Archives of Clinical Gastroenterology
Celiac disease: Definition, classification, historical and epistemological profiles, anatomopathological aspects, clinical signs, differential diagnosis, treatments and prognosis. Proposed diagnostic scheme for celiac disease (DSCNC)
Giulio Perrotta1* and Emanuele Guerrieri2
2Physician and “Emergency and Urgency Medicine”, Polytechnic University of Marche, Via Tronto n. 10/a, 60126, Torrette, Ancona, Italy
Cite this as
Perrotta G, Guerrieri E (2022) Celiac disease: definition, classification, historical and epistemological profiles, anatomopathological aspects, clinical signs, differential diagnosis, treatments and prognosis. Proposed diagnostic scheme for celiac disease (DSCNC). Arch Clin Gastroenterol 8(1): 008-019. DOI: 10.17352/2455-2283.000106Copyright License
© 2022 Perrotta G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Celiac disease is an immune-mediated enteropathy, caused (in genetically predisposed or susceptible individuals) by the ingestion of gluten, the complex of water-insoluble proteins found in cereal grains such as wheat, rye and barley. In terms of terminology, it is the complex natural history and extremely polymorphous clinical presentation that has created some confusion. In fact, to date, at least three different forms of celiac disease are known, in addition to the simple non-celiac gluten sensitivity, since in common clinical practice most patients do not present the classic symptoms such as malabsorptive syndrome with diarrhoea, steatorrhoea, weight loss and nutritional deficiency, but rather an anaemia, asthenia, meteorism, abdominal tension, osteoporosis and infertility, thus painting an extremely varied and complex symptomatic picture that is linked to enteric microbiota and microbiome issues. Celiac disease affects the mucosa of the small intestine, while it generally spares the submucosa, muscolaris propria and serosa; if the disease does not involve the whole of the small intestine but only part of it, it is usually more serious in the proximal than the distal tract. The simultaneous presence of shortened villi, crypt hyperplasia, the abnormal cytological appearance of the absorbent surface and increased lamina propria cells is required for the diagnosis of celiac disease. Based on these findings, several forms of celiac disease have been identified in the clinic: typical, atypical (and in turn silent, latent, and potential), and sensitive non-celiac. Based on these considerations a specific diagnostic scheme is suggested to frame the celiac universe more functionally and structurally (so-called Diagnostic Scheme for Celiac Disease and Nonceliac Gluten Sensitivity, DSCNC), identifying at least eight clinical hypotheses based on the serological, genetic, bioptic and allergological tests suggested). From a pharmacological and integrative point of view, the protocols shared by the scientific community remain in place: gluten-free diet, vitamin and salt supplementation if appropriate, pharmacological therapy (antibiotics, antihistamines, corticosteroids and immunosuppressants) if necessary, also in the future with the majority orientation oriented towards oral glutenase able to counteract the effects of gliadin in sensitive subjects, the use of larazotide acetate to remedy the increase in intestinal permeability and tTG inhibitors to reduce the toxic effects of gluten intake. The state of the art on celiac disease is not yet able to explain the precise aetiology and atypical forms of the disease, as well as the real impact of genetic predisposition on clinical manifestations. Research continues and seems to point the way to a complete resolution of this enteropathy that has been so prevalent over the last two decades.
Definitions
Celiac disease is an immune-mediated enteropathy, caused (in genetically predisposed or susceptible individuals) by the ingestion of gluten, the complex of water-insoluble proteins found in the cereal grains such as wheat, rye and barley. Other clinical terms for celiac disease are celiac sprue, gluten-dependent enteropathy, non-tropical sprue, celiac syndrome and primary malabsorption [1].
In terms of terminology, it is the complex natural history and the extremely polymorphous clinical presentation that has created some confusion. In fact, to date, at least three different forms of celiac disease are known, in addition to the simple non-celiac sensitivity, since in common clinical practice most patients do not present the classic symptoms (of the typical form) such as malabsorptive syndrome with diarrhoea, steatorrhoea, weight loss and nutritional deficiency, but rather anaemia, asthenia, meteorism, abdominal tension, osteoporosis and infertility, thus painting an extremely varied and complex symptomatic picture that is linked to the enteric microbiota and microbiome issue [2,3].
Historical and epidemiological profiles
The first historical evidence of this condition came from Areteus of Cappadocia in the 1st century BC, while the term ‘sprue’ was not coined until the 18th century in Dutch circles due to the presence of mouth ulcers in patient’s mouths. In 1888, Samuel Gee published a study on celiac disease, stating that the disease affected all age groups indiscriminately and that diet was the only viable solution, but only a Dutch paediatrician named Willem Karel Dicke, in the middle of the 20th century, linked the celiac disease to the ingestion of certain graminaceous cereals, particularly wheat; This was confirmed and supported by subsequent studies (also in those years) by Paulley and van de Kamer, who identified gluten as the cause of mucosal lesions in celiacs. In the 1980s, Howell, Lundin and Molberg, in three different studies based on medical genetics and immunology, demonstrated that the celiac condition is associated with the presence of specific HLA-DQ2 haplotypes, that HLA-DQ2 preferentially presents gliadin-derived peptides to T lymphocytes in the intestinal mucosa, and consequently the enzyme tissue transglutaminase type 2 (tTG2) was recognised as an autoantigen of the coeliac condition. Thus, tTG2-induced gliadin modifications enhance the celiac disease-specific T-cell response, demonstrating that the identification of tTG-specifically Deaminated Gliadin Peptides (DGPs) as major epitopes of alpha-gliadin on T-cells has prompted the dogma that tTG2 plays a crucial role in the pathogenesis of the celiac disease, in recent years, researchers have turned to studies aimed at implementing the gluten-free diet with targeted and specific therapies, such as gluten detoxification and the use of glutenase, tTG2 inhibitors and other immunomodulation-based treatments to control gluten intolerance [1] table 1.
Epidemiologically, with the introduction of modern antibody screening tests, it has been possible to demonstrate that celiac disease is a very frequent condition in Caucasian populations, affecting both adults and children. In particular, the European prevalence of the typical form is around 1% of the population, while in Finland it is as high as 2.4%, as the following factors play a role: the frequency of the HLA haplotype in the population, the concentration of gliadin in powdered milk, and variations in the data found during intestinal biopsies. In the rest of the world, the incidence is higher in those populations that use wheat more than rice, i.e. north-east India, north-west Africa, North America and Europe itself, reaching 2-3% [1,5-7].
However, this data is not up to date on a global scale and therefore it is assumed to be an underestimate about the last twenty years, where commercial wheat-based products have increased due to low cost, and therefore if we look at the average incidence of atypical, silent, potential and latent celiac conditions, as well as susceptible but not celiac forms, the overall impression is that there is a clear underestimation of the real impact of the disease.
Anatomopathological profiles and pathogenesis
Celiac disease [1,8]. Affects the mucosa of the small intestine, while generally sparing the submucosa, muscolaris propria, and serosa; if the disease does not involve the entire small intestine but only a portion of it, there is usually greater severity in the proximal than the distal tract. On microscopic examination, the analysed portion (to be suggestive of celiac disease) must show based on severity and extent.
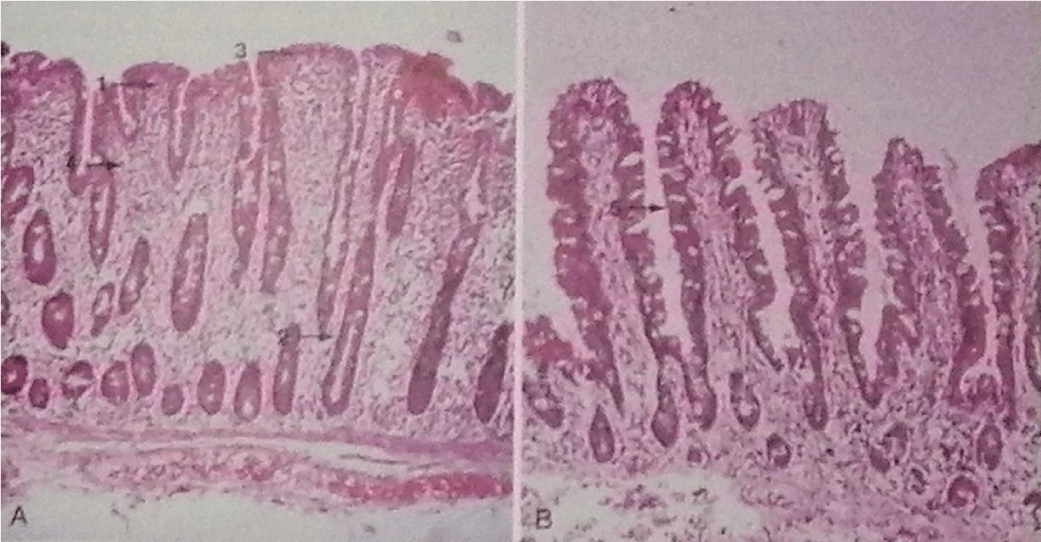
a) Loss of normal villous structure, with flattening to complete disappearance (severe atrophy) and hyperplasia of the crypts, significantly decreasing the available absorbent surface area devoted to digestion and absorption. Studies of epithelial cell kinetics indicate that it is inappropriate to speak of ‘villous atrophy’, as there is evidence of an actual increase in erythropoiesis in the crypts; indeed, the mucosa of a celiac produces six times the number of cells that a normal intestine does, while the cell cycle is halved due to premature breakdown of the mucosa. This atrophic effect of the villi is probably due to the direct toxic effect on mature enterocytes which causes them to flake prematurely in the intestinal lumen and produces a compensatory increase in the replication of crypt enterocytes.
b) Enterocyte alteration, which appears cuboidal or more rarely squamoid, rather than columnar, with a markedly more basophilic (i.e., more RNA-rich) cytoplasm, altered basal polarity of the nucleus, markedly reduced brush border and a marked increase in the number of free ribosomes, confirming cytoplasmic basophilia and vacuolization of the cytoplasm, mitochondria and the presence of numerous large lysosomes. The endoplasmic reticulum is rarefied, in turn causing the reduced synthesis of digestive enzymes, including disaccharidases and peptidases.
c) Increased permeability of the mucosal barrier, due to the presence of structural abnormalities of the tight junctions.
d) Intense inflammation of the lamina propria and epithelium. In particular, in celiac disease, the number of cells in the lamina propria is higher in the tract affected by the disease and the cellular infiltrate consists mainly of plasma cells and lymphocytes, with a 2- to 6-fold increase in cells producing IgA, IgM and IgG. Polymorphonuclear leukocytes, eosinophils and mast cells also contribute to the increase in cellularity. The number of intraepithelial lymphocytes (IEL) is higher, as is the density of CD4+ (helper/inducer) and CD8+ (cytotoxic/suppressor), while the distribution is still normal in the lamina propria; however, the increase in intraepithelial lymphocytes alone is not sufficient to make a histological diagnosis of celiac disease, as this condition is also common to other pathological entities such as bacterial overproliferation syndrome (SIBO), peptic duodenitis, Helicobacter Pylori (Hp) infection, use of non-steroidal anti-inflammatory drugs and various autoimmune and inflammatory diseases.
The diagnosis of celiac disease requires the simultaneous presence of shortened villi, crypt hyperplasia, an abnormal cytological appearance of the absorbent surface and an increase in the cells of the lamina propria [8].
Based on these considerations, the sequential progression of the celiac lesion in the intestinal mucosa has been theorised: it starts with normal, pre-infiltrative mucosa (stage 0) and the first event is the increase of intraepithelial lymphocytes, followed by the infiltration of lymphocytes into the lamina propria (stage 1); this is followed by crypt hyperplasia (stage 2), which precedes villous atrophy (stage 3), which is only seen in the presence of lymphocytosis of the lamina propria, suggesting that increased intraepithelial lymphocytes alone cannot induce mucosal architectural change; finally, total mucosal atrophy develops (stage 4), characterised by complete loss of villi, increased apoptosis and crypt hyperplasia [9] Figure 1.
In terms of etiopathogenesis, the scientific literature is unanimous in its belief that the interaction of the water-insoluble proteins (gluten) contained in certain cereals with the mucosa of the small intestine of predisposed individuals is the central event in the pathogenesis of celiac disease since celiac disease is an immune-mediated enteropathy triggered by gliadin in genetically predisposed individuals. However, the clinical manifestations are the result of a complex relationship between environmental, genetic and immunological factors, although it is still unclear how these factors control the expression of the disease and the transition from latent to full-blown disease [1,10].
It is well known that gluten comprises two major protein fractions: gliadins and glutenins. The gliadins, which are certainly toxic, are alcohol-soluble and can be differentiated by modern electrophoretic techniques into more than 100 components, which in turn are grouped into four main types (ω5-, ω1,2-, α/β-, γ-). Similar to gliadins in terms of their amino acid composition are prolamins, i.e. the alcohol-soluble components of barley (hordein), rye (secalin) and oats (avenin), the latter of which is still of doubtful toxicity, since it is often well-tolerated by celiacs due to its probable lower quantity of toxic prolamins, provided that the quantity ingested during the day is modest and in any case does not exceed 40-60 g/day. Glutenins are alcohol-insoluble, their toxicity has recently been confirmed and they can be further subdivided into high and low molecular weight subunits. Gluten is characterised by a unique amino acid composition, rich in glutamine and proline. These two amino acids give glutenins particular antigenic properties, such as their ability to bind to HLA class II molecules, their indigestibility by luminal enzymes and the brush border, and their rigid α-helical conformation. Complete degradation of gliadins to single amino acids deprives them of their toxicity, while digestion by pepsin and trypsin generates peptides that are still toxic. Recently, two α-gliadin oligopeptides, 31-49 and 51-70 have been identified that can induce flattening of the intestinal mucosa both in vivo and in vitro. Oligopeptide 51-70 has also been shown to activate gliadin-specific and HLA DQ2-restricted T lymphocytes, which, as we shall see, play a key pathogenetic role. Lastly, the 57-73 oligopeptide of α-gliadin, which is very similar to 51-70, is said to be the peptide towards which the first immune response is triggered, which then spreads to other gliadin peptides through an epitope spreading process. The importance of genetic factors in the pathogenesis of the celiac disease is evidenced by studies conducted on relatives of celiac patients, which found a prevalence of celiac disease of 10-15% among first-degree relatives and 30% if HLA-identical collaterals are considered. The much higher concordance for disease among monozygotic twins than among HLA-identical siblings confirms that non-HLA genes are also implicated in the pathogenesis of this condition, reaching an incidence of 70%. The association between celiac disease and genes of the major histocompatibility system, i.e. genes coding for HLA molecules, is very strong. More than 90% of celiac patients carry the HLA DQ2 molecule encoded by the HLA alleles DQA1*0501 and DQB1*0201. The remaining patients who do not carry the DQ2 molecule express, in most cases, the DQ8 molecule encoded by the HLA alleles DQA1*0301 and DQB1*0302. However, it should be made clear that these alleles are a necessary but not sufficient condition for the development of this disease, as confirmed by the presence of these alleles in 25-30% of the general population. In addition, a number of non-HLA genes involved in the regulation of the immune response, located on the long arm of chromosome 5, have been associated with the disease. Subsequent studies have also identified gene variants related to the costimulatory molecule CTLA-4, the myosin IXB gene and the IL-2 and -21 coding regions. This topic is still highly controversial; although the current heterogeneity of results may likely reflect the genetic heterogeneity of various populations, there is no doubt that celiac disease should be considered a polygenic condition. Tissue transglutaminase is known to be the autoantigen to which anti-endomysium antibodies are directed. Tissue transglutaminase, or type 2 transglutaminase, is a member of the transglutaminase family, calcium-dependent enzymes that catalyse the transfer of peptide chains from one protein to another (cross-linking) and are involved in the wound repair process. Tissue transglutaminase catalyses transamidation reactions between the amide group of a glutamine residue and the amino group of a lysine residue, releasing an ammonia molecule and forming an ε-(γ glutamine)-lysine bond. Through this function, transglutaminase catalyses the cross-linking of proteins in the extracellular matrix and thus the repair of wounds. In the case of celiac disease, this leads to the formation of a gliadin-transglutaminase complex with the creation of a neoheptum. In the absence of an amine donor or the presence of an acidic pH, transglutaminase instead catalyses a deamidation reaction of the glutamine residue, which reacts with a water molecule to form a glutamic acid residue. The importance of tissue transglutaminase in the pathogenesis of celiac disease is demonstrated not only by the fact that it is the target of anti-endomysium antibodies, but also by the fact that, among other proteins, gliadin is a preferred substrate. By specifically deamidating the glutamine residues in which gliadins are particularly rich, transglutaminase increases the binding affinity between gliadins and HLA molecules DQ2 and DQ8. Thus deamidated, gliadin is presented by antigen-presenting cells to T lymphocytes in a form that more effectively activates them. Numerous demonstrations confirm that cells comprising HLA DQ2/DQ8-restricted innate immunity (macrophages, dendritic cells, enterocytes) present gliadin, which has penetrated the intestinal wall transcellular (through enterocytes) or paracellularly (between enterocytes), to the antigen receptor of CD4+ lymphocytes in an unmodified or, more frequently, deamidated form. The resulting T-cell activation results in the hyperproduction of proinflammatory Th1 cytokines (interferon-γ, IL-2 and -15), which, together with the direct cytotoxic action of intraepithelial CD8+ lymphocytes and the activation of metalloproteases, induces a drastic acceleration of enterocyte apoptosis, resulting in villus atrophy and cellular hyperproliferation with crypt hyperplasia-hypertrophy. Although there is no doubt that cell-mediated immunity plays a very important role in the pathogenesis of celiac disease, there is ample evidence to suggest that humoral immunity is involved. In untreated celiac disease, high serum levels of anti-gliadin and tissue transglutaminase (or anti-endomysium) antibodies are found. Both are gluten-dependent, i.e. they become negative after exclusion of gluten from the diet. It has been proposed that the pathogenetic mechanism leading to the production of these antibodies is triggered by the formation of gliadin-transglutaminase complexes, which are also presented to the immune system; although it is not yet clear whether the role played by humoral immunity is pathogenically primary or secondary, it is undeniable that the search for these antibodies is a great diagnostic aid [1,11-15] table 2.
Clinical profiles
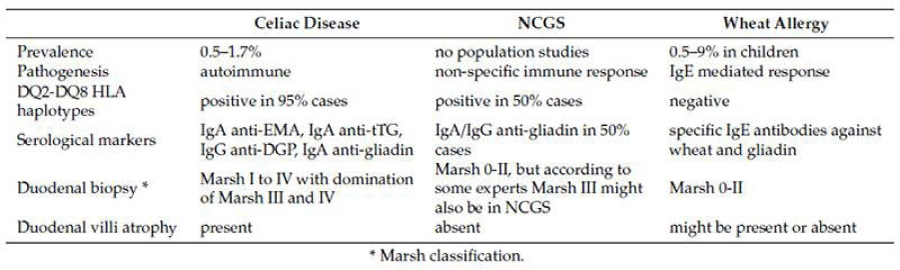
In the literature, there is no unanimity about the classification of the pathological forms except for the best known: the ‘typical’ (classical or evident) form [10]. This form, whether it begins in childhood or in adulthood, presents diarrhoea (less frequent in overweight or obese subjects) with consequent loss of electrolytes and liquids, dehydration, weight loss, malabsorption, vitamin deficiency, steatorrhoea, abdominal cramps and extraintestinal symptoms [16-18]. With skin manifestations (ecchymoses, petechiae, oedemas, atopic or seborrhoeic or herpetiform dermatitis, hyperkeratosis, stomatitis, mouth ulcers) [19-26]. Endocrinological (amenorrhoea, infertility, impotence, hyperparathyroidism, hypotestosteronism, hypothyroidism, diabetes, hypercortisolemia, metabolic syndrome, obesity) [27-31]. Haematological (anaemia, haemorrhage, thrombocytosis, hyperhomocysteinemia) [32-43]. Hepatic (hepatic steatosis, autoimmune hepatitis and hepatic-biliary morbidity [44-47]. Muscular (atrophy, muscle weakness and tetany) [10,48-50]. Hypovitaminic (especially calcium, iron, magnesium, b-complex including folates, vitamin k and vitamin d) [51-53]. Neurological and psychiatric (ataxia ataxia, seizures, peripheral neuropathy and demyelinating lesions of the central nervous system, mood disorders and psychosis) [54-57]. Immune (allergies, immunodeficiencies and immune vulnerability) [10,58-60]. And skeletal (rickets in infants and children, osteopenia, osteoporosis, osteoarthropathy and pathological fractures) [61,62]. The “typical form” of celiac disease has positivity for one or all of the following antibody markers: anti-transglutaminase antibodies (anti-tTG, IgA), anti-endomysium (anti-EMA, IgA) which is considered a confirmatory test for the former, and anti-gliadin deaminated peptides (anti-DPG, IGG). The onset of symptoms is generally gradual and characterised by a time interval of months or years after the introduction of gluten unless the condition is so severe that it is already disabling from the first gluten intake; however, in patients who have been on a gluten-free diet for a long time, the occasional ingestion of gluten may cause immediate symptoms, with vomiting and/or abdominal colic [63,64].
Celiac disease, in its typical form, can also be
a) Responding, if within 6 months there is improvement in symptoms in association with the gluten-free diet.
b) Resistant (or non-responsive), if symptoms tend to recur within 6-12 months despite perfect adherence to the gluten-free diet. In this case, there is likely comorbidity with other morbid conditions that facilitate or aggravate the celiac condition, such as SIBO, tropical sprue, eosinophilic enteritis, food intolerances, ulcers of the small intestinal tract, microscopic colitis, lymphomas, collagenous sprue, diverticulitis and Crohn’s disease: it is necessary to act on the cause and work on maintaining a gluten-free diet, associating where necessary pharmacological therapy with antibiotics, antihistamines and/or cortisone, and in the worst cases with methotrexate and/or cyclosporine.
c) Refractory, if it does not respond to the gluten-free diet even after 1 year. The most probable causes are a clonal increase and/or the presence of aberrant T lymphocytes and merit special clinical attention, with possible immunological therapy with immunosuppressants.
In addition to the ‘typical form’, other so-called ‘atypical’ pathological forms [1,10,65 -71]. Have been identified in recent years, mainly on an asymptomatic (and clinical) basis and as a consequence of gluten intake:
1. “Silent”, which includes both intestinal and extraintestinal symptoms, but with histological manifestations that are less marked than in the case of the overt disease, chronic and resistant constipation (replacing diarrhoea and dehydration, which occur rarely or are completely absent) and greater presence of gastroenterological symptoms such as gastritis and chronic duodenitis, reflux oesophagitis, incontinence of the cardia and pylorus, biliary disorders, intestinal microbiota alterations causing colitis and diverticulitis, abdominal bloating and vagal hyperexcitability symptoms, which are almost always typical of moderate to severe intestinal dysbiosis. Serological markers are inactive, but genetic positivity (HLA-DQ2/8) and an increased total IgE value are almost always present, as is the presence of histological damage that is not, however, clearly attributable to celiac disease.
2. “Latent”, which includes both the symptoms of the typical and the silent form, but which are even more attenuated and nuanced. Serological markers may be active, as well as genetic positivity (HLA-DQ2/8), but in the absence of histological damage unless typical of mild-to-moderate intestinal dysbiosis. IgE may be higher than normal values.
3. “Potential”, which includes both the symptoms of the typical and the silent form, but even more attenuated and nuanced. Serological markers may be active, in the presence or absence of genetic positivity (HLA-DQ2/8), but in the absence of histological damage if not typical of mild-to-moderate intestinal dysbiosis. IgE may be higher than normal values.
Distinct from celiac disease is the so-called “non-celiac gluten sensitivity” (NCGS) [72-77]. Which is neither an allergic reaction nor an autoimmune disease. To better understand this new disease entity, however, it is necessary to take a step back and better contextualise the pathological universe of ‘gluten’. In fact, while wheat allergy is a true allergic reaction (diagnosed by positivity to specific IgE antibodies), celiac disease is an enteropathy on an autoimmune basis in response to the intake of gluten (diagnosed by serological, genetic and histological tests by biopsy of the small intestine tract), non-celiac gluten sensitivity, on the other hand, is an inflammatory reaction resulting from gluten intake by mechanisms that are not yet fully understood, but with symptoms very close to the attenuated forms of celiac disease, without histological damage but with anti-gliadin IgA/IgG marker positivity in 50% of cases; for this reason, in the opinion of the writers, this condition can still be considered an intermediate form between the celiac condition and the specific allergic reaction, in which IL1/2/6, TNF-alpha and INF-Gamma are the components that activate the non-specific immune response, which is composed of macrophages and neutrophils, more rarely monocytes, and the intestinal release of IgE is the cause of mast cell degranulation, effectively triggering a systemic histamine cascade. It is no coincidence that in wheat we find at least 3 groups of substances that can be troublesome for our immune system: a) gluten, composed of gliadin and glutenin, which with its residues rich in proline and glutamine, leaves peptides that cannot be broken down due to the lack of specific enzymes. These peptides increase the increase in zonulin, open up junctions and stimulate immune activation both through antigens present in the cells and through IL1-beta inflammasome-mediated; b) Amylase-Trypsin Inhibitors (ATIs), a class of albumins representing 4% of wheat proteins, which are highly protease-resistant and can inhibit the function of pancreatic amylases and proteases, greatly worsening the digestive outcome. These proteins are also responsible for activating innate immunity, especially TRL4, in both celiacs and NCGS, and are the main causes of non-response to the gluten-free diet. Their measurement is being used to predict the efficacy of response to biotechnological drugs. These proteins can mediate the release of Il8/2, TNF, MCP1 that profoundly stimulate local immune activity, which as we now know reverberates on systemic immune activity; c) FODMAPs, especially mono/di/oligosaccharides and fibres that are not digestible by us, but which can glycate, activating RAGE (receptors for glycated proteins) and favour unwanted microbial species that thrive and can ferment, causing bloating and meteorism, and further stimulate inflammation table 3.
Celiac disease and intestinal microbiota
Starting from the premise, therefore, that the celiac condition is an autoimmune-based enteropathy affecting the small intestine and triggered by the ingestion of foods containing gluten in genetically predisposed individuals, it is important to study its correlations with intestinal dysbiosis (understood as quantitative and/or qualitative alterations in the enteric flora), as it is known that an alteration in the intestinal microbiota can promote or anticipate the onset of celiac disease or aggravate its symptoms, by promoting damage to the intestinal mucosa and its ability to permeate the environment from the passage of microorganisms from the intestinal lumen to other internal pathways in the body. Evaluations of the microbiota in patients with celiac disease on a gluten-free diet have, however, revealed significant alterations, especially in those in whom, despite the diet, an adequate state of well-being was not being achieved. The majority of duodenal biopsies from patients with celiac disease compared to those from healthy patients showed an increase in Staphylococcus spp. and Bacteroides fragilis bacteria, and a subsequent reduction in Bifidobacterium (especially longum), and to a lesser extent Prevotella and Lactobacillus. Thus there is a lack of micro-organisms producing short-chain fatty acids, which have an anti-inflammatory effect on the intestinal mucosa, or which promote the presence of mucus as a defence factor. It, therefore, becomes important to treat with both prebiotics and specific probiotics (e.g. Bifidobacterium longum ES1, which can reduce activated T lymphocytes and inflammatory markers such as TNF-alpha) that favour the colonisation of bacteria producing short-chain fatty acids. Diet and lifestyle are key to rebalancing the microbiota. In the scientific literature, however, it is not yet clear whether the alterations in the microbiota are one of the causes activating the celiac condition or whether it is the celiac condition that triggers the inflammatory mechanism altering the microbiota [2,3,78-82].
Psychological profiles
Recent clinical studies have shown that there is a direct correlation between untreated celiac disease and the worsening of psychological and psychiatric symptoms such as anxiety disorders, mood disorders, ADHD, the autistic spectrum, depression and eating disorders, whereas there does not seem to be a correlation with bipolar forms, the schizophrenic spectrum and personality disorders, although a distinction should be made between celiac disease-related symptoms and those caused by intestinal dysbiotic forms, which are also related to psychotic symptoms. It is therefore important to provide celiac sufferers with psychological and psychiatric support, especially in cases where some psychiatric comorbidities or symptoms have not been treated pharmacologically and/or with psychotherapy [83-119].
Celiac disease in emergency medicine
Prolonged exposure to gluten due to a lax agglutination diet and/or late diagnosis is the most important factor in the development of complications of celiac disease. It is known that the mortality rate for celiacs diagnosed in childhood and since then under strict dietary treatment is no higher than that of the general population, whereas in celiacs diagnosed in adulthood the mortality rate is doubled and increases 6-fold in the case of frequent dietary transgressions. The increased mortality that characterises celiac disease in adults is underpinned by the onset of several complications that should be suspected when there is no clinical or histological response to the diet, or when, despite a strict diet, symptoms such as abdominal pain, fever, diarrhoea, haemorrhages and anaemia reappear. These complications have a high mortality rate and can occur many years after the diagnosis of celiac disease, but fortunately, they are rare. On the other hand, the risk of developing these complications is directly proportional to the age at diagnosis of celiac disease and they are almost exclusively found in patients with a major form of celiac disease. Intestinal lymphoma is the most prominent complication in terms of frequency and severity. It is a high-grade, blastic, enteropathy-associated T-cell lymphoma (EATCL), often occurring between the fifth and seventh decade of life. The clinical picture in most cases is characterised by abdominal pain, weight loss, fever and diarrhoea; in other cases, the onset may also be intestinal obstruction or perforation. Ulcerative jejunitis is a rarer complication and is characterised by multiple, transverse ulcers of the small intestine, followed by scar retraction, often leading to stenosis of the affected tract. Histologically, the lesions have a non-specific, non-granulomatous inflammatory infiltrate and varying degrees of villous atrophy in the mucosa adjacent to the ulceration. The clinical features are similar to those of EATCL with which ulcerative jejunitis is often associated. Mortality in the latter exceeds 70% and is due to intestinal occlusions, perforations and haemorrhages. Refractory celiac disease is characterised, as mentioned above, by duodenal mucosa with severe villous atrophy despite the agglutination diet, which is clinically accompanied by a recurrence of symptoms. Since the aberrant phenotype of intraepithelial lymphocytes and the monoclonal rearrangement of the TRC γ-chain are often present in refractory celiac disease and ulcerative jejunitis in addition to EATCL, it has been proposed that these three complications may represent three different stages of a single developmental spectrum with a poor prognosis. Other neoplastic complications of celiac disease are adenocarcinoma of the small intestine and squamous cell carcinoma of the pharynx and oesophagus. Non-neoplastic complications are atrophy of the spleen with functional hyposplenism, potentially leading to fatal infections, and collagenous sprue, which is characterised by the deposition of thick bands of subepithelial collagen. Suspicion of these conditions necessitates instrumental investigations aimed at visualising the entire intestine. An important tool for both direct observations of lesions and biopsy of distal sections of the small bowel is ‘double-balloon enteroscopy. The recent introduction of the endoscopic video capsule, a simple and practical method of investigation, allows the visualization of the small intestine along its entire length, reaching intestinal areas inaccessible to traditional endoscopic manoeuvres. This method, therefore, provides more detailed information on the extent and number of lesions present and, although it does not allow biopsies to be taken, it is a preliminary investigation to enteroscopy. Finally, the enema of the small intestine allows the detection of stenosis, which is indirect evidence of ulceration [10,120-123].
Diagnostic profiles and differential hypotheses
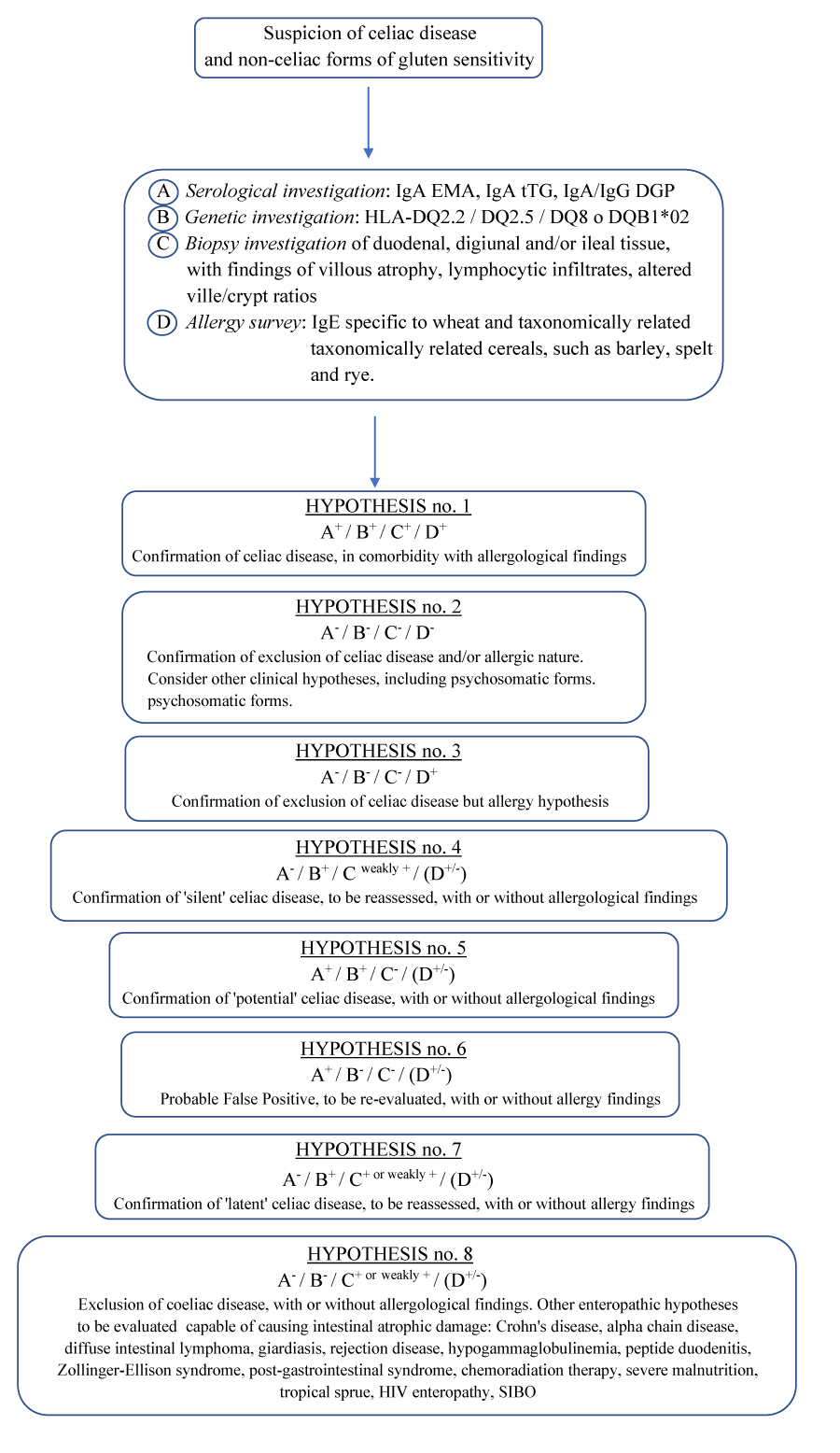
As mentioned above, laboratory data and symptoms vary according to the severity and extent of the intestinal damage [1]. Celiac-specific laboratory tests (IgA EMA, IgA tTG, IgA/IgG DGP) and intestinal biopsy are the most reliable tools for diagnosis. Faecal and haematological examinations and radiology studies, while showing abnormalities, rarely direct the diagnosis because similar alterations are common with other malabsorptive diseases. Genetic testing of HLA DQ2 and DQ8 is useful in ruling out celiac disease in specific clinical cases, but other serological and instrumental tests may be useful in making the final diagnosis:
- vitamin and salt deficiency;
- ultrasound scan of the spleen, looking for atrophy;
- hypercholesterolemia and transaminases;
- Physico-chemical stool test;
- xylose and lactulose-mannitol tests.
However, the latter tests, lacking specificity, cannot be included in routine tests in suspected celiac disease.
Of interest, although rarely prescribed because marked features of interest are not always found, is the barium meal X-ray, which in cases of celiac disease would show dilation of the loops of the small bowel with the digiunal foliar morphology markedly thickened or flattened; excessive fluid secretion in the proximal tenue, then, associated with insufficient absorption of intraluminal contents causes dilation of the barium with a decrease in contrast in the more distal bowel [1].
Diseases associated with celiac disease include dermatitis herpetiformis, seborrheic and atopic dermatitis, type 1 diabetes mellitus, epilepsy, IgA nephropathy, Yashimoto’s syndrome, pericarditis, Down’s syndrome, and sarcoidosis. Also of doubtful correlation: various autoimmune conditions, schizophrenia and psychotic disorders, Addison’s disease and chronic pneumopathies [1].
In 2015, 30 of the world’s leading experts in gluten-related disorders jointly outlined the new guidelines for the diagnosis of Non-Celiac Gluten Sensitivity (SGNC). “The Salerno Experts’ Criteria”, in the absence of biomarkers, represents the first internationally shared recommendation for a model diagnostic protocol for confirming the condition. According to the latest review of studies, from the epidemiological point of view, it is estimated that it is more frequent than celiac disease (1% of the population) and that it affects mainly women, compared to men. In general, the onset of symptoms appears a few hours or days after gluten intake. As for the therapy, the answer of experts is that this is represented by a gluten-free diet, exactly as in the case of celiac disease [124].
Taking into account the global prevalence [125]. The various aspects characterizing celiac disease [126,127]. Including the hypothesis of non-celiac gluten sensitivity and all the slow-reactive forms, potential with or without minimal lesions and seronegative [128,129]. Here below we suggest a diagnostic scheme for the suspicion of celiac disease and non-celiac forms of gluten sensitivity (Diagnostic Scheme for Celiac Disease and Nonceliac Gluten Sensitivity, DSCNC) table 4.
Treatments and prospects
The standard treatment for celiac disease is a gluten-free diet, which must be very strict and followed scrupulously throughout life, requiring not only the elimination of all foods containing wheat and its derivatives but also those containing cereals taxonomically related to wheat. Based on this assumption, we consider it appropriate to perform a control biopsy after 12-18 months of removal of gluten from the diet (sufficient time for the lesions to normalise). A control duodenal biopsy is essential if there is no clinical response to the gluten-free diet. In such cases, the initial diagnosis of celiac disease must first be re-evaluated to ensure that the patient has enteropathy and that this enteropathy is gluten-dependent, and then it must be ensured that the patient is following a sufficiently strict diet. Vitamin and salt supplements are suggested when necessary, as well as pharmacological therapies in the most resistant cases or those refractory to the gluten-free diet: specific antibiotic drugs, antihistamines and corticosteroids, the latter for a short cycle of 3-5 days at a low dosage, in the hypothesis of severe gastroenteric episodes or refractory celiac disease, up to immunosuppressive drugs such as methotrexate and cyclosporine. The prognosis is excellent if the prescribed therapy is carried out correctly and rare complications do not occur; indeed, some studies show that it is a condition that can disappear on its own after a medium to a long period of abstention from gluten intake. In the future, too, the focus is on finding new products to combat the disease, such as oral glutenases to counteract the effects of gliadin in sensitive individuals, as well as the use of larazotide acetate to counteract increased intestinal permeability and tTG inhibitors to reduce the toxic effects of gluten intake [1,130-133].
Conclusions
The state of the art on celiac disease is still unable to explain the precise aetiology and atypical forms of the disease, as well as the real impact of genetic predisposition on clinical manifestations. Research continues and seems to point the way to a complete resolution of this enteropathy that has been so prevalent over the last two decades.
- Kelly CP (2018) Celiachia. In Malattie gastrointestinali ed epatiche. Fisiopatologia, diagnosi e trattamento (Sleisenger e Fordtran), II vol., C. 107, X ed., trad. ita, Edra Ed.
- Perrotta G (2021) The intestinal microbiota: towards a multifactorial integrative model. Eubiosis and dysbiosis in morbid physical and psychological conditions. Arch Clin Gastroenterol 7: 024-035. Link: https://bit.ly/3jJH6gb
- Perrotta G (2021) Intestinal dysbiosis: definition, clinical implications, and proposed treatment protocol (Perrotta Protocol for Clinical Management of Intestinal Dysbiosis, PID) for the management and resolution of persistent or chronic dysbiosis. Arch Clin Gastroenterol 7: 056-063. Link: https://bit.ly/3voWpjS
- Kasanda DD, Okita TW, Bernardin JE, Baecker PA, Nimmo CC, et al. (1984) Nucleic acid (cDNA) and amino acid sequences of alfa-type gliadins from wheat (Triticum aestivum). Proc Natl Acad Sci USA 81: 4712-4716. Link: https://bit.ly/3JP2uv3
- El-Metwally A, Toivola P , AlAhmary K, Bahkali S, AlKhathaami A, et al. (2020) The epidemiology of Celiac Disease in the general population and high-risk group in arab countries: a systematic review. Biomed Res Int. Link: https://bit.ly/37WsyHd
- Ierodiakonou D, Garcia-Larsen V, Logan A, Groome A, Cunha S, et al. (2016) Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA 316: 1181-1192. Link: https://bit.ly/3JObiBa
- Safi MA (2019) Celiac disease among at-risk individuals in Saudi Arabia. Saudi Med J 40: 9-18. Link: https://bit.ly/3EjJhR2
- Villanacci V, Vanoli A, Leoncini G, Arpa G, Salviato T, et al. (2020) Celiac disease: histology-differential diagnosis-complications. A practical approach. Pathologica 112: 186-196. Link: https://bit.ly/3KJDvum
- Ensari A, Marsh MN (2019) Diagnosing celiac disease: A critical overview. Turk J Gastroenterol 30: 389-397. Link: https://bit.ly/3KRxHyU
- Rugarli C (2021) Medicina Interna Sistematica.VIII ed., Edra Ed.
- Bamonte F (2012) Celiachia: nuove opportunità diagnostiche. Tesi di specializzazione in Biochimica clinica, Università degli Studi di Pisa.
- Crowe SE (2020) Putting celiac disease in perspective: Pathogenesis, comorbidity and transition of care. United European Gastroenterol J 8: 129-130. Link: https://bit.ly/3uMlciz
- D’Avino P, Serena G, Kenyon V, Fasano A (2021) An updated overview on celiac disease: from immuno-pathogenesis and immuno-genetics to therapeutic implications. Expert Rev Clin Immunol 17: 269-284. Link: https://bit.ly/36oAVew
- Capittini C, De Silvestri A, Rebuffi C, Tinelli C, Poddighe D (2019) Relevance of HLA-DQB1*02 Allele in the genetic predisposition of children with celiac disease. Medicina (Kaunas) 55: 190. Link: https://bit.ly/3vnfnqX
- De Silvestri A, Capittini C, Poddighe D, Valsecchi C, Marseglia G, et al. (2018) HLA-DQ genetics in children with celiac disease: a meta-analysis suggesting a two-step genetic screening procedure starting with HLA-DQ β chains. Pediatr Res 83: 564-572. Link: https://bit.ly/3k1tzAV
- Garnier-Lengliné H, Cerf-Bensussan N, Ruemmele FM (2015) Celiac disease in children. Clin Res Hepatol Gastroenterol 39: 544-551. Link: https://bit.ly/3EhD8ok
- Vackovà Z (2020) Celiac disease in adults. Vnitr Lek 66: 116-120. Link: https://bit.ly/3MaGXi8
- Jericho H, Sansotta N, Guandalini S (2017) Extraintestinal manifestations of Celiac Disease: Effectiveness of the Gluten-Free Diet. J Pediatr Gastroenterol Nutr 65: 75-79. Link: https://bit.ly/36oBhSo
- Therrien A, Kelly CP, Silvester JA (2020) Celiac Disease: Extraintestinal manifestations and associated condictions. J Clin Gastroenterol 54: 8-21. Link: https://bit.ly/3jGVSnQ
- Lebwohl B, Söderling J, Roelstraete B, Lebwohl MG, Green PHR, et al. (2020) Risk of skin disorders in patients with celiac disease: A population-based cohort study. J Am Acad Dermatol Link: https://bit.ly/3Mcyc7g
- Lu C-Y, Hsieh MS, Wei KC, Ezmerli M, Kuo CH, et al. (2020) Gastrointestinal involvement of primary skin disease. J Eur Acad Dermatol Venereol 34: 2766-2774. Link: https://bit.ly/3vlE1YX
- Klemm N, Gooderham MJ, Papp K (2021) Could it be gluten? Additional skin condictions associated with celiac disease. Int J Dermatol. Link: https://bit.ly/3vngnvd
- Muddasani S, Amanda M, Katherine L (2021) Gluten and skin disease beyond dermatitis herpetiformis: a review. Int J Dermatol 60: 281-288. Link: https://bit.ly/3M89btF
- Persechino F, Galli G, Persechino S, Valitutti F, Zenzeri L, et al. (2021) Skin manifestations and Coeliac Disease in paediatric population. Nutrients 13: 3611. Link: https://bit.ly/3M8s3Zt
- Bell KA, Pourang A, Mesinkovska NA, Cardis MA (2021) The effect of gluten on skin and hair: a systematic review. Dermatol Online J 27. Link: https://bit.ly/3Ejkyw8
- Cruz I-T-S-A, Fraiz FC, Celli A, Amenabar JM, Assunção LR (2018) Dental and oral manifestations of Celiac Disease. Med Oral Patol Oral Cir Bucal 23: e639-e645. Link: https://bit.ly/3KIcoQp
- Freeman HJ (2016) Endocrine manifestations in celiac disease. World J Gastroenterol 22: 8472-8479. Link: https://bit.ly/3Mr1LlF
- Starchl C, Scherkl M, Amrein K (2021) Celiac Disease and the Thyroid: Highlighting the roles of Vitamin D and Iron. Nutrients 13: 1755. Link: https://bit.ly/3O99XIG
- Demirdere H, Caklili OT, Yarman S (2021) Serologic testing for Celiac Disease in Graves’ Hyperthyroidism: Should we act early? Endocr Res. Link: https://bit.ly/3JMSaDQ
- Del Vecchio M, Bizzoco F, Lapolla R, Gentile A, Carrozza C, et al. (2021) Iodine absorption in Celiac children: A longitudinal pilot study. Nutrients 13: 808. Link: https://bit.ly/3xyB0Y6
- Sahin Y, Evliyaoglu O, Erkan T, Cokugras FC, Ercan O, et al. (2018) The frequency of celiac disease in children with autoimmune thyroiditis. Acta Gastroenterol Belg 81: 5-8. Link: https://bit.ly/3801gQA
- Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW (2021) Iron deficiency. Lancet 397: 233-248. Link: https://bit.ly/380GlNe
- Pinto-Sanchez MI, Seiler CL, Santesso N, Alaedini A, Semrad C, et al. (2020) Association between Inflammatory Bowel Diseases and Celiac Disease; A systematic review and meta-analysis. Gastroenterology 159: 884-903.e.31. Link: https://bit.ly/3rBtGaj
- Airaksinen L, Myllymäki L, Kaukinen K, Saavalainen P, Huhtala H, et al. (2021) Differences between familial and sporadic celiac disease. Dig Dis Sci 66: 1981-1988. Link: https://bit.ly/38ZV0sr
- Jiménez Sànchez J, Ruiz Moreno M, Martínez Crespo JJ. (2021) Balanced by iron. Hereditary hemochromatosis and celiac disease. Rev Esp Enferm Dig 113: 305-306. Link: https://bit.ly/38ZV2R5
- Kurki A, Kemppainen E, Laurikka P, Kaukinen K, Lindfors K (2021) The use of peripheral blood mononuclear cells in celiac disease diagnosis and treatment. Expert Rev Gastroenterol Hepatol 15: 305-316. Link: https://bit.ly/3rxECpD
- Ralbovsky NM, Lednev IK (2021) Analysis of individual red blood cells for Celiac disease diagnosis. Talanta 221: 121642. Link: https://bit.ly/3Era5if
- Hadithi M, Mulder CJ, Stam F, Azizi J, Crusius JB, et al. (2009) Effect of B vitamin supplementation on plasma homocysteine levels in celiac disease. World J Gastroenterol 15: 955-960. Link: https://bit.ly/3viKVhy
- Valente FX, Nascimento Campos T, Sousa Moraes L, Miranda Hermsdorff HH, Morais Cardoso L, et al. (2015) B vitamins related to homocysteine metabolism in adults celiac disease patients: a cross-sectional study. Nutr J 14: 110. Link: https://bit.ly/380ffWf
- Freeman HJ (2015) Iron deficienty anemia in celiac disease. World J Gastroenterol 21: 9233-9238. Link: https://bit.ly/37XQ2M8
- Balaban DV, Alina Popp, Florentina Ionita Radu, Mariana Jinga (2019) Hematologic manifestations in Celiac Disease – A practical review. Medicina 55: 373. Link: https://bit.ly/3rzaYA3
- Baydoun A, Maakaron JE, Halawi H, Abou Rahal J, Taher AT (2012) Hematologic manifestations in Celiac Disease. Scand J Gastroenterol 47: 1401-1411. Link: https://bit.ly/3rxjaB5
- Dima A, Jurcut C, Manolache A, Balaban DV, Popp A, et al. (2018) Hemorrhagic events in adult celiac disease patients. J Gastrointestin Liver Dis 27: 93-99. Link: https://bit.ly/3uPSehR
- Valvano M, Longo S, Stefanelli G, Frieri G, Viscido A, et al. (2020) Celiac Disease, Gluten-Free Diet, and Metabolic and Liver Disorders. Nutrients 12: 940. Link: https://bit.ly/3Mdb5sW
- Marciano F, Savoia M, Vajro P (2016) Celiac disease-related hepatic injury: Insights into associated conditions and underlying pathomechanisms. Dig Liver Dis 48: 112-119. Link: https://bit.ly/3MaYu9U
- Dembinski J, Robert B, Sevestre MA, Freyermuth M, Yzet T, et al. (2021) Celiac axis stenosis and digestive disease: Diagnosis, consequences and management. J Visc Surg 158: 133-144. Link: https://bit.ly/3uQD1NG
- Zhang J, Li L, Jani V, Cramer JW, Fletcher SE, et al. (2021) Increased Hepatic Stiffness in Young Adults After Biventricular Repair of Congenital Heart Disease. Ann Thorac Surg 112: 1335-1341. Link: https://bit.ly/3jOtErq
- Sharawat IK, Sharma S, Suthar R, Thapa BR (2020) Celiac disease in a boy with Duchenne Muscular Dystrophy: A double Jeopardy! Ann Indian Acad Neurol 23: 731-732. Link: https://bit.ly/3xzjJxP
- Spagnoli C, Pisani F, Di Mario F, Leandro G, Gaiani F, et al. (2018) Peripheral neuropathy and gastroenterologic disorders: an overview on an underrecognized association. Acta Biomed 89: 22-32. Link: https://bit.ly/3McXMsB
- Gadiparthi C, Hans A, Potts K, Ismail MK (2018) Gastrointestinal and hepatic disease in the inflammatory myopathies. Rheum Dis Clin North Am 44: 113-129. Link: https://bit.ly/3JSuNsC
- Vici G, Belli L, Biondi M, Polzonetti V (2016) Gluten free diet and nutrient deficiencies: A review. Clin Nutr 35: 1236-1241. Link: https://bit.ly/37jc0JT
- Aydemir Y, Erdogan B, Türkeli A (2021) Vitamin D deficiency negatively affects both the intestinal epithelial integrity and bone metabolism in children with Celiac disease. Clin Res Hepatol Gastroenterol 45: 101523. Link: https://bit.ly/392JM6F
- Vici G, Camilletti D, Polzonetti V (2020) Possible role of vitamina D in Celiac Disease onset. Nutrients 12: 1051. Link: https://bit.ly/37lcijp
- Casella G, Bordo BM, Schalling R, Villanacci V, Salemme M, et al. (2016) Neurological disorders and celiac disease. Minerva Gastroenterol Dietol 62: 197-206. Link: https://bit.ly/3KZgZOs
- Mearns ES, Taylor A, Thomas Craig KJ, Puglielli S, Leffler DA, et al. (2019) Neurological manifestations of Neuropathy and Ataxia in Celiac Disease: A systematic review. Nutrients 11: 380. Link: https://bit.ly/3xFsiaJ
- Trovato CM, Raucci U, Valitutti F, Montuori M, Villa MP, et al. (2019) Neuropsychiatric manifestations in celiac disease. Epilepsy Behav 99: 106393. Link: https://bit.ly/3M6JsBQ
- Cavusoglu D, Olgac Dundar N, Oztekin O, Arican P, Gencpinar P, et al. (2021) A Neurological Appearance of Celiac Disease: Is There Any Associated Factor? Pediatr Emerg Care 37: 303-307. Link: https://bit.ly/37XZLlC
- Cabanillas B (2020) Gluten-related disorders: Celiac disease, wheat allergy, and nonceliac gluten sensitivity. Crit Rev Food Sci Nutr 60: 2606-2621. Link: https://bit.ly/3ruyPAP
- Jang S, Lebwohl B, Abrams JA, Green PHR, Freedberg DE, et al. (2020) Celiac disease serology and gut microbiome following proton pump inhibitor treatment. Randomized Controlled Trial Medicine (Baltimore) 99: e21488. Link: https://bit.ly/3xAvaFz
- Roca M, Donat E, Rodriguez Varela A, Carvajal E, Cano F, et al. (2021) Fecal Calprotectin and Eosinophil-Derived Neurotoxin in Children with Non-IgE-Mediated Cow's Milk Protein Allergy. J Clin Med 10: 1595. Link: https://bit.ly/3OjXfXz
- Micic D, Rao VL, Semrad CE (2020) Celiac Disease and Its Role in the Development of Metabolic Bone Disease. J Clin Densitom 23: 190-199. Link: https://bit.ly/37k0VrX
- Zanchetta MB, Longobardi V, Bai JC (2016) Bone and Celiac Disease. Curr Osteoporos Rep 14: 43-48. Link: https://bit.ly/3Ojry0I
- Auricchio R, Troncone R (2021) Can Celiac Disease be prevented? Front Immunol 12: 672148. Link: https://bit.ly/37XYlrA
- Viscido A, Latella G (2021) Lymphocytic gastritis and celiac disease. Pol J Pathol 72: 87-88. Link: https://bit.ly/3jKOHLq
- Karabiber H, Celiloğlu C, Selimoğlu MA (2011) Atypical presentations of celiac disease. Turk J Pediatr 53: 241-249. Link: https://bit.ly/3JKZ5gM
- Çaltepe G. (2018) The hidden danger: Silent celiac disease. Turk J Gastroenterol 29: 530-531. Link: https://bit.ly/37vu57g
- Popp A, Mäki M (2019) Gluten-Induced Extra-Intestinal Manifestations in Potential Celiac Disease-Celiac. Nutrients 11: 320. Link: https://bit.ly/3xAi3nV
- Mulak A, Kowalski K, Jasińska M, Paradowski L (2017) Diagnostic challenges in celiac disease. Adv Clin Exp Med 26: 729-737. Link: https://bit.ly/3JOJ89l
- Byrne G, Feighery CF (2015) Celiac Disease: Diagnosis. Methods Mol Biol 1326: 15-22. Link: https://bit.ly/3jPUiA2
- Schedel J, Rockmann F, Bongartz T, Woenckhaus M, Schölmerich J, et al. (2005) Association of Crohn's disease and latent celiac disease: a case report and review of the literature. Int J Colorectal Dis 20: 376-380. Link: https://bit.ly/3ryESEK
- Sánchez-Castañon M, Castro BG, Toca M, Santacruz C, Arias-Loste M, et al. (2016) Intraepithelial lymphocytes subsets in different forms of celiac disease. Auto Immun Highlights 7: 14. Link: https://bit.ly/3xEhs4y
- Roszkowska A, Pawlicka M, Mroczek A, Bałabuszek K, Nieradko-Iwanicka B (2019) Non-Celiac Gluten Sensitivity: A Review. Medicina (Kaunas) 55: 222. Link: https://bit.ly/3vs6bSk
- Lebwohl B, Ludvigsson JF, Green PH (2015) Celiac disease and non-celiac gluten sensitivity. BMJ 351: h4347. Link: https://bit.ly/3KM72nf
- Gibson PR, Skodje GI, Lundin KE (2017) Non-coeliac gluten sensitivity. J Gastroenterol Hepatol 32: 86-89. Link: https://bit.ly/3jNxH7f
- Sergi C, Villanacci V, Carroccio A (2021) Non-celiac wheat sensitivity: rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: a review. BMC Gastroenterol 21: 5. Link: https://bit.ly/3M6LLoo
- Cárdenas-Torres I, Cabrera-Chávez F, Figueroa-Salcido OG, Ontiveros N (2021) Non-Celiac Gluten Sensitivity: An Update. Medicina (Kaunas) 57: 526. Link: https://bit.ly/3ryFZ7o
- Holmes G (2021) Co-morbidities associated with non-coeliac gluten sensitivity. Gastroenterol Hepatol Bed Bench 14: 291-294. Link: https://bit.ly/3JSc68v
- Cenit MC, Olivares M, Codoñer-Franch P, Sanz Y (2015) Intestinal Microbiota and Celiac Disease: Cause, Consequence or co-evoluzion? Nutrients 7: 6900-6923. Link: https://bit.ly/3ryGa2y
- Chibbar R, Dieleman LA (2019) The Gut Microbiota in Celiac Disease and probiotics. Nutrients 11: 2375. https://bit.ly/3KRgfLa
- Caio G, Lungaro L, Segata N, Guarino M, Zoli G, et al. (2020) Effect of Gluten-Free Diet on Gut Microbiota composition in patients with Celiac Disease and Non-Celiac Gluten / Wheat Sensitivity. Nutrients 12: 1832.Link: https://bit.ly/3vswpE6
- Pecora F, Persico F, Gismondi P, Fornaroli F, Iuliano S, et al. (2020) Gut Microbiota in Celiac Disease: Is there any role for Probiotics? Front Immunol 957. Link: https://bit.ly/3OjaOqw
- Jedwab CF, Roston BCMB, Toge ABFS, Echeverria IF, Tavares GOG, et al. (2021) The role of probiotics in the immune response and intestinal microbiota of children with celiac disease: a systematic review. Rev Paul Pediatr 40:e2020447. Link: https://bit.ly/38ZT43d
- Clappison E, Hadjivassiliou M, Zis P (2020) Psychiatric manifestations of Coeliac Disease, a systematic review and meta-analysis. Nutrients 12: 142. Link: https://bit.ly/3MfzgqD
- Cossu G, Carta MG, Contu F, Mela Q, Demelia L, et al. (2017) Coeliac disease and psychiatric comorbidity: Epidemiology, pathophysiological mechanisms, quality-of-life, and gluten-free diet effects. Int Rev Psychiatry 29: 489–503. Link: https://bit.ly/3vquTCr
- Zysk W, Głąbska D, Guzek D (2018) Social and Emotional Fears and Worries Influencing the Quality of Life of Female Celiac Disease Patients Following a Gluten-Free Diet. Nutrients 10: 1414.Link: https://bit.ly/3MhKnzH
- Wolf RL, Lebwohl B, Lee AR, Zybert P, Reilly NR, et al. (2018) Hypervigilance to a gluten-free diet and decreased quality of life in teenagers and adults with celiac disease. Dig Dis Sci 63: 1438–1448. Link: https://bit.ly/3M6NtGk
- Leffler DA, Acaster S, Gallop K, Dennis M, Kelly CP, et al. (2017) A novel patient-derived conceptual model of the impact of celiac disease in adults: Implications for patient-reported outcome and health-related quality-of-life instrument development. Value Health 20: 637–643. Link: https://bit.ly/3xAs987
- Singh P, Arora A, Strand TA, Leffler DA, Catassi C, et al. (2018) Global prevalence of celiac disease: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 16: 823–836. Link: https://bit.ly/3OjbVGI
- Roncoroni, Bascuñán KA, Doneda L, Scricciolo A, Lombardo V, et al. (2018) A low FODMAP gluten-free diet improves functional gastrointestinal disorders and overall mental health of celiac disease patients: A randomized controlled trial. Nutrients 10: 1023. Link: https://bit.ly/36me17l
- Mirijello A, d'Angelo C, De Cosmo S, Gasbarrini A, Addolorato G (2019) Management of celiac disease in daily clinical practice: Do not forget depression! Eur J Intern Med 62: e17. Link: https://bit.ly/3rukqVq
- Nardecchia S, Auricchio R, Discepolo V, Troncone R (2019) Extraintestinal manifestations of coeliac disease in children: Clinical features and mechanisms. Front. Pediatr 7: 56. Link: https://bit.ly/3Mu6hQn
- Grode L, Bech BH, Plana-Ripoll O, Bliddal M, Agerholm IE, et al. (2018) Reproductive life in women with celiac disease; a nationwide, population-based matched cohort study. Hum Reprod 33: 1538–1547. Link: https://bit.ly/3843yy8
- Zingone F, Swift GL, Card TR, Sanders DS, Ludvigsson JF, et al. (2015) Psychological morbidity of celiac disease: A review of the literature. United Eur Gastroenterol J 3: 136–145. Link: https://bit.ly/3KUvLpA
- Porcelli B, Verdino V, Ferretti F, Bizzaro N, Terzuoli L, et al. (2018) A study on the association of mood disorders and gluten-related diseases. Psychiatry Res 260: 366–370. Link: https://bit.ly/37rfiL0
- Parisi P (2018) The relationship between mucosal damage in celiac disease and the risk of neurological and psychiatric conditions is much more complex than previously thought. Eur J Neurol 25: 797-798. Link: https://bit.ly/3KRk9ni
- Perrotta G (2019) Autism Spectrum Disorder: Definition, contexts, neural correlates and clinical strategies. J Neurol Neurother 2019; 4: 136. Link: https://bit.ly/3rAbcXL
- Perrotta G (2019) Attention Deficit Hyperactivity Disorder: definition, contexts, neural correlates and clinical strategies. J Addi Adol Beh. Link: https://bit.ly/3uRVzgz
- Perrotta G (2019) Tic disorder: definition, clinical contexts, differential diagnosis, neural correlates and therapeutic approaches. J Neurosci Rehab. Link: https://bit.ly/3jNDRnT
- Perrotta G (2019) Anxiety disorders: definitions, contexts, neural correlates and strategic therapy. J Neurol Neurosci 6: 046. Link: https://bit.ly/3OjpRjN
- Perrotta G (2019) Neural correlates in eating disorders: Definition, contexts and clinical strategies. J Pub Health Catalog 2: 137-148.
- Perrotta G (2019) Post-traumatic stress disorder: Definition, contexts, neural correlations and cognitive-behavioural therapy. J Pub Health Catalog 2: 40-47. Link: https://bit.ly/3uPJ6tu
- Perrotta G (2019) Sleep-wake disorders: Definition, contexts and neural correlations. J Neurol Psychol 7: 09. Link: https://bit.ly/3jKXYmI
- Perrotta G (2019) Depressive disorders: Definitions, contexts, differential diagnosis, neural correlates and clinical strategies. Arch Depress Anxiety 5: 009-033. Link: https://bit.ly/3M9RERV
- Perrotta G (2019) Panic disorder: definitions, contexts, neural correlates and clinical strategies. Curr Tr Clin & Med Sci 1. Link: https://bit.ly/3Em6Bxs
- Perrotta G (2019) Obsessive-Compulsive Disorder: definition, contexts, neural correlates and clinical strategies. Journal of Neurology. Link: https://bit.ly/36q2fZQ
- Perrotta G (2019) Behavioral addiction disorder: definition, classifications, clinical contexts, neural correlates and clinical strategies. J Addi Adol Beh 2. Link: https://bit.ly/3rxusoR
- Perrotta G (2019) Delusions, paranoia and hallucinations: definitions, differences, clinical contexts and therapeutic approaches. Journal of Neurology (CJNE) 22-28. Link: https://bit.ly/3EnnBU4
- Perrotta G (2019) Bipolar disorder: definition, differential diagnosis, clinical contexts and therapeutic approaches. J. Neuroscience and Neurological Surgery 5. Link: https://bit.ly/3Oiu3QK
- Perrotta G (2019) The reality plan and the subjective construction of one's perception: the strategic theoretical model among sensations, perceptions, defence mechanisms, needs, personal constructs, beliefs system, social influences and systematic errors. J Clinical Research and Reports 1. Link: https://bit.ly/3EnS5Fy
- Perrotta G (2020) Suicidal risk: definition, contexts, differential diagnosis, neural correlates and clinical strategies. J. Neuroscience and Neurological Surgery 6:114. Link: https://bit.ly/3Mf8hLQ
- Perrotta G (2020) Borderline Personality Disorder: definition, differential diagnosis, clinical contexts and therapeutic approaches. Ann Psychiatry Treatm 4: 043-056
- Perrotta G (2020) Narcissism and psychopathological profiles: definitions, clinical contexts, neurobiological aspects and clinical treatments. J Clin Cases Rep 4: 12-25. Link: https://bit.ly/3EloXPi
- Perrotta G (2020) Psychotic spectrum disorders: definitions, classifications, neural correlates and clinical profiles. Ann Psychiatry Treatm 4: 070-084. Link: https://bit.ly/36nDX2B
- Perrotta G (2021) Maladaptive stress: Theoretical, neurobiological and clinical profiles. Arch Depress Anxiety 7: 001-007. Link: https://bit.ly/3xArNP6
- Perrotta G (2021) The state of consciousness: from perceptual alterations to dissociative forms. Defining, neurobiological and clinical profiles, J Neuro Neurol Sci Disord 7: 006-018.
- Perrotta G (2020) The strategic clinical model in psychotherapy: theoretical and practical profiles. J Addi Adol Beh 3. Link: https://bit.ly/37V0tjI
- Perrotta G (2020) Accepting "change" in psychotherapy: from consciousness to awareness. J Addiction Research and Adolescent Behaviour 3. Link: https://bit.ly/3OiFPL7
- Perrotta G (2021) Strategic psychotherapy and the "decagonal model" in clinical practice. Ann Psychiatry Treatm 5: 028-035. Link: https://bit.ly/3KUR0HB
- Perrotta G (2021) Perrotta Psychotherapeutic Protocol for Disorder of the Neurotic Area (PPP-DNA): proposal of protocol, profiles and clinical applications. Open J Trauma 5: 010-018. Link: https://bit.ly/3rxw8Pb
- Marafini I, Monteleone G, Stolfi C (2020) Association between Celiac Disease and Cancer. Int J Mol Sci 21: 4155. Link: https://bit.ly/3KUOfGv
- Kato K, Iwamoto M, Kusumi Y, Masuda S, Nakayama T, et al. (2021) Celiac Disease Diagnosed after Gastrectomy for Gastric Cancer. Intern Med. Link: https://bit.ly/3vqBugb
- Lebwohl B, Green PHR, Emilsson L, Mårild K, Söderling J, et al. (2021) Individuals With Celiac Disease: A Nationwide Cohort Study. Clin Gastroenterol Hepatol.Link: https://bit.ly/3rAAHs6
- Wang M, Yu M, Kong WJ, Cui M, Gao F (2021) Association between intestinal neoplasms and celiac disease: A review. World J Gastrointest Oncol 13: 1017-1028. Link: https://bit.ly/3KVc1Sz
- Catassi C, Bonaz B, Bouma G, Carroccio A, Castillejo G, et al. (2015) Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts' Criteria. Nutrients 7: 4966-4977. Link: https://bit.ly/3JWr3Gh
- Singh P, Arora A, Strand TA, Leffler DA, Catassi C, et al. (2018) Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 16: 823-836.e2. Link: https://bit.ly/3xAyYXh
- Caio G, Volta U¸ Sapone A, Leffler DA, De Giorgio R, et al. (2019) Celiac disease: a comprehensive current review. BMC Med 17: 142. Link: https://bit.ly/3xFFoop
- Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, et al. (2020) European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J Pediatr Gastroenterol Nutr 70: 141-156. Link: https://bit.ly/3rxBCJO
- Leonard MM, Sapone A¸ Catassi C, Fasano A (2017) Celiac Disease and Nonceliac Gluten Sensitivity: A Review. JAMA 318: 647-656. Link: https://bit.ly/3vtcXqX
- Catassi C, Alaedini A¸ Bojarski C, Bonaz B, Bouma G, et al. (2017) The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 9: 1268. Link: https://bit.ly/3OmWa1d
- Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, et al. (2019) Celiac disease: a comprehensive current review. BMC Med 17: 142. Link: https://bit.ly/3M8MMwk
- Rubin JE, Crowe SE (2020) Celiac Disease. Ann Inter Med 172: ITC1-ITC16. Link: https://bit.ly/3KVHsMw
- Vaquero L, Rodríguez-Martín L, León F, Jorquera F, Vivas S (2018) New coeliac disease treatments and their complications. Gastroenterol Hepatol 41: 191-204. Link: https://bit.ly/36sJVzi
- Sahin Y (2021) Celiac disease in children: A review of the literature. World J Clin Pediatr 10: 53-71. Link: https://bit.ly/38NDbww
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
