Archives of Organ Transplantation
Contrary to expectation: Preserved renal function after using PD-1 Inhibitor Cemiplimab-rwlc in a kidney transplant recipient
Muhannad Leghrouz1*, Svetomir N Markovic2 and Aleksandra Kukla2
2Mayo Clinic, Rochester, MN, USA
Cite this as
Leghrouz M, Markovic SN, Kukla A (2022) Contrary to expectation: Preserved renal function after using PD-1 Inhibitor Cemiplimab-rwlc in a kidney transplant recipient. Arch Organ Transplant 7(1): 009-010. DOI: 10.17352/2640-7973.000020Copyright
© 2022 Leghrouz M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction
The use of immune checkpoint inhibitors in transplant recipients with malignancy is associated with the risk of graft failure due to acute rejection. There is limited literature regarding the use of Cemiplimab-rwlc (Libtayo) in kidney transplant patients. Here we present a case of using Cemiplimab-rwlc (Libtayo), a Programmed Death receptor-1 (PD-1) blocking antibody for locally advanced and metastatic Cutaneous Squamous Cell Carcinoma (CSCC), in a kidney transplant recipient.
Case description
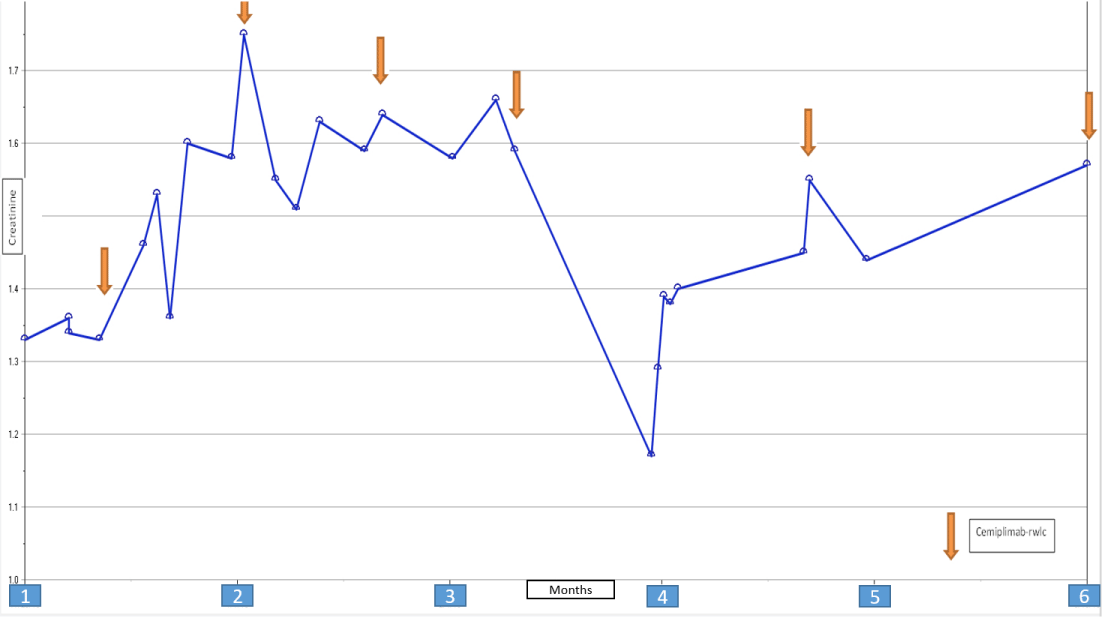
A 48 years old male patient with a history of End Stage Renal Disease (ESRD) secondary to Autosomal Dominant Polycystic Kidney Disease (ADPKD), received a 5/6 ABDR HLA mismatch living donor kidney transplant in May of 2016. He had class II DSAs (DSA to DR4 with MFI of 1975). He received Thymoglobulin induction and maintenance immunosuppression with Tacrolimus, MMF, and Prednisone. Protocol allograft biopsy at 2 years post-transplant showed Banff borderline acute cellular rejection. The patient continued on the same immunosuppression regimen at that time. At 2 years post-transplant, he was diagnosed with metastatic CSCC of the head & neck, HPV positive. Tacrolimus and Mycophenolate Mofetil (MMF) were discontinued and the patient was switched to Sirolimus. Treatment included the Mohs procedure, chemotherapy (paclitaxel/carboplatin/cetuximab) for 4 cycles, and radiation for 5 months. He had a good response to the cervical lymph nodes metastasis but had a minimal sustained response to skull metastasis. Subsequently, he was switched from intratumoral 5-fluorouracil to intratumoral IL2 and was started on immunotherapy with cemiplimab (Libtayo). Prednisone was discontinued. He underwent excision of scalp lesion and right cortical mastoid metastasectomy while on the full dose of Sirolimus, which he tolerated well [1-5]. His kidney function remained stable throughout over 5 months follow-up period (Figure 1).
Conclusion
Literature reports a substantial risk of rejection in kidney transplant recipients who are treated with immunotherapy. However, as our case shows, Cemiplimab (Libtayo) a PD-1 inhibitor can be used with preserving allograft function on a Sirolimus-based immunosuppression regimen. More data is needed to guide clinicians and appropriately counsel patients regarding the risks and benefits of immunotherapy medications.
- Rossi E, Schinzari G, Maiorano BA, Esposito I, Acampora A, Romagnoli J, Stefani AD, Regno LD, Lancellotta V, Fionda B, Tagliaferri L, Peris K, Tortora G. Immune-checkpoint inhibitors in renal transplanted patients affected by melanoma: a systematic review. Immunotherapy. 2022 Jan;14(1):65-75. doi: 10.2217/imt-2021-0195. Epub 2021 Nov 9. PMID: 34751039.
- Lai HC, Lin JF, Hwang TIS, Liu YF, Yang AH, Wu CK. Programmed Cell Death 1 (PD-1) Inhibitors in Renal Transplant Patients with Advanced Cancer: A Double-Edged Sword? Int J Mol Sci. 2019 May 3;20(9):2194. doi: 10.3390/ijms20092194. PMID: 31058839; PMCID: PMC6540260.
- Lebas E, Marchal N, Rorive A, Nikkels AF. Cemiplimab for locally advanced cutaneous squamous cell carcinoma: safety, efficacy, and position in therapy panel. Expert Rev Anticancer Ther. 2021 Apr;21(4):355-363. doi: 10.1080/14737140.2021.1876567. Epub 2021 Feb 7. PMID: 33554680.
- Orte Cano C, Van Meerhaeghe T, Tannous J, Lienard D, Van Gestel D, Cuylits N, Luce S, Carlot S, Le Moine A, Aspeslagh S, Del Marmol V. Advanced cutaneous squamous cell carcinoma of the head in two renal transplanted patients treated with cemiplimab. J Eur Acad Dermatol Venereol. 2022 Jan;36 Suppl 1:53-58. doi: 10.1111/jdv.17658. PMID: 34855244.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
