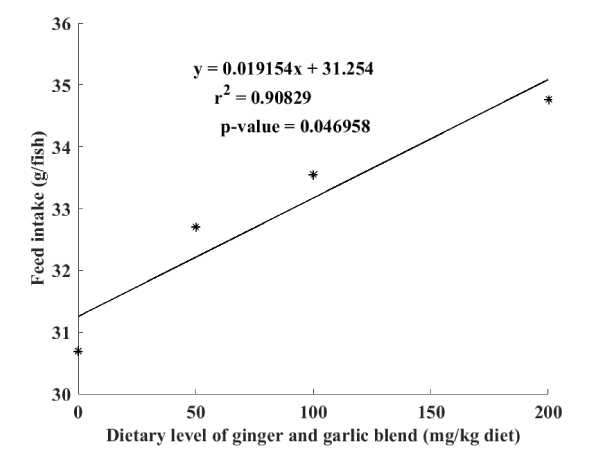
Daily observations without measurement during feeding made it possible to note an acceptance of all the feeds by the fry which fed voraciously during the whole feeding period. This is reflected in Table 3 below by a significant increase in feed intake and therefore protein intake, depending to the level of dietary phytogenic additive blend inclusion. Moreover, the linear regression curve illustrated in Figure 3 show a great relationship between feed intake and dietary inclusion level of garlic and ginger blend powders (R2 = 0.90). On the other hand, it is noticed a significant decrease in the value of the condition factor K in function to the level of dietary phytogenic additive blend inclusion. The best values of feed conversion ratio (FCR= 1.25±0.11) as well as protein efficiency ratio (PER=1.52±0.02) obtained with diet D3 were significantly different to the rest of treatments D0 (FCR=2.03±0.03; PER=1.10±0.02), D1 (FCR=2.03±0.09; PER=1.09±0.04) and D2 (FCR=1.81±0.07; PER=1.23±0.04).
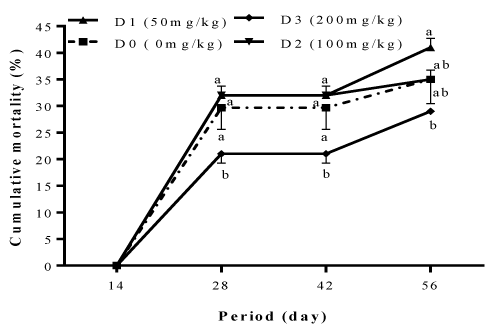
The mortalities recorded in different treatments during the study and presented in Figure 4 were not related to the inclusion level of garlic and ginger blend in the diet of Clarias gariepinus fry. The highest mortality rate registered at the end of the feed trial was observed with diet D2 (41±1.73%), followed by fish fed with diets D0 (35±4.58%) and D1 (35±1.73%), while the lowest mortality of 29±0.00% was observed in fish fed with diet D3.
Proximate whole-body composition and nutrients retention
Table 4 present the whole-body composition of Clarias gariepinus after 56 days of feeding. When compared to fish body composition at the start of the study, it is observed that apart from the moisture rate that dropped down significantly (p<0.05) in fish fed with the control diet D0 and experimental diets D1, D2 and D3. The values of others biochemical elements like ash, protein, lipid and energy increased significantly (p<0.05), particularly in fish fed with the experimental diets. Moreover, whole-body composition of fish between experimental treatments revealed that apart fish fed on diet D1 that have presented the highest lipid content (4.26 ± 0.01%), those fed with diet D3 have expressed the highest levels in terms of protein (27.10 ± 0.06%), ash (2.21 ± 0.06%) and energy (6.98 ± 0.01kJ/g WW); resulting therefore in significantly high retention of these macro-nutrients in fish carcass.
Figure 5 illustrates the linear regression between the retention of macro-nutrients throughout the body of fish and the level of dietary inclusion of the ginger-garlic mixture. Regardless of the type of nutrient, there is clear relationship between nutrient retention and the level of dietary supplementation of the mixture of ginger and garlic powders. This is illustrated by the values of the coefficient of determination indicating that the relationship between protein retention and the level of phytogenic additives mixture inclusion is the highest (R2 = 0.95) followed by ash retention (R2 = 0.94), then by energy retention (R2 = 0.89) and finally by lipid retention, with R2 = 0.77.
Discussion
The performances of fish to feeds are due to more than just nutritional factors. Indeed, water quality parameters, which are the result of the rearing system have a great influence on fish survival as well as growth, especially at the fry stage. The physico-chemical parameters of the rearing medium such as temperature and dissolved oxygen recorded during the present study were observed to be within the range recommended for freshwater fish culture [32,33]. [33] recommended temperature values between 25 and 30°C for rearing of C. gariepinus. In addition, dissolved Oxygen level greater than 5mg/l is necessary to support good fish production. The values of the acidity degree registered during the study were oscillated between 6.6 and 6.7. [34] reported that the ideal pH level is between 7.5 and 8.5 and any value above or below this could be stressful to the fishes, especially to fry. According to [35], when the pH value drops below 7, it has a negative influence on the nitrification process. However, the nitrite concentration recorded in the present study shows a range between 0.0mg/L and 0.2mg/L. According to [36] concerning water quality for aquaculture pond and based on the research work carried out by [37] whose observations led to the development of guidelines on the useful water quality for the management of pond fish culture, the nitrite threshold for freshwater fish fry is 0.2mg/l. Excess nitrite compounds in water caused by faeces and unconsumed feeds are termed as an invisible killer of fish because it oxidizes haemoglobin to methemoglobin in the blood, turning the blood and gills brown and hindering respiration. Accordingly, the concentration of nitrite recorded during the present study still meets the proper range for optimum growth of catfish fry. Moreover, nitrite being a transitional form between ammonia and nitrate during nitrification process, and between nitrate and nitrogen gas during denitrification process is relatively less toxic [38]. The results of the present study have indicated that the concentration of nitrate ranged between 0.00mg / L and 10mg/L. This concentration still meets the proper range for optimum growth of catfish fry [39,40]. According to the above observations, the mortalities accounted in all the treatments would be attributed more to the poor conditions of the breeding environment than to the diet. Indeed, the absence of automatic siphoning of feed residues and faeces would be partly responsible for the acidity of the rearing medium. Moreover, water in the rearing tanks was half renewed each morning before feeding. Unfortunately, this daily action has being carried out sometimes manually due to power cuts to run the water pump. Thus, the low pH value mentioned above associated with the daily renewal of the water in the rearing tanks constitute potentially stressful conditions for the fry of C. gariepinus which would probably have caused a dysfunction of their physiological balance and would certainly have induces an increase in the production of free radicals whose effects on vital functions are known to cause death. On the other hand, it was observed a significant decrease of the mortality rate after feeding fry with the diet containing the ginger-garlic mixture at the dose of 200mg/kg diet as compared to the fry fed with the control diet or other experimental diets. This could be attributed to the antioxidant properties of the phyto-additives contained in the feed. According to [20], spices such as ginger and garlic are useful in aquaculture industry not only to improve the palatability of feed and as a flavouring agent but also because of their high antioxidative content. The main bioactive compounds associated with spices comprise a diverse array of components such as terpenoid components, flavonoids, phenolic compounds, saponins, glycosides and other bioactive [41]. So, the significant decrease in mortalities observed in fry fed with the D3 diet, compared to other diets, could be partially attributed to the synergistic or cumulative actions of the bioactive molecules mentioned above contained in the two phyto-additives used. These bioactive compounds are known to not only improve fish health through their ability to eliminate free radicals, prevent lipid peroxidation and promote endogenous antioxidant defences, but also through their ability to improve palatability and feed consumption [42].
Direct observations during feeding permitted to notice that fry fed with the control diet were the smallest and less active in the hapas, compared to those fed on basal diet enriched with the ginger-garlic mixture. It was also observed that enriched diets with the mixture of ginger and garlic were more attractable to the fry than the control diet. This, because fry in the experimental hapas in addition to being the most vigorous and agitated during feeding, have also expressed the highest feed consumption values, compared to control. This indicate that inclusion of ginger and garlic blend in the diet of C. gariepinus fry would have improved the feed taste, leading to an increase in appetite stimulation. These observations corroborate the previous research works conducted by [17] during which it was observed that inclusion of the ginger-garlic mixture in the diet of C. gariepinus post-larvae in fertilized pond increased feed intake with respect to the inclusion level. Plant derived products or by-products used as additives in fish feed are known to enhance feed taste or palatability as well as appetite stimulation, thereby inducing a positive effect on growth [43,44].
In the present study, growth performance and feed utilization were significantly improved in fish fed on dietary supplementation of ginger-garlic mixture as compared to those fed with control diet; with the highest response being observed in fish fry fed with the highest level of phyto-additives combination. These results are in contradiction with those obtained by [29] who observed no improvement in growth and feed utilization in broilers fed a diet containing the mixture of ginger and garlic as compared to control. The conflicting results of the present study with those of [29] could therefore be attributed to the different between the species used, feeding program and even the culturing conditions. On the other hand, these results agrees with the findings of [45,30] as well as those of [17] where the mixture of ginger and garlic powders was included in the diet of Oncorhychus mykiss juveniles, Huso huso juveniles and Clarias gariepinus post-larvae respectively. The improvement in growth and utilization of feed nutrients in C. gariepinus fry induced by the ginger-garlic blend inclusion over the performances of those fed with the control diet could have certainly emanated from the presence of bioactive compounds contained in both phytogenic additives. Indeed, ginger is known to stimulate the appetite, the secretion of pancreatic enzymes and the bile from the liver in addition to intervening in the balance of intestinal bacteria [46-48]. Garlic for its part is known to promote feed intake, the performance of the intestinal flora, enhance digestion as well as energy utilization [49]. Thus, it is easy to suggest that the presence of the mixture of ginger and garlic in the diets would have boosted nutrient utilization characterized by low feed conversion ratio and high protein efficiency ratio; which was reflected by significantly higher weight gain as well as length gain. Moreover, because of the pharmacological properties of ginger and garlic on blood glucose reduction, it has been suggested that they would partially stimulate growth by increasing the inflow of glucose into tissues [50]. According to the above mentioned physiological and pharmacological properties of ginger and garlic on the digestion and glucose metabolism, it can be agree that enhancement of growth performance as well as feed nutrient utilisation in C. gariepinus fry fed with experimental diets as compared to the control could be partially attributed to the combined effects of the bioactive compounds contained in each phytogenic additive which would have acted in synergy to increase the digestibility of macronutrients such as proteins, lipids and ash which are part of the major constituents of the feed. The probable improvement in digestibility induced by the synergistic effects of the bioactive molecules of the plant additives could be justified by the low value of the feed conversion ratio obtained in C. gariepinus fry fed on the experimental diet, particularly that containing the highest level of the phyto-additives mixture. In addition to the possible involvement of ginger and garlic bioactive molecules in the digestion mechanism as well as glucose metabolism of experimental fry, other studies have indicated that the beneficial effects of both phytogenic additives on the health status of fish when they are used as feed additive are also due to their antioxydant properties [51]. Biochemical analysis of the feeds showed no difference between different diets. Thus, significant improvement in C gariepinus fry growth in the experimental treatments compared to control can only be explained by the physiological and pharmacological properties of the bioactive components contained in the mixture of ginger and garlic. These bioactive molecules would thus have induced their biological effects through their synergistic actions on the functioning of the digestive system, but also by their hypoglycaemic and antioxidant properties, which would have contributed to an improvement in general body homeostasis. This therefore, allow the fry to make the best use of the nutrients contained in the consumed feed, justifying thus the significant improvement in the macro-nutrients retention as well as biochemical composition of the whole-body of the juveniles obtained at the end of the study as compared to control.
Conclusion
The results of the present study revealed that, independently of the parameter evaluated, the dietary inclusion of the ginger-garlic mixture induced significantly increased in the growth performance, feed nutrient utilization and retention as well as whole-body composition of the juveniles produced; the highest effect being obtained with the highest level of inclusion. These observed beneficial effects have been attributed to the synergistic actions of the bioactive contents of ginger and garlic than to their nutritional properties.
The authors thank Mr Tcheudjie Tchianga Joël for providing the technical installations. Thanks also to Dr Anselme Mama for the statistical analyses.
- Opiyo MA, Orina P, Charo-Karisa H (2017) Fecundity, growth parameters and survival rate of three African Catfish (Clarias gariepinus) strains under hatchery conditions. Journal of Aquaculture Engineering and Fisheries Research 3: 75-81. Link: https://bit.ly/3yCeTxX
- Dauda AB, Natrah I, Karim M, Kamarudin MS, Bichi AH (2018) African Catfish Aquaculture in Malaysia and Nigeria: Status, Trends and Prospects. Fish Aqua J 9: 237. Link: https://bit.ly/3wzQV4F
- FAO (2020) The state of world fisheries and aquaculture 2020. Sustainability in action, Rome. Link: https://bit.ly/3yGiE5G
- Obiero K, Meulenbroek P, Drexler S, Dagne A, Akoll P, Odong R, Kaunda-Arara B, Waidbacher H (2019) The Contribution of Fish to Food and Nutrition Security in Eastern Africa: Emerging Trends and Future Outlooks. Sustainability 11: 1636. Link: https://bit.ly/3yExF88
- Adeleke B, Robertson-Andersson D, Moodley G, Taylor S (2021) Aquaculture in Africa: A Comparative Review of Egypt, Nigeria, and Uganda Vis-À-Vis South Africa. Reviews in Fisheries Science and Aquaculture 292: 167-197. Link: https://bit.ly/3bX5mb0
- Temegne NC, Momo NJ (2019) Review on Aquaculture Research in Cameroon: Fish Farming. Int J Oceanogr Aquac 3: 000170. Link: https://bit.ly/3uojz7t
- Ngota BL (2020) Advancing the contextual factors impeding the development of aquapreneurship in Cameroon. Entrepreneurial Business and Economics Review 8: 65-82. Link: https://bit.ly/3oOEVd1
- Dada AA (2015) Improvement of Tilapia (Oreochromis niloticus Linnaeus, 1758) growth performance fed three commercial feed additives in diets. J Aquac Res Development 6: 325. Link: https://bit.ly/34gbLKg
- Alemayehu AT, Geremew A, Getahun A (2018) The Role of Functional Feed Additives in Tilapia Nutrition. Fish Aqua J 9: 249. Link: https://bit.ly/3uoYJVr
- Ogunkalu OA (2019) Effects of Feed Additives in Fish Feed for Improvement of Aquaculture. Eurasian Journal of Food Science and Technology 3: 49-57. Link: https://bit.ly/3vAa3Q9
- Hodar A, Vasava RRJ, Mahavadiya DR, Joshi NH, Nandaniya VV, et al. (2021) Herbs and herbal medicines : A prominent source for sustainable aquaculture. J Exp Zool India 24: 719-732. Link: https://bit.ly/3yCfIa5
- Bai SC, Katya K, Yun H (2015) Additives in aquafeed: an overview. In: Feed and Feeding Practices in Aquaculture. Woodhead Publishing Series in Food Science, Technology and Nutrition 171-202. Link: https://bit.ly/3fK8fxc
- Ebru Y, Cengiz K (2016) Feed additives in aquafeeds. Scientific Papers Animal Science 66: 155-160. Link: https://bit.ly/3bT5KHN
- Marlowe CA, Caipang, Mabuhay-Omar J, Gonzales-Plasus MM (2019) Plant and fruit waste products as phytogenic feed additives in aquaculture. AACL Bioflux 12: 261-264. Link: https://bit.ly/3fldRPm
- Ndakalimwe NG (2019) Review on the progress in the role of herbal extracts in tilapia culture. Cogent Food & Agriculture 5: 1619651. Link: https://bit.ly/34n3dBa
- Shin SH, Kim MK (2004) Effect of dried powders or ethanol extracts of garlic flesh and peel on lipid metabolism and antithrombogenic capacity in 16-month-old rats. Journal of Nutrition and Health 37: 515-524. Link: https://bit.ly/2RDU8BD
- Nyadjeu P, Ekemeni RGM, Tomedi MET (2020) Growth Performance, Feed Utilization and Survival of Clarias gariepinus Post-larvae Fed with a Dietary Supplementation of Zingiber officinale-Allium sativum Mixture. HSOA Journal of Aquaculture & Fisheries 4: 028. Link: https://bit.ly/34iqZyp
- Chi MS, Koh ET, Stewart TJ (1982) Effects of garlic on lipid metabolism in rats fed cholesterol or lard. J Nutr 112: 241-248. Link: https://bit.ly/3fmqY2F
- Corzo-Martinez M, Corzo N, Villamiel M (2007) Biological properties of ions and garlic. Trends in Food Science Technology 18: 609-625. Link: https://bit.ly/3yP7c7U
- Shahidi F, Hossain A (2018) Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J Food Bioact 3: 8–75. Link: https://bit.ly/3vmW683
- Yilmaz S, Ergün S (2012) Effects of Garlic and Ginger Oils on Hematological and Biochemical Parameters of Sea Bass, Dicentrarchus labrax. Journal of Aquatic Animal Health 24: 219-224. Link: https://bit.ly/3bWsWET
- Zadeh JB, Kor NM (2014) Physiological and pharmaceutical effects of Ginger (Zingiber officinale Roscoe) as a valuable medicinal plant. Euro J Exp Bio 4: 87-90. Link: https://bit.ly/3hYrhT5
- Yashin A, Yashin Y, Xia X, Nemzer B (2017) Antioxidant activity of spices and their impact on human health. A review. Antioxidants 6: 1-18. Link: https://bit.ly/2RL23Nn
- Shakya SR (2015) Medicinal uses of ginger (Zingiber officinale roscoe) improves growth and enhances immunity in aquaculture. Int J Chem Stud 3: 83–87. Link: https://bit.ly/3bUi8Hi
- Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Doan VH (2020) Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish Immunol 99: 267–273. Link: https://bit.ly/3wEZz21
- Lawal AR, Olayinka BU, Murtadha RA, Ayinla A, Etejere EO (2018) Comparative Analysis of Phytochemical and Proximate Composition of Allium sativum L. and Zingiber officinale Rosc. Nigerian Journal of Basic and Applied Science 26: 82-87. Link: https://bit.ly/34hhors
- Mekuriya W, Mekibib B (2018) Review on the Medicinal Values of Ginger for Human and Animal Ailments. J Vet Sci Technol 9: 519. Link: https://bit.ly/3hTjKoB
- Motlagh AH, Paolucci M, Lashkarizadeh BM, Safari O (2020) Sexual parameters, digestive enzyme activities, and growth performance of guppy (Poecilia reticulata) fed garlic (Allium sativum) extract supplemented diets. J World Aquacult Soc 1-11. Link: https://bit.ly/3wArGz4
- Karangiya VK, Savsani HH, Patil SS, Garg DD, Murthy KS, et al. (2016) Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Veterinary World 9: 245-250. https://bit.ly/34wu9il
- Gabor EF, Şara A, Benţea M, Creţa C, Baciu A (2012) The Effect of Phytoadditive combination and growth performances and meat quality in Rainbow Trout (Oncorhychus mykiss). Animal Science and Biotechnologies 45: 43-47. Link: https://bit.ly/2SuGK2I
- AOAC (Association of Official Analytical Chemist) (1990) Official methods of analysis association of official analytical chemists. 15th ed. Washington, DC, Association of Analytical Chemists 1298. Link: https://bit.ly/3uovcLo
- Saraswathy R, Muralidhar M, Sundaray JK, Lalitha N, Kumararaja JK (2015) Water quality management in fish hatchery and grow-out systems. Advances in marine and brackishwater aquaculture 2017-2225. Link: https://bit.ly/3yF7ttR
- Bhatnagar A, Jana SN, Garg SK, Patra BC, Singh G, et al. (2004) Water quality management in aquaculture, In: Course Manual of summer school on development of sustainable aquaculture technology in fresh and saline waters. CCS Haryana Agricultural, Hisar (India) 203- 210.
- Santhosh B, Singh NP (2007) Guidelines for water quality management for fish culture in Tripura, ICAR Research Complex for NEH Region, Tripura Center, Pu.
- Molleda MI (2007) Water quality in recirculating aquaticultures systems for arctic charr (Salvelinus aplpinus L.) culture. United Nation University. Iceland 48: 36-42. Link: https://bit.ly/34hUvnR
- Boyd CE (1998) Water Quality in Ponds for Aquaculture. Auburn University Press, Birmingham. Link: https://bit.ly/2TaDTMw
- Bhatnagar A, Devi P (2013) Water quality guidelines for the management of pond fish culture. International Journal of Environmental Sciences 3. Link: https://bit.ly/3g1fjpp
- Kroupova H, Machova J, Svobodova Z (2005) Nitrite influence on fish: a review. Vet Med Czech 50: 461–471. Link: https://bit.ly/2RCZmxr
- Devi PA, Padmavathy P, Aanand S, Aruljothi K (2017) Review on water quality parameters in freshwater cage fish culture. International Journal of Applied Research 3: 114- 120. Link: https://bit.ly/3vrj2CX
- Zidni I, Iskandar, Buwono ID, Mahargyani BP (2019) Water Quality in the Cultivation of Catfish (Clarias gariepinus) and Nile Tilapia (Oreochromis niloticus) in the Aquaponic Biofloc System. Asian Journal of Fisheries and Aquatic Research 4: 1-6. Link: https://bit.ly/3vrj3qv
- Parthasarathy VA, Chempakam B, Zachariah TJ (2008) Chemistry of spices. Oxfordshire, CABI. Link: https://bit.ly/3hUc6KA
- Udu-Ibiam OE, Ogbu O, Ibiam UA, Nnachi AU, Agah MV, et al. (2014) Phytochemical and Antioxidant Analyses of Selected Edible Mushrooms, Ginger and Garlic from Ebonyi State, Nigeria. Journal of Pharmacy and Biological Sciences 9: 86-91. Link: https://bit.ly/2SrAw3F
- Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433:50-61. https://bit.ly/2SvBhs9
- Kuebutornye FKA, Abarike ED (2020) The contribution of medicinal plants to tilapia aquaculture: a review. Aquaculture International. https://bit.ly/34jQmQw
- Kanani HG, Nobahar Z, Kakoolaki S, Jafarian H, Gholipour KH (2014) Effect of ginger and garlic-supplemented diet on growth performance, some hematological parameters and immune responses in juvenile Huso huso. Fish Physiol Biochem 40: 481-490. Link: https://bit.ly/3umPKEe
- Platel K, Srinivasan K (2004) Digestive stimulant action of spices: A myth or reality. Indian J Med Res 119:167-179. Link: https://bit.ly/2SuB6h3
- Reinbeck HC, Martinussen T, Møller P (2010) Effects of hot spices on energy intake, appetite and sensory specific desires in humans. Food Quality and Preference 21: 655–661. Link: https://bit.ly/3urnKiB
- Ibidunni AS, Olubodun OS, Ikililu A (2017) Metabolic activities and health indices of African catfish (Clarias gariepinus) fed varying levels of Zingiber officinale root. J App Biol Biotech 5: 021-028. Link: https://bit.ly/2RKgRM4
- Adeniji CA, Wusu D, Falana EO (2019) “Individual and Combined Effects of Moringa Leaf and Garlic Powder on Growth and Plasma Biochemical Indices of Clarias gariepinus Juveniles.” American Journal of Food Science and Technology 7: 137-145. Link: https://bit.ly/3bVCfVx
- Badreldin HA, Blunden G, Tanira MO, Nemmar A (2008) Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol J 46: 409-420. Link: https://bit.ly/2RL3Lyh
- Mahmoud R, Aziza A, Marghani B, Eltaysh R (2019) Influence of ginger and garlic supplementation on growth performance, whole body composition and oxidative stress in the muscles of Nile tilapia (O. niloticus). Adv Anim Vet Sci 7: 397-404. Link: https://bit.ly/3wwlaJK .

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
