Open Journal of Bacteriology
Biologically active peptides from marine proteobacteria: Discussion article
Komal Anjum*
Cite this as
Anjum K (2021) Biologically active peptides from marine proteobacteria: Discussion article. Open J Bac 5(1): 005-012. DOI: 10.17352/ojb.000018Marine bioactive peptides are deliberated as an abundant source of natural products that may give long-hual fitness, in comparison to other resources. Numerous literature concerning bioactive peptides from marine proteobacteria has been summarized, which shows the possibleness of therapeutic efficacy comprehensive wide spectra of bioactivities against many infectious agents. Their antimicrobial, antitumor, antiviral, and other bioactivities have gained an attention for the medicine development toward a new flow of drug explicate, for therapy and control of several diseases. Nonetheless, the execution of the action of several peptides has been still unexplored. So in this glance, this mini-review is focused on some peptides by which they intervene with microbial infection. This compilation is one of the main extract to be implicit particularly for the conversion of biomolecules into desired medicines.
Introduction
The marine microorganisms are considered an unexplored living creature. It has been noticed that these marine bacteria and their biologically active metabolites are possible sources to be used as sustainable food and pharmaceutical constituent. Disdain great progression in medicine, contagious diseases elicited by bacteria, fungi, and viruses are quite a important exemplary to community health. Due to the insufficiency of medicine and increase of considerable resistance, people are especially impressed in developing countries. The expansion in the rate of disease causing microbes that have gained antibiotic resistance takes place in the flourishing concern in novel and influential antimicrobial compounds. Particularly, the report of the anti-infective impact of marine peptides has appeal affection from researchers and marine peptides are much advised anti-infective compounds. For this intention, marine life is designated as the chief option for the discovery of new and bioactive medicinal factors, peculiarly marine peptides, as marine peptides might be proactive in boost production and diminishing disease [1]. By-products of marine fish, algae, mollusk, crustacean, and others are the major root of these peptides. It seems that the antimicrobial peptides may also have the potential to boost an immunity proportional to other appropriate drugs.
Antimicrobial peptides from bacteria are classified depending on their route of arrangements (tissue pathway) for instance the ribosomal (bacteriocin) route or non-ribosomal route. Polyketide Synthase (PKS) and Non-ribosomal Peptide Synthetase (NRPS) are the major messenger of secondary metabolites. This current mini-review convergent on the bioactive capability of peptides, extracted from the marine resources and various compounds by which these peptides fight against human pathogenesis.
Marine proteobacteria
Nonetheless, Proteobacteria is the utmost bountiful phylum in marine habitat (profusion between 50% to 80%), while very less bioactive components have been disclosed from those microorganisms [2]. Various types of natural products from marine microbes are presented in the biography, several of them are peptides [3]. Many published reports have been dedicated to the characterization of marine bacterial compounds. Nonetheless, very few studies have concentrated on bioactive peptides from Proteobacteria. Contempt the accessibility of novel adequate compounds, just some specific classes of antibacterial compounds have been specified for human consumption. A few familiar proteobacterial genera comprising oceanobulbus, pheaobacter, photobacterium, myxococcus, paraliomyxa, chondromyces, pseudoalteromonas, vibrio, and pseudomonads were studied in the marine environment (Table 1). Freshly, some unreported species of Proteobacteria have been analyzed and it is oriented to the evolution of Candidatus-Proteobacteria class [4].
Marine proteobacteria as a point of bioactive peptides
In the marine ecosystem, Proteobacteria is the near flooding phylum and its spare rate is from 50% to 80% merely unfortunately, exclusively a fewer bioactive compounds were reported from such microbes [5]. Marine proteobacteria have been explored for the amount of bioactive metabolites, and several of them are cataloged in Table 2 [2]. In this minireview, we will chiefly be focused on stuff from the α-, γ-and δ-Proteobacteria classes. There is no report of molecules from β-, ε- and δ-Proteobacteria so far. An immense figure of bioactive compounds have been purified from diametric genera/species of proteobacteria along with moiramide A, andrimide, holomycin, kahalalides (eg. kahalalide A), unnarmicins, and ngercheumicins (A-E), indigoidine, solonamides (Solonamide A), thiomarinols, cyclic-depsipeptides (eg. chondramide C and miuraenamides), myxovalargins, althiomycin, myxothiazols, purified from Vibrio sp., Photobacterium halotolerans, Photobacterium sp. MBIC06485, and Photobacterium sp., Oceanibulbus, Phaeobacter, Pseuodoalteromonas, Pseudomonas, Myxococcus, Paraliomyxa sp.,. Some other examples of bioactive compounds are enlisted in Table 2.
Protocols and strategies for peptides purification
The current methods for peptide purification are flash chromatography, RP-HPLC chromatographym, hydrophobic interaction chromatography, ion-exchange chromatography, size exclusion chromatography, gel filtration chromatography, and hydrophilic interaction chromatography [18-21]. These methods permit acquire products with high-purity. Nevertheless, they use up larger amounts of solvent, and in many cases, produce low output and involve lengthy purification times, which cause an increase in production costs. The modification of new methodologies depend on Solid-Phase Extraction (SPE) made this method more skilled, allowing pretreatment of whatever kind of sample in a broad concentration range [22]. SPE chromatographic separation is depend on the same rule as Liquid Chromatography (LC). Frontal chromatography is the chief process in the extraction step, while displacement chromatography is the procedure that regulate the analyte desorption [23]. The main standard for choosing the chromatography mode is the analyte’s physicochemical attribute [22,23]. SPE is regarded as a separation protocol with advantages over other protocols, allowing a collection of applications together with reproducibility, speed, and efficiency [22]. Reverse-phase SPE (RPSPE) is the method most used. A sample dissolved in a polar mobile phase is applied onto the column, and then the non-retained impurities are wash by washing with the identical polar mobile phase. The analyte is then eluted with a less polar mobile phase comprising on organic modifier. This elution may be isocratic or gradient [23-25].
Chemical structures and binding properties of some reported peptides
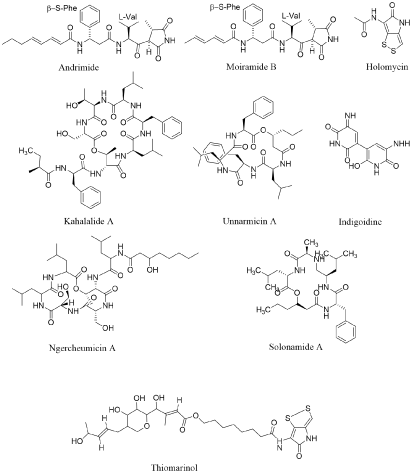
Andrimide and its analogue moiramide are plausibly the most reported pseudopeptide antibiotics. It was first isolated from Vibrio in 1994, and reported to be synthesized by a bacterium Vibrio (isolated from a marine sponge) (Hyatella sp.) ( [10] (Figure 1A). These bioactive peptides appear to be distributed in the γ-Proteobacteria as various Vibrio species [10,26,27], Pantoea agglomerans [28], the marine Pseudomonas fluorescens [29] and a symbiotic planthopper Enterobacter [30] have been reported to produce them. Andrimid exist in four component parts: (1) A β-Phe moiety; (2) An unsaturated fatty-acid chain; (3) A pyrrolidinedione and (4) A L-Val derived β-ketoamide moiety. Andrimide and moiramide dissent just in the size of their fatty acid chains. A broad antibacterial activity has been represented for andrimid against Gram-positive and Gram-negative bacteria [29]. The broad antibacterial spectrum is probably coupled to the molecular target of these compounds, i.e., the broadly preserved Acetyl-CoA Carboxylase (ACC) [31].
By chemical synthetic studies, Pohlmann, et al. verified that a pyrrolidinedione head part is accountable for andrimid antimicrobial capability and also its fatty acid chain aid in cell perception. Two interior elements of moiramide, that is the L-Val-derived β-ketoamide and the β-(S)-Phe, have been reported by the same person, in 2006. Survey proved that Phe replacement by a non-aromatic amino acid consequence in a lack of antibacterial potential. Replacement with lipophile on the phenyl ring consequence in the β-(S)-Phe amended antibacterial potential, praise that this component is accountable for cell penetration of the molecule and also the fatty acid chain. Alteration of an isopropyl chain of the L-Val-derived β-ketoamide betters its bioactive potential against Gram-positive bacteria [32].
Holomycin is synthesized by the Photobacterium halotolerans S2753 [27]. It is the closing product of a Cys-Cys dipeptide substance that showed a broad compass of antibacterial potential [33] (Figure 1A). This pyrrothine compound is an superior illustration of how natural amino acid residues can be adapted to form “exotic” molecule. Preceding to this survey, this pyrrothine antibiotic had only been separated from Gram-positive bacteria and particularly from the actinomycete Streptomyces clavuligerus [34]. Holomycin possess a bacteriostatic consequence on both Gram-negative and Gram-positive bacteria [35]. It results speedy inhibition of elongation of RNA chain [35,36].
Kahalalides is a family of depsipeptides with changeable size and peptide ordering, ranging from C31 (tripeptide) to C77 (tridecapeptide) and possessing various fatty acid chains [13]. These compounds, first purified from the herbivorous marine mollusc (Elysia) and its algal food origin (Bryopsis pennata) have also been represented in some Vibrio sp. strains separated from the same mollusc [37]. Although the beginning of kahalalides still ambivalent, Hill, et al. Propose that Elysia rufescens adopt kahalalide-producing microbes from the overhead of Bryopsis and then carry these microbes as symbionts. This family of depsipeptides has been studied [13]; hence, here only the main properties of these peptides have been presented. Kahalalides and particularly kahalalide F are notable for their antifungal and antitumour activities. Phase II clinical trials are in process in regard to the former (Figure 1A).
Unnarmicins are depsipeptides separated from the extraction broth of a marine Photobacterium sp. MBIC06485. Unnarmicins comprise two Leu, two Phe, and one 3-hydroxyoctanoyl moiety or one 3hydroxyhexanoyl group in unnarmicins A and C, respectively (Figure 1A). These compounds employ an antibacterial effects only towards species of the Pseudovibrio genus, one of the nearly common Proteobacteria genera in the marine environment [38]. To date, five ngercheumicins A–E, depsipeptides, have been separated and characterized. Ngercheumicins A and B have a depsipeptide macrocycle comprising one Phe and two Leu parts with contrary fatty acid tails. Ngercheumicins B–D have a macrocycle consisted of three Leu, two Thr and one Ser with no fatty acid tail. Although these compounds lonely victim bacteria for which none pathogenesis has been represented to date, as notable for unnarmicins, ngercheumicins can be amended to culture media to boost slowly-growing marine bacteria [14].
A blue pigment, indigoidine, has reported to be related to the cognition of Phaeobacter sp. Y4I to suppress Vibrio fischeri. This pigment can synthesize by the condensation of two glutamine portion via a NRPS-based biosynthetic pathway [6]. Solonamides, in terms of structural similarity are intimately concomitant to unnarmicins and ngercheumicins, with a macrocycle containing one L-Ala, one L-Phe, one L-Leu and one D-Leu part with a hydroxyoctanoyl or a hydroxyhexanoyl moiety in solonamides B and A, respectively. In the similar study, no antibacterial activity has been discovered for solonamides A and B nonetheless the activity has only been observed against Vibrio anguillarum and S. aureus. However, solonamide B has been studied to decrease the expression of both hla and rnaIII of Methicillin Resistant Staphylococcus aureus (MRSA) [12]. Thiomarinols have effective activity against both Gram-positive and Gram-negative bacteria, particularly against MRSA. Liquid-liquid filtration process of the cell-free supernatant has present that Pseudo alteromonas sp. SANK 73390 produces seven thiomarinol analogue [39-41] (Figure 1A).
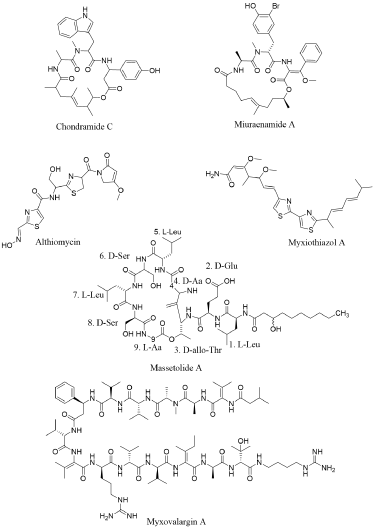
Chondramide’s structure has a similarity with jaspamide (Figure 1B). Both structures comprise of three amino acids, i.e., one Ala, one (α-methoxy) β-Tyr, and one N-methyl-Trp [42]. The only difference comprises in the length and arrangement of the polyketide chain. Numerous biological activities have been presented for these jasmine-accompanying peptides. Their capability as antibacterial or antifungal agents has particularly been foreground since their finding. One auspicious area of research pertain their antitumour activity, which is well written for jaspamide, a microfilament inhibitor [43]. Chondramide A stimulate the same effect as jaspamide on actin polymerisation [44] (Figure 1B). Corresponding cytotoxic or anti-proliferative ability have also been incontestable for miuraenamide A [45], seragamide A [46], doliculide [47] and geodiamolide H [48].
Ojika, et al. has noticed the similarity between miuraenamides (isolated from myxobacterium) with other cyclic depsipeptides from marine microbes such as the geodiamolides [49,50], doliculide (Ishiwata, et al. 1998), and seragamides [51], which have all been separated from invertebrates. By considering their similarity and their source of invertebrate isolation, many jaspamide-accompanying peptides at first attributed to marine invertebrates may in reality be synthesize by unknown marine myxobacteria [51] (Figure 1B). Massetolides are cyclic lipopeptides synthesized by other γ-Proteobacteria that have anti-mycobacterial activity. Massetolides A–D were initially extracted from a culture of a marine Pseudomonas MK90E85 separated from an unidentified red alga received from Masset Inlet, BC, Canada (Figure 1B). Viscosin and Massetolides E–H were first extracted from Pseudomonas MK91CC8 separated from an unidentified tube worm that was received from Moira Island, BC, Canada [53]. Massetolides are comprised on nine amino acids with alternating L- or D-configurations and a changeable length of fatty acid chains of uncertain length. Massetolides are cyclised through with an ester linkage between the carboxyl group of the last amino acid and the hydroxyl of the third amino acid residue.
Myxovalargins are linear peptides ranging from 1500 to 1700 Da, containing Arg, Val, and several unusual amino acids [16] (Figure 1B). Because of its most active quality, myxovalargin A was further investigated. Myxovalargins comprises on 14 amino acids in addition of 3-methylbutyric acid and agmatine moieties, nonetheless its structure has not been completely elucidated. Myxovalargin A possess bioactivity against both Gram-positive and Gram-negative bacteria and powerfully reduce protein synthesis in reference bacteria at low concentrations, however, its principal effect at high concentrations is because of its membrane permeabilisation [54]. Althiomycin is another peptide isolated from M. fulvus along with the antibiotic myxopyronins [16]. Nevertheless, althiomycin was primitively represented in the actinobacteria Streptomyces althioticus [55] (Figure 1B). It is a pentapeptide comprising on two Cys, two Gly, and one Ser and displays broad spectrum of antibiotic activity against Gram-positive and Gram-negative bacteria by inhibiting protein synthesis [56]. Myxothiazols possess anti-fungal potential [57,58] due to intervention with cytochrome b and suppression of respiration. The production of myxothiazols in M. fulvus has not been examined. Nevertheless, Perlova, et al. Expound the mechanism concerning the NRPS in some other myxobacteria, Stigmatella aurantiaca [59] (Figure 1B).
Proteobacteria were also deliberate to possess antitumor and anti-fungal activity. Several purified compounds including miuraenamides and kahalalides fermented by Paraliomyxa miuraensis and Vibrio sp. respectively own antifungal potential, and kahalalides also have anti-tumor activity [6,13]. Some other examples are cataloged in Table 2.
Discussion
The unrivaled collection of marine sourced natural products concerted with an precocious worldwide knowingness about the scarcity of novel medications for anti-infectious agents. Nevertheless, marine product leads have continuum been withstanding with galore hindrance, considering an everlasting render and large-scale manufacture. These two barriers are an actual dispute for the consecutive alteration of marine natural products into medical applications, and substitute scheme for economically attainable and environmentally good provision are surely required. Today, a technical disadvantage connected with natural products has been diminished, and there is a superior possibility to investigate the bio-activity of antecedently unapproachable origin of natural products [60,61]. In light of the proof that chemical assortment of natural products is well appropriate to concede the centre arrangement for forthcoming drugs, there will be additional progress in the use of new natural products of marine root and chemical collection hinge on natural products, in the process of drug revelation.
Conclusion
Bioactive peptides from marine Proteobacteria are specifically acquiring from Nonribosomal Pathways (NRP). They show influential antibacterial and/or anti-fungal potentials. As a consequence, they may represent helpful way to face the demand of MDR strains. The deficiency of standardized cultivation methods has restricted research into their biochemistry. New culturing protocols have freshly verified impressive and can provide an approach to the antecedently unculturable. This mini-review has believably sole fair grazed upon the perk of the iceberg.
- Kang HK, Seo CH, Park Y (2015) Marine peptides and their anti-infective activities. Mar Drugs 13: 618-654. Link: https://bit.ly/3tBAKmX
- Bhatnagar I, Se-Kwon K (2010) Immense essence of excellence: Marine microbial bioactive compounds. Mar Drugs 8: 2673- 2701. Link: https://bit.ly/3rguj6G
- Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2004) Marine natural products. Nat Prod Rep 21: 1- 49.
- Wipf P, Reeves JT, Day BW (2004) Chemistry and biology of Curacin A. Curr Pharm Des 10: 1417-1437. Link: https://bit.ly/2YLIDYz
- Emerson D, Rentz JA, Lilburn TG, Davis RE, Aldrich H, et al. (2007) A novel lineage of Proteobacteria involved in formation of marine Fe-Oxidizing Microbial Mat Communities. PLoS One 2: 667. Link: https://bit.ly/3oJPKve
- Pohlmann J, Lampe T, Shimada M, Nell PG, Pernerstorfer J, et al. (2005) Pyrrolidinedione derivatives as antibacterial agents with novel mode of action. Bioorg Med Chem Lett 15: 1189-1192. Link: https://bit.ly/3atvPLK
- Slightom RN, Buchan A (2009) Surface colonization by marine roseobacters: integrating genotype and phenotype. Applied and Environmental Microbiology 75: 6027- 6037. Link: https://bit.ly/36IZv6M
- Iizuka T, Fudou R, Jojima Y, Ogawa S, Yamanaka S, et al. (2006) Miuraenamides A and B, novel antimicrobial cyclic depsipeptides from a new slightly halophilic myxobacterium: taxonomy, production, and biological properties. J Antibiot (Tokyo) 59: 385-391. Link: https://bit.ly/2YJghON
- Semon BA (2016) Dietary cyclic dipeptides, apoptosis and psychiatric disorders: a hypothesis. A Hypothesi 82: 740.
- Oclarit JM, Okada H, Ohta S, Kaminura K, Yamaoka Y, Lizuka T, et al. (1994) Anti-bacillus substance in the marine sponge, Hyatella species, produced by an associated Vibrio species bacterium. Microbios 78: 7- 16. Link: https://bit.ly/3aET6uk
- Oku N, Gustafson KR, Cartner LK, Wilson JA, Shigematsu N, et al. (2004) Neamphamide A, a New HIV-Inhibitory Depsipeptide from the Papua New Guinea Marine Sponge Neamphius huxleyi. J Nat Prod 67: 14071411. Link: https://bit.ly/3pTix1H
- Mansson M, Gram L, Larsen TO (2011) Production of bioactive secondary metabolites by marine Vibrionaceae. Mar Drugs 9: 1440- 68. Link: https://bit.ly/39LYNrg
- Gao J, Hamann MT (2011) Chemistry and biology of kahalalides. Chem Rev 111: 3208-3235. Link: https://bit.ly/39PPlTR
- Adachi K, Kawabata Y, Kasai H, Katsuta M, Shizuri Y (2007) Novel Ngercheumicin or its salt useful for treating infection caused by Pseudovibrio denitrificans. Japanese Patent JP2007230911-A.
- Li ZF, Li X, Liu H, Liu X, Han K, et al. (2011) Genome Sequence of the Halotolerant Marine Bacterium Myxococcus fulvus HW-1. J Bacteriol 193: 5015-5016. Link: https://bit.ly/3rlNR9K
- Irschik H, Gerth K, Höfle G, Kohl W, Reichenbach H (1983) The myxopyronins, new inhibitors of bacterial rna synthesis from Myxococcus fulvus (myxobacterales). J Antibiot 36: 1651-1658. Link: https://bit.ly/3rtA0ON
- Schieferdecker S, Exner TE, Gross H, Roth M, Nett M (2014) New myxothiazols from the predatory bacterium Myxococcus fulvus. Journal of Antibiotics 67: 519. Link: https://go.nature.com/3robD54
- Aguilar MI (2004) Methods in Molecular Biology, HPLC of Peptides and Proteins, Methods and Protocols. Humana Press Inc.; Totowa, NJ, USA. Link: https://bit.ly/36LVHlf
- Ren HLJ, Zheng XQ, Liu XL (2010) Purification and characterization of antioxidant peptide from sunflower protein hydrolysate. Food Technology and Biotechnology 48: 519- 523. Link: https://bit.ly/2MXujcZ
- Cytryńska M, Mak P, Zdybicka-Barabas A, Suder P, Jakubowicz T (2007) Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 28: 533-546. Link: https://bit.ly/39Nwf0A
- Pingitore EV, Salvucci E, Sesma F, Nader-Macias ME (2007) Different Strategies for Purification of Antimicrobial Peptides from Lactic Acid Bacteria (LAB) In: Mendez Vilas A., editor. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. FORMATEX; Badajoz, Spain: 557- 568. Link: https://bit.ly/3avhQVW
- Poole F (2003) New trends in solid-phase extraction. Trends in Analytical Chemistry 22: 362-373. Link: https://bit.ly/36J0Daw
- Hennion MC (1999) Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography. J Chromatogr A 856: 3- 54. Link: https://bit.ly/3oRWCH1
- Herraiz T, Casal V (1995) Evaluation of solid-phase extraction procedures in peptide analysis. J Chromatogr A 708: 209-221. Link: https://bit.ly/3rr4cds
- Palmblad M, Vogel JS (2005) Quantitation of binding, recovery and desalting efficiency of peptides and proteins in solid phase extraction micropipette tips. J Chromatogr B Analyt Technol Biomed Life Sci 814: 309-313. Link: https://bit.ly/3pPNWCk
- Graff JR, Forschner-Dancause SR, Menden-Deuer S, Long RA, Rowley DC (2013) Vibrio cholerae Exploits Sub-Lethal Concentrations of a Competitor-Produced Antibiotic to Avoid Toxic Interactions. Front Microbiol 4: 8. Link: https://bit.ly/39RXG9D
- Wietz M, Mansson M, Gotfredsen CH, Larsen TO (2010) Antibacterial compounds from marine Vibrionaceae isolated on a global expedition. Mar Drugs 8: 2946-2960. Link: https://bit.ly/3pSP3ku
- Jin M, Fischbach MA, Clardy J (2006) A biosynthetic gene cluster for the acetyl-CoA carboxylase inhibitor andrimid. J Am Chem Soc 128: 10660-10661. Link: https://bit.ly/3jrt0iE
- Singh MP, Mroczenski-Wildey MJ, Steinberg DA, Andersen RJ, Maiese WM, et al. (1997) Biological activity and mechanistic studies of andrimid. J Antibiot (Tokyo) 50: 270-273. Link: https://bit.ly/3pUE2iH
- Fredenhagen A, Tamura SY, Kenny PTM, Komura H, Naya Y, et al. (1987) Andrimid, a new peptide antibiotic produced by an intracellular bacterial symbiont isolated from a brown planthopper. J Am Chem Soc 109: 4409- 4411. Link: https://bit.ly/3jkPgdJ
- Freiberg C, Brunner NA, Schiffer G, Lampe T, Pohlmann J, et al. (2004) Identification and characterization of the first class of potent bacterial acetyl-CoA carboxylase inhibitors with antibacterial activity. J Biol Chem 279: 26066-26073. Link: https://bit.ly/3jhoR0C
- Desriac F, Jégou C, Balnois E, Brillet B, Le Chevalier P, et al. (2013) Antimicrobial peptides from marine Proteobacteria. Mar Drugs 11: 3632-3660. Link: https://bit.ly/39RFdtQ
- Li B, Walsh CT (2010) Identification of the gene cluster for the dithiolopyrrolone antibiotic holomycin in Streptomyces clavuligerus. Proc Natl Acad Sci U S A 107: 19731-19735. Link: https://bit.ly/3traJGF
- Kenig M, Reading C (1979) Holomycin and an antibiotic (MM 19290) related to tunicamycin, metabolites of Streptomyces clavuligerus. J Antibiot (Tokyo) 32: 549-554. Link: https://bit.ly/3cJBgZJ
- Oliva B, O'Neill A, Wilson JM, O'Hanlon PJ, Chopra I (2001) Antimicrobial properties and mode of action of the pyrrothine holomycin. Antimicrob Agents Chemother 45: 532-539. Link: https://bit.ly/3pXgfik
- Khachatourians GG, Tipper DJ (1974) In vivo effect of thiolutin on cell growth and macromolecular synthesis in Escherichia coli. Antimicrob Agents Chemother 6: 304-310. Link: https://bit.ly/2MDNfxx
- Hill RT, Enticknap J, Rao KV, Hamann MT (2004) Kahalalide-Producing Bacteria 2004. EP1689848. European Patent Application.
- Oku N, Kawabata K, Adachi K, Katsuta A, Shizuri Y (2008) Unnarmicins A and C, new antibacterial depsipeptides produced by marine bacterium Photobacterium sp. MBIC06485. J Antibiot 61: 11-17. Link: https://bit.ly/39NDPIn
- Shiozawa H, Kagasaki T, Kinoshita T, Haruyama H, Domon H, et al. (1993) Thiomarinol, a new hybrid antimicrobial antibiotic produced by a marine bacterium. Fermentation, isolation, structure, and antimicrobial activity. J Antibiot (Tokyo) 46: 1834-1842. Link: https://bit.ly/39NDqFS
- Shiozawa H, Kagasaki T, Torikata A, Tanaka N, Fujimoto K, et al. (1995) Thiomarinols B and C, new antimicrobial antibiotics produced by a marine bacterium. J Antibiot (Tokyo) 48: 907- 909. Link: https://bit.ly/39NBHAq
- Thiomarinols B and C, new antimicrobial antibiotics produced by a marine bacterium. J Antibiot (Tokyo) 48: 907- 909. Link: https://bit.ly/39NBHAq
- Shiozawa H, Shimada A, Takahashi S (1997) Thiomarinols D, E, F and G, new hybrid antimicrobial antibiotics produced by a marine bacterium; isolation, structure, and antimicrobial activity. J Antibiot (Tokyo) 50: 449-452. Link: https://bit.ly/2YPbv2d
- Rachid S, Krug D, Kunze B, Kochems I, Scharfe M, et al. (2006) Molecular and biochemical studies of chondramide formation-highly cytotoxic natural products from Chondromyces crocatus Cm c5. Chem Biol 13: 667-681. Link: https://bit.ly/3jjhN3v
- Ebada SS, Wray V, de Voogd NJ, Deng Z, Lin W, et al. (2009) Two new jaspamide derivatives from the marine sponge Jaspis splendens. Marine Drugs 7: 434- 444. Link: https://bit.ly/3pSefYz
- Sasse F, Kunze B, Gronewold TM, Reichenbach H (1998) The chondramides: cytostatic agents from myxobacteria acting on the actin cytoskeleton. J Natl Cancer Inst 90: 1559-1563. Link: https://bit.ly/3pVuR1u
- Sumiya E, Shimogawa H, Sasaki H, Tsutsumi M, Yoshita K, et al. (2011) Cell-morphology profiling of a natural product library identifies bisebromoamide and miuraenamide A as actin filament stabilizers. ACS Chem Biol 6: 425-431. Link: https://bit.ly/3pPxB0g
- Tanaka C, Tanaka J, Bolland RF, Marriott G, Higa T (2006) Seragamides A–F, new actin-targeting depsipeptides from the sponge Suberites japonicus thiele. Tetrahedron 62: 3536- 3542. Link: https://bit.ly/2MyRR7W
- Matcha K, Madduri AV, Roy S, Ziegler S, Waldmann H, et al. (2012) Total synthesis of (-)-doliculide, structure-activity relationship studies and its binding to F-actin. Chembiochem 13: 2537-2548. Link: https://pubmed.ncbi.nlm.nih.gov/23129522/
- Freitas VM, Rangel M, Bisson LF, Jaeger RG, Machado-Santelli GM (2008) The geodiamolide H, derived from Brazilian sponge Geodia corticostylifera, regulates actin cytoskeleton, migration and invasion of breast cancer cells cultured in three-dimensional environment. J Cell Physiol 216: 58394. Link: https://pubmed.ncbi.nlm.nih.gov/18330887/
- Ojika M, Inukai Y, Kito Y, Hirata M, Iizuka T, et al. (2008) Miuraenamides: antimicrobial cyclic depsipeptides isolated from a rare and slightly halophilic myxobacterium. Chem Asian J 3: 126-133. Link: https://pubmed.ncbi.nlm.nih.gov/18022981/
- Chan WR, Tinto WF, Manchand PS, Todaro LJ (1987) Stereo structures of geodiamolides A and B, novel cyclodepsipeptides from the marine sponge Geodia sp. J Org Chem 52: 3091- 3093. Link: https://pubs.acs.org/doi/10.1021/jo00390a023
- Ishiwata H, Nemoto T, Ojika M, Yamada K (1994) Isolation and stereo structure of doliculide, a cytotoxic cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. J Org Chem 59: 4710- 4711. Link: https://pubs.acs.org/doi/10.1021/jo00096a002
- Weissman KJ, Müller R (2009) A brief tour of myxobacterial secondary metabolism. Bioorg Med Chem 17: 2121-236. Link: https://pubmed.ncbi.nlm.nih.gov/19109025/
- Gerard JM (1992) Ph.D. Thesis. University of British Columbia; Vancouver, Canada: Antibiotic Secondary Metabolites of Bacteria Isolated from the Marine Environment. Link: https://open.library.ubc.ca/cIRcle/collections/ubctheses/831/items/1.0059606
- Irschik H, Reichenbach H (1985) The mechanism of action of myxovalargin A, a peptide antibiotic from Myxococcus fulvus. J Antibiot (Tokyo) 38: 1237-1245. Link: https://pubmed.ncbi.nlm.nih.gov/2415501/
- Yamaguchi H, Nakayama Y, Takeda K, Tawara K, Maeda K, et al. (1957) A new antibiotic, althiomycin. J Antibiot (Tokyo) 10: 195- 200. Link: https://bit.ly/3aEBZJ0
- Fujimoto H, Kinoshita T, Suzuki H, Umezawa H (1970) Studies on the mode of action of althiomycin. J Antibiot (Tokyo) 23: 271-275. Link: https://bit.ly/3jklVAc
- Thierbach G, Reichenbach H (1981) Myxothiazol, a new antibiotic interfering with respiration. Antimicrob Agents Chemothe 19: 504-507. Link: https://bit.ly/3ay0vM9
- Ahn JW, Jang KH, Yang HC, Oh KB, Lee HS, et al. (2007) Bithiazole metabolites from the myxobacterium Myxococcus fulvus. Chem Pharm Bull (Tokyo) 55: 477-449. Link: https://bit.ly/36MsBC2
- Perlova O, Fu J, Kuhlmann S, Krug D, Stewart AF, et al. (2006) Reconstitution of the myxothiazol biosynthetic gene cluster by Red/ET recombination and heterologous expression in Myxococcus xanthus. Appl Environ Microbio 72: 7485- 7494. Link: https://bit.ly/36IYBqU
- Kotoku N, Kato T, Narumi F, Ohtani E, Kamada S, et al. (2006) Synthesis of 15, 20-triamide analogue with polar substituent on the phenyl ring of arenastatin A, an extremely potent cytotoxic spongean depsipeptide. Bioorg Med Chem 14: 7446-7457. Link: https://bit.ly/3oQ1gVV

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
