Open Journal of Chemistry
Chemical and physical stability of selected drugs for aerosol therapy with Sirmione thermal water
Francesco Saverio Robustelli della Cuna1,2*, Carlo Sturani3, Sarah Galfrè1, Luisa Gervasio2, Cristina Sottani4 and Elena Grignani4
2Casimiro Mondino National Neurological Institute, Pavia, Italy
3Terme di Sirmione, Colombare di Sirmione, (BS), Italy
4Environmental Research Center, ICS Maugeri SPA SB, Institute of Pavia, IRCCS, Via Salvatore Maugeri, 10-27100 Pavia (PV), Italy
Cite this as
Robustelli della Cuna FS, Sturani C, Galfrè S, Gervasio L, Sottani C, et al (2021) Chemical and physical stability of selected drugs for aerosol therapy with Sirmione thermal water. Open Journal of Chemistry 7(1): 029-033. DOI: 10.17352/ojc.000026In this work, we investigated the chemical and physical stability of ambroxol, beclomethasone dipropionate, budesonide and N-acetylcysteine after dilution with Sirmione thermal water, stored in ampoules for aerosol, at room temperature. The chemical stability of all active drugs was evaluated by Ultra-High-Performance Liquid Chromatography tandem mass spectrometry (UHPLC/MS/MS). All samples were analyzed in triplicate at room temperature under normal fluorescent light at 0h, 1h, 6h, 12h, and 24 h. According to the Official Pharmacopoeia of the Italian Republic, the degradation of a molecule that exceeds more than 10% is considered unacceptable. After dilution with thermal water of Sirmione, ambroxol, beclomethasone dipropionate, budesonide, and N-acetylcysteine, were found to be physically stable over the entire study (degradation <3%) at room temperature without any loss of activity.
Introduction
Respiratory diseases represent an important public and private expense. However, the impact is not only economic, but other factors must be considered: in pediatric patients, diseases such as rhinosinusitis, rhinitis, asthma, and upper and lower respiratory tract infections have extremely relevant social and clinical implications. In Italy, a quarter of the medical consultations relating to primary care in pediatric patients concern respiratory problems [1]. The airway allows the medication to arrive directly at the nasal fossa without systemic distribution, with a high therapeutic effect and low undesirable effects [2-4]. The most-commonly prescribed nebulizers for the treatment of upper and middle respiratory tract diseases are corticosteroids, mucolytics agents, and antibiotics [5-7]. In order to improve the drug administration, the molecules were mingled with 0.9% or 3% sodium chloride solutions and subsequently administered via aerosol. The use of these sodium chloride solutions enhances not only the inhaled drug’s permeability and bioavailability [8], but also the therapeutic effect, leading to the improvement of the mucociliary clearance time [9]. Currently, in common clinical practice, Sirmione thermal water is also used as a diluent, especially for mucolytic agents and corticosteroids. Because of the high concentration of sodium chloride, carbonates and calcium salts, the salty-bromine-iodine thermal water of Sirmione exhibits hypertonic properties that lead to a fluidifying and cleansing action (Table 1).
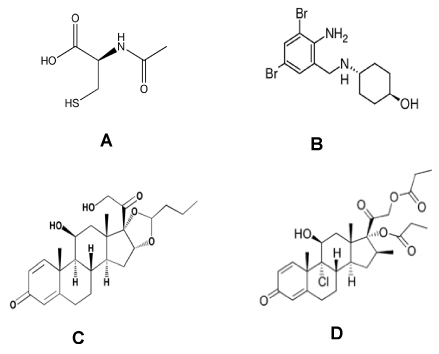
The water of Sirmione has an adjuvant action in the treatment of both infectious and allergic rhinitis and sinusitis thanks to: a) its mucolytic and unblocking action, due to the sulfur content, especially present as hydrogen sulfide, moreover, it has a vasodilating and stimulating action on the secretory IgA of the nasal mucus and it can improve the rheological characteristics of the mucus, b) its antiseptic action, due to iodine content, c) its moisturizing action, due to the mineral complex [10]. These properties make the thermal water of Sirmione suitable not only for aerosol therapy, but it can also be used as a diluent for active drugs for inhalation use. This study aims to evaluate the stability in aerosol containers of two mucolytic agents, N-acetylcysteine (300 mg/3 ml) and ambroxol (15 mg /2ml), and two corticosteroids beclomethasone dipropionate (800 μg /2ml) and budesonide (0.50 mg /2ml and 0.25 mg /2ml), after dilution with Sirmione thermal water stored in plastic ampoules, for aerosol therapy. A large number of analytical methods for the determination and quantification of N-acetylcysteine [11,12], ambroxol [13,14], budesonide [15] and beclomethasone dipropionate [16,17] in pharmaceutical preparations have been developed. However, in spite of the structural diversity and different chemical-physical properties of these drugs (Figure 1), mainly due to the presence of excipients (sodium edetate, polysorbate 80, polysorbate 20, and sorbitan laurate) that can interfere with the separation process, no method was found for their simultaneous analysis. Ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) allowed the optimization of the method and the separation of the four drug in a short analysis time.
Materials and methods
Chemicals
For chromatography, acetonitrile, formic acid, and ammonium formate solution in LC–MS-grade water (Merck House, Poole, UK) were used. Other organic solvents (i.e. methanol) were purchased from Merck (KGa A64271 Darmstadt, Germany). Analytical-grade N-acetylcysteine, ambroxol, beclomethasone diproprionate, and budesonide powder used for validation and quality control of the UHPLC/MS/MS assay were purchased from Ultra scientific, Bologna, Italy. Commercially available formulations were employed to prepare the solutions in aerosol ampoule (Meds Neosol ampoule, Farmac Zabban, Calderara Di Reno, Italy, lot 201808).
Ambroxol (Fluibron® (15 mg/2ml) was purchased from Chiesi Farmaceutici S.p.A, Parma, Italy, lot1099365). Beclometasone dipropionate (Clenil® (800 μg/2ml) was purchased from Chiesi Farmaceutici S.p.A, Parma, Italy, lot1094230). Budesonide (Aircort® (0.25 mg/2ml and 0.50 mg/2ml) was purchased from Italchimici S.p.A., Pomezia, Italy, lot 055119. N-acetylcysteine (Fluimucil® (300 mg/3 ml) was purchased from Farma 1000 S.r.l., Milan, Italy, lot FL9072a), Thermal water of Sirmione in multidose dose vials (Acqua di Sirmione®) was purchased from Terme di Sirmione, Sirmione, Italy, lot 0154A).
Sample preparation
Triplicate samples of tested drugs were analyzed immediately after dilution with thermal water of Sirmione (final volume 5 ml) at 1 hour, 6 hours, 12 hours and 24 hours. Samples were stored in aerosol ampoules at room temperature and exposed to normal fluorescent light. The final concentration of the solutions obtained were 30 mg/ml for ambroxol, 0.16 mg/ml for beclomethasone dipropionate, 0.05 mg/ml and 0.10 mg/ml for budesonide and 60 mg/ml for N-acetylcysteine, respectively. To avoid the solvent’s evaporation, the aerosol ampoules were sealed with Parafilm®. The pH of the solutions was determined before each analysis using a pH meter (HBasic 20, Crison Instruments S.p.A., Modena, Italy). Analyzes were performed in triplicate (n = 45).The pH was measured immediately after dilution with thermal water of Sirmione at 1 hour, 6 hours, 12 hours and 24. The sterility of the samples was not assessed.
Quantitative analysis using UHPLC/MS/MS
The UHPLC system consisted of an Agilent Technologies 1200 series system equipped with a degasser, binary pump and a high-performance autosampler (HiP ALS SL+) with a thermostatic column compartment. Separation was carried out using a reversed-phase Zorbax Eclipse Plus, 2.1 x 100 mm, 1.8 μm (Agilent, Santa Clara, CA, USA), operating at 0.3 ml/min. The mobile phase consisted of 10mM ammonium formate/formic acid 0.1% in acetonitrile 80:20 (v/v) as solvent A and 10mM ammonium formate/formic acid 0.1% in acetonitrile 20:80 (v/v) as solvent B. The initial conditions were 95% A and 5% B. The mobile phase B linear gradient was applied as follows: mobile phase B (5%) was held for 2 min, then it was increased to 95% in 6.0 min and held at this percentage for 0.5 min. This procedure was followed by the reconstitution of starting conditions within 0.1 min, and by the equilibration with 5% of B for 0.5 min, resulting in a total analysis time of 11 min. UHPLC flow rate was set at 400 μL/min and the column temperature at 40 °C.The injection volume was 5 μl. The analysis was performed with the UHPLC tandem 6460 triple quadrupole mass spectrometer (Agilent Technologies, Inc.). An Agilent Mass Hunter workstation was used for the control of equipment, data acquisition, and analysis. The 6460 Agilent featuring an electro- spray ionization (ESI) interface served for MS/MS analysis. The source operated with the Jet Stream technology and a super-heated sheath gas helped to enhance sensitivity. The ion source parameters for negative mode were as follows: vaporizer temperature, 250 °C; sheath gas, 11 mL/min; temperature, 250 °C; nozzle voltage, 400 V and capillary voltage, 3000 V. Finally, nitrogen was used as nebulizer gas and was set at 45 psi with a flow rate of 5 L/min.
Data analysis
The initial concentration was considered as 100% and the subsequent concentrations were expressed as a percentage of the initial value. Following the acceptability criteria indicated by the Official Italian Pharmacopoeia, the degradation of a molecule greater than 10% of the initial concentration is considered unacceptable in terms of loss of activity [18]. The accuracy and precision of our assay met standards set for bioanalytical method validation [19].
Results and discussion
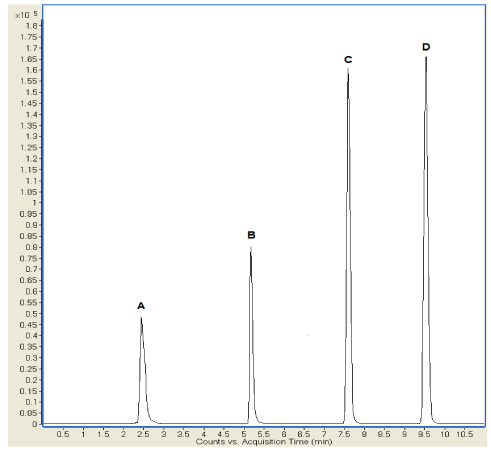
The development of the analytical method allowed the simultaneous analysis of the four different drugs with a total analysis time of 11 minutes. In this study, a triple quadrupole mass spectrometer was used as a detector that allowed the optimization of the mass transitions of the individual compounds. In particular, the following selected reaction monitoring (SRM) transitions were used: N-acetylcysteine 164→59, ambroxol 376→261, budesonide 521→503, and beclomethasone dipropionate 431→413. Figure 2 shows the separation of the analytes in the Total Ion Chromatogram (TIC).
The method was found to be linear in concentrations between 500-5000 ng/ml for ambroxol, budesonide and beclomethasone dipropionate (r2 = 0.9975, 0.9964 and 0.9975, respectively) and between 50-500 ng/ml for N-acetylcysteine (r2 = 0.9962). Peak areas were used for the determination of the different drug concentrations. The coefficients of regression (r2) for all of the standard curves were found to be between 0.98 and 0.99, during the whole study. Intraday and interday coefficients of variation for acetylcysteine samples were ranked from < 6% at 80 μg/mL to < 2% at 220 μg/mL. Table 1 shows the stability data of the four molecules analyzed in aerosol ampoules at room temperature under light exposure, after dilution with Sirmione thermal water. As we can see in Table 2, all drugs exhibited optimal stability at +1 hours, +6 hours, +12 hours, and +24 hours.
The average percentage of degradation found for each molecule was: 0.3% for N-acetylcysteine, 0.6% for ambroxol, 0.7% for budesonide (0.25 mg/ml), 0.5% for budesonide (0.50 mg/ml) and 0.2% for beclomethasone dipropionate. All solutions had a pH of 6.82+0.4 for the entire study. UHPLC belongs to a new category of separative techniques based on established principles of liquid chromatography. The stationary phase is composed by particles smaller than 2 μm. Furthermore, the mobile phase can operate at high speeds and this positively influences the resolution, sensitivity, and speed of the analysis. Due to its speed and sensitivity, this technique has gained considerable attention in recent years in the field of pharmaceutical and biomedical analysis [20]. A large number of factors can affect the stability of a pharmaceutical product, that includes manufacturing process, stability of the molecule, storage conditions (temperature, exposure to light and humidity), as well as chemical reactions such as oxidation, reduction, hydrolysis, and racemization, which could occur after dilution of the drug before administration. The analyses carried out allowed not only the determination of the stability of the four drugs studied, but also the exclusion of degradation products, and impurities already known in literature for all tested drugs [12,15,16,21] (Figure 3).
Conclusion
The proposed method was successfully developed and allowed to define an efficient, simple, rapid, sensitive, and economical protocol for the evaluation of the stability of ambroxol (15 mg/2ml), beclomethasone dipropionate (800 μg/2ml), budesonide (0.50 mg/2ml and 0.25 mg/2ml) and N-acetylcysteine (300 mg/3 ml), after dilution with Sirmione thermal water in ampoules for aerosol therapy. All the active drugs showed good stability under the analyzed conditions, for 24 hours at room temperature.
Author contributions
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.
- Ahrens RC (2005) The role of the MDI and DPI in pediatric patients: “Children are not just miniature adults”. Respir Care 50: 1323-1328. Link: https://bit.ly/3qWWeJO
- Bettoncelli G (2007) Criteri per l’utilizzo della terapia inalatoria nelle patologie ostruttive delle vie aeree. Rivista SIMG 5: 11-14. Link: https://bit.ly/3qYFcv5
- Al-Subu AM, Hagen S, Eldridge M, Boriosi J (2017) Aerosol therapy through high flow nasal cannula in pediatric patients. Expert Rev Respir Med 11: 945-953. Link: https://bit.ly/3AL4O2H
- Terzano C, Allegra L, Grassi C, Dal Negro RW, Passali S, et al. (1999) Linee guida in aerosol terapia. Giornale Italiano delle Malattie del Torace 6: 463-490. Link: https://bit.ly/36ruP9o
- Barnes NC (2007) The properties of inhaled corticosteroids: similarities and differences. Prim Care Respir J 16: 149-154. Link: https://bit.ly/3yD2CIP
- Fashner J, Ericson K, Werner S (2012) Treatment of the common cold in children and adults. Am Fam Physician 86: 153-159. Link: https://bit.ly/3e1cIvb
- Maselli DJ, Keyt H, Restrepo MI (2017) Inhaled Antibiotic Therapy in Chronic Respiratory Diseases. Int J Mol Sci 18: 1062. Link: https://bit.ly/3e28lA3
- Papsin B, McTavish A (2003) Saline nasal irrigation. Its role as an adjunct treatment. Can Fam Physician 49: 168–173. Link: https://bit.ly/3dVwZCo
- Ural A, Oktemer TK, Kizil Y, Ileri F, Uslu S (2009) Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. J Laryngol Otol 123: 517-521. Link: https://bit.ly/2TP5Fig
- Amabile G, Pignataro L, Pignataro O (2000) Valutazione strumentale dell’efficacia della crenoterapia solfureo-salso-bromo-iodica nelle rinosinusiti croniche. Otorinolaringologia 50: 27-34. Link: https://bit.ly/2SYiaaV
- Fohl AL, Johnson CE, Cober MP (2011) Stability of extemporaneously prepared acetylcysteine 1% and 10% solutions for treatment of meconium ileus. Am J Health Syst Pharm 68: 69-72. Link: https://bit.ly/3hqyyKB
- Kiser TH, Oldland AR, Fish DN (2007) Stability of acetylcysteine solution repackaged in oral syringes and associated cost savings. Am J Health Syst Pharm 64: 762-766. Link: https://bit.ly/3htPlNe
- Trivedi RK, Patel MC, Jadhav SB (2011) A Rapid, Stability Indicating RP-UPLC Method for Simultaneous Determination of Ambroxol Hydrochloride, Cetirizine Hydrochloride and Antimicrobial Preservatives in Liquid Pharmaceutical Formulation. Scientia Pharmaceutica 79: 525-543. Link: https://bit.ly/3e0GDUf
- Muralidharan S, Kumar JR, Dhanara SA (2013) Development and validation of an high-performance liquid chromatographic, and an ultraviolet spectrophotometric method for determination of Ambroxol hydrochloride in pharmaceutical preparations. J Adv Pharm Technol Res 4: 65-68. Link: https://bit.ly/3xG7m04
- Hou S, Hindle M, Byron PR (2001) A stability-indicating HPLC assay method for budesonide. J Pharm Biomed Anal 24: 371-380. Link: https://bit.ly/3k1133o
- Sambandan E, Kathavarayan T, Sellappan S, Shiea J, Ponnusamy VK (2019) Identification and characterization of unknown degradation impurities in beclomethasone dipropionate cream formulation using HPLC, ESI-MS and NMR. J Pharm Biomed Anal 167: 123-131. Link: https://bit.ly/3hNJf9m
- Taylor RL, Grebe SK, Singh RJ (2004) Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin Chem 50: 2345-2352. Link: https://bit.ly/3e2iQmS
- Norme di Buona Preparazione, Farmacopea Ufficiale Italiana XII ed (2018) Roma, Istituto Poligrafico e Zecca dello Stato.
- Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, et al. (2000) Bioanalytical method validation—a revisit with a decade of progress. Pharm Res 17: 1551-1557. Link: https://bit.ly/3wtEXcj
- Nováková L, Vlcková H (2009) A review of current trends and advances in modern bio-analytical methods: chromatography and sample preparation. Anal Chim Acta 656: 8-35. Link: https://bit.ly/3dVNzSv
- Thummala VR, Ivaturi MR, Nittala SR (2014) Isolation, Identification, and Characterization of One Degradation Product in Ambroxol by HPLC-Hyphenated Techniques. Sci Pharm 82: 247-263. Link: https://bit.ly/3yCH0MB
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
