Open Journal of Tropical Medicine
Assessment of malaria parasitemia in people living with HIV/AIDS in Kumba, Cameroon
Eyong Clinton Achere1, Adamu Ndongho Ndifontiayong2, Fokunang Charles Ntungwen3 and Nsagha Dickson Shey1*
2Faculty of Science, Research Unit of Microbiology and Antimicrobial Substances, University of Dschang, P.O. Box 67: Dschang, Cameroon
3Faculty of Medicine and Biomedical Sciences, Department of Pharmacotoxicology and Pharmacokinetics, University of Yaoundé 1, P.O Box 1364, Yaounde, Cameroon
Cite this as
Achere EC, Ndifontiayong AN, Ntungwen FC, Shey ND (2022) Assessment of malaria parasitemia in people living with HIV/AIDS in Kumba, Cameroon. Open J Trop Med 6(1): 001-010. DOI: 10.17352/ojtm.000021Copyright
© 2022 Kodama A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Malaria parasitemia and HIV infections are globally important public health concerns. People residing in regions where these two infections are endemic are prone to develop co-infection. Sub-Saharan Africa has the greatest burden of both diseases and Cameroon particularly the South West Region has been reported as one of the regions with the highest malaria and HIV prevalence. Hence, there is a need for continuous monitoring and epidemiologic inquiry to generate updated data on the burden of malaria parasitemia on HIV/AIDS. The aim of this study was to determine the prevalence of malaria parasitemia and the association between viral load and malaria parasite density in people living with HIV/AIDS in Kumba, Cameroon.
Methods: A cross-sectional study was conducted involving 250 people living with HIV/AIDS selected from five main Community Based Organizations in Kumba during a period of 5 months from April to August 2021. Participants’ consent was obtained followed by socio-demographic and other useful data via a standardized questionnaire. Capillary blood samples were collected and Giemsa-stained blood films were examined to detect malaria parasitemia. The recent viral loads were collected from the participants’ medical files. Pearson’s chi-square was used for the comparison of proportions and correlation analysis to determine the association between parasite density and viral load. Statistical significance was set at p < 0.05.
Results: An overall prevalence of malaria parasitemia in people living with HIV/AIDS in Kumba was 27.2%; [95% CI: 21.8% – 33.2%]. Among 215 participants who were HAART-experienced, 49(22.79%); [95% CI: 17.6% – 27.9%] were found to be positive for malaria parasitemia while 19(54.29%); [95% CI: 48.1% – 60.5%] out of the 35 HAART naïve individuals were positive for malaria parasitemia. This difference in prevalence was statistically significant (X2 = 15.078, df = 1, N = 250, p < 0.000). Also, malaria parasite density was significantly dependent on viral load (X2 = 61.065, df = 6, N = 49, p < 0.000). HAART-experienced participants with high viral load(>1000copies/ml) had significantly higher malaria parasite density(>400trophozoites/µL) than HAART-experienced participants with ‘not detectable’ viral load.
Conclusion: The prevalence of malaria parasitemia in the study population was 27.2%. HAART naïve individuals had significantly higher malaria parasitemia prevalence and malaria parasite density than HAART-experienced individuals. Malaria parasite density was significantly dependent on viral load in HAART-experienced individuals.
Introduction
People residing in regions where HIV and malaria parasitemia are endemic are prone to develop co-infections. Thus there is a growing interest in whether this dual infection intensifies each other and attributes to complications in the clinical spectra and treatment outcomes. Over two-thirds of people living with HIV/AIDS (PLWHA) are found in Sub-Saharan Africa (SSA), a region in which malaria is endemic [1]. The Joint United Nations program on HIV/AIDS (UNAIDS) reported sub-Saharan Africa in 2017 as the most severely affected region, with nearly 1 in every 25 adults (4.1%) living with HIV and accounting for nearly two-thirds of the people living with HIV worldwide [1]. According to the National AIDS Control Committee/Central Technical Group, there are 141 new HIV infections per day in Cameroon [2], which means six newly infected persons each hour, daily.
Malaria is also a life-threatening infection of global concern. It is a vector-borne parasitic disease found in 91 countries globally (predominantly the tropics) transmitted through the bite of a female anopheles ‘mosquito [3-5]. Only 6 out of the more than 120 plasmodium species that infect birds, mammals, and reptiles, are known to regularly infect humans; Plasmodium falciparum, P. vivax, P. malariae, P. ovale curtisi, P. ovale wallikeri and P. knowlesi[4]. Nevertheless, the predominant species globally are Plasmodium falciparum and P. vivax. According to the World malaria report of 2017, there were 216 million cases of malaria in 2016 of which 91% of deaths were from the African region [5]. Children below 5 years of age, pregnant women and persons with immune compromised status such as HIV/AIDS bear the greatest burden of the illness [6]. In view of the considerable geographical overlap of HIV and malaria in Sub-Saharan Africa, coinfections are prevalent [6]. Recent data show that combined malaria and HIV cause more than 2 million deaths annually [7].
The prevalence of Plasmodium/HIV co-infection in Cameroon varies from one region to the other; ranging from 24.5% in Bamenda [8] to 29.5% in Douala [9]. Despite these reports, there is need for continuous monitoring and epidemiologic enquiry to accommodate the current distribution of malaria which does not seem to be influenced only by the geographical setting but also changes overtime with variations in climatic conditions [10]. The objective of this study was to determine the prevalence of malaria parasitemia in people living with HIV/AIDS in Kumba, Cameroon, compare the prevalence of malaria parasitemia between HAART-experienced and HAART-naïve individuals and as well determine the association between viral load and malaria parasite density in the study population.
Materials and methods
Study area
This study was carried out in Kumba, Meme Division, South West Region of Cameroon. Kumba (4°38′N 9°26′E) has an estimated population of about 400,000 inhabitants with about three quarters of this population falling within the youthful age group [11]. Figure 1 shows the map of the study area.
Kumba is in the tropical rain forest, which is thick and evergreen. The climate is equatorial, characterized by two seasons: rainy and dry season. The rainy season last for eight months and is from March to November while the dry season lasts from November to March. There is a break in rainfall for a period of two weeks in the month of August commonly called the August break. The average annual temperature is 31 0C in Kumba and the annual amount of precipitation is 3512 mm [13]. The main occupation of the population is farming (cocoa, coffee, palms and food crops). There are also many people involved in petty trading like the sale of electronic equipment and other goods from neighboring Nigeria and Douala. it is therefore, an ideal site to assess malaria parasitemia in PLWHA
Study duration
Data was collected for a period of five months from April to August 2021.
Study design and setting
This was a cross-sectional hospital and community based study that was conducted between April and August 2021, involving PLWHA. Participants were recruited at the different support groups’ sites and Community-Based Organizations in Kumba (‘Strength of Men and Women’, ‘Starlight’, ‘Courage’, ‘Tabela” and ‘Solidarity’). Starlight Association and Tabela were the main Community Based Organizations (CBO) in Kumba that distributed free Antiretroviral (ARV) drugs in the community. They had close to three hundred (300) registered patients in the community with all the groups receiving about 20 newly diagnosed HIV- positive individuals every month, coming from all over Kumba and its environ.
Study population
Target population: PLWHA were recruited at the different support group sites and Community-Based ARV Dispensation centers in Kumba. The study population consisted of HIV/AIDS patients on treatment (HAART-experienced) and newly diagnosed cases (HAART-naïve) within the study period.
Inclusion criteria: PLWHA both male and female of all ages who gave their consent, on HAART and those not yet on HAART attending any of the support group sites or Community-Based ARV Dispensation centers in Kumba within the study period. For minors and those who could not sign the informed consent, their parents or guardians signed on their behalf.
Exclusion criteria: PLWHA both male and female of all ages who did not give their consent to participate in this study, and participants who were on any anti-malarial medication one week prior to the study. Also, minors and those who could not sign the informed consent whose parents, guardians, or next of kin did not consent on their behalf were excluded from the study.
Sample size estimation: Using the following formula for sample size calculation [14];
Where n: is the minimum sample size of the study
Z: the power of the study
P = prevalence of malaria parasitemia among PLWHA = 24.5% [8]
For a 95% confidence level, Z = 1.96
e = selected sample error margin 5%
This implied:
n = 246
Thus, this gave a minimum sample size of 246 participants.
Sampling technique
A simple random probability sampling technique was used. PLWHA were consecutively recruited into the study as they came to collect their ARV drugs. Table 1 shows the different support groups/CBO and the number of persons who participated in each group.
Study procedures
After ethical and administrative authorizations, a questionnaire was administered to all the consented participants enrolled in the study to obtain socio-demographic data such as age, sex, level of education, marital status; while clinical data such as HIV status, ART usage, duration on treatment, recent viral load was collected from each patient’s file at the HIV treatment centers in Kumba. The questionnaire also assessed the utilization of insecticide-treated nets and insecticide residual spray as preventive methods against malaria. Capillary blood was collected following aseptic techniques according to CDC recommended procedure and malaria parasitemia was determined by blood smear microscopy.
Thick and thin blood films were prepared and stained with 10% Giemsa and examined using standard methods [15]. Blood films were read by two expert microscopists who were blinded from the results of the other. In case of any discrepancy with the results, a third one was brought in and the results he/she gave was considered as final. The thick films were screened for at least 200 fields using the 100X (oil immersion) objective. If asexual parasites were observed, the density was then determined by counting the number of parasites against 500 leucocytes. A blood sample was considered as negative when no parasite was observed after examining at least 200 fields.
Data management and analysis
Data collected was entered into an Excel spreadsheet and imported to the IBM Statistical package for the Social Science (SPSS) version 20.0 software (IBM-SPSS Inc., Illinois, USA). Data was summarized into means and standard deviation (SD) and percentages were used in the evaluation of the descriptive statistics. The statistical tests performed included the Pearson’s Chi-square for comparison of proportions, the Student’s T-test and ANOVA for the comparison of group means and correlation analysis to determine the association between parasite density and viral load. Statistical significance was set at p < 0.05.
Ethical considerations
This study was undertaken after an ethical approval Ref No: 2021/1403-04/UB/SG/IRB/FHS from the Faculty of Health Sciences-Institutional Review Board, University of Buea. Administrative authorizations were gotten from the South West Regional Delegation of Public Health with Ref R11/MINSANTE/SWR/RDPH/PS/814/919, Kumba Health District with Ref No 04/021/MINSANTE/RDPHSW/KHD/DMO/032, from Kumba Bafaw Traditional Council with Ref 01/April/2021 and Kumba District Hospital with Ref No: 071/DHK/M/04/2021. Participation in the study was strictly voluntary. Strict anonymity and confidentiality was maintained in the handling of patient’s data by ensuring that only the principal and co-investigators investigator had access to the data. No names were put on the questionnaires. Prior to collection of data, all participants were fully schooled about the objective of the study and the procedures that they had to go through in the process. Written consent was led read and approved by the participants. There was strict respect of the Helsinki Declaration of the fundamental ethical principles of respect, beneficence, maleficence and minimum risk infliction on participants.
Results
Demographic characteristics of plwha in kumba
A total of 250 participants were enrolled in the study from five main support groups/CBO as shown in Table 2. The CBO Tabela had the highest number of participants 85(34%); [95% CI: 28.1% – 40.2%] while Solidarity had the lowest number of participants 20(8%); [95% CI: 5.0% – 12.1%]. Two thirds 166(66.4%); [95% CI: 60.2% - 72.2%] of those who participated in the study were females. Most of the participants were aged from 41-60 years 163(65.2%); [95% CI: 58.9% – 71.1 %] and those who were of age >60years were least in number 11(4.4%); [95% CI: 2.2% – 7.7 %]. Half of the participants 131(52.4%); [95% CI: 46.0% – 58.7 %] were married with the least being divorced 6(2.4%); [95% CI: 0.9% – 5.2 %].
Distribution of participants according to use of haart regimens, use of bactrim, preventive method against malaria and recent viral load
Use of Highly Active Anti-Retroviral Therapy (HAART): More than three quarters 215 (86%); [95% CI: 81.1% – 90.1 %] out of 250 participants were on HAART while 35 (14%); [95% CI: 9.9% – 18.9%] were newly diagnosed not yet on HAART as shown in Figure 2.
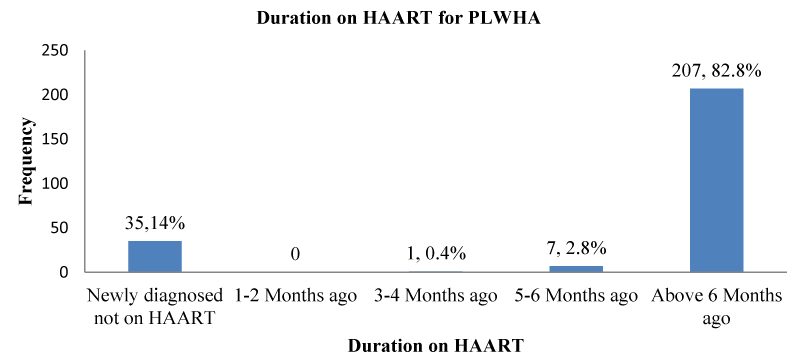
Duration of use of HAART for PLWHA in Kumba, 2021: Of those who were on HAART, 207(82.8%); [95% CI: 77.5% – 87.3 %] had been on HAART for more than six months as shown in Figure 3.
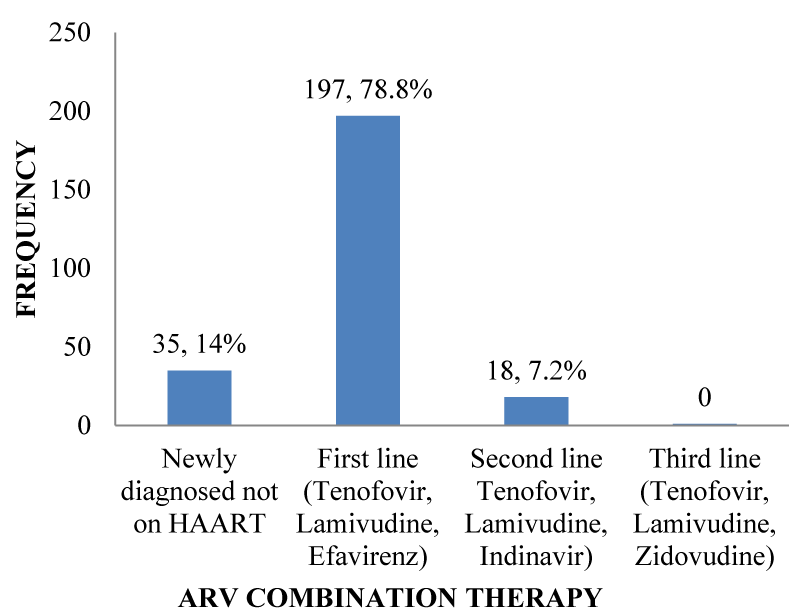
HAART Regimens: More than two-thirds of 197(78.8%); [95% CI: 72.8% – 83.3%] out of 250 participants were on First line HAART. There was no participant in Third line treatment. Those on first-line treatment were on a combination of Tenofovir, Lamivudine and Efavirenz while those on second-line treatment were on a drug combination of Tenofovir, Lamivudine and Indinavir as shown in Figure 4.
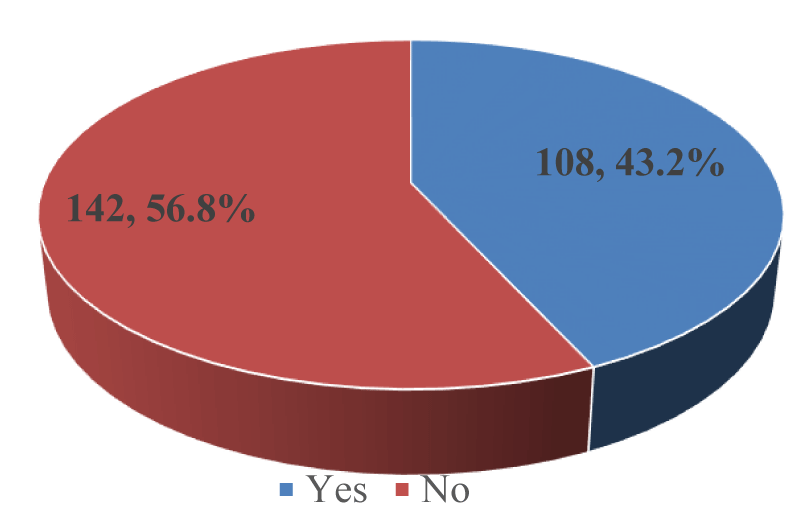
Use of Bactrim (Co-trimoxazole) among PLWHA in Kumba: More than half, 142 (56.8%); [95% CI: 50.4% – 63.0%] out of 250 participants were not on Bactrim as shown in Figure 5.
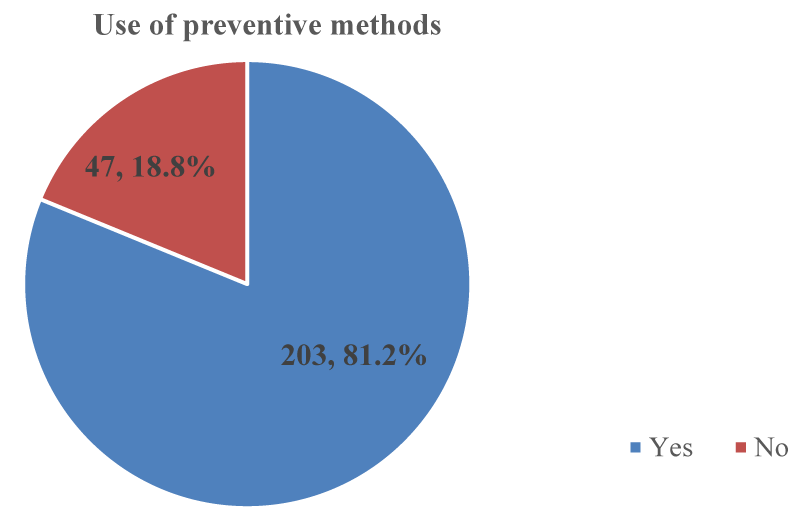
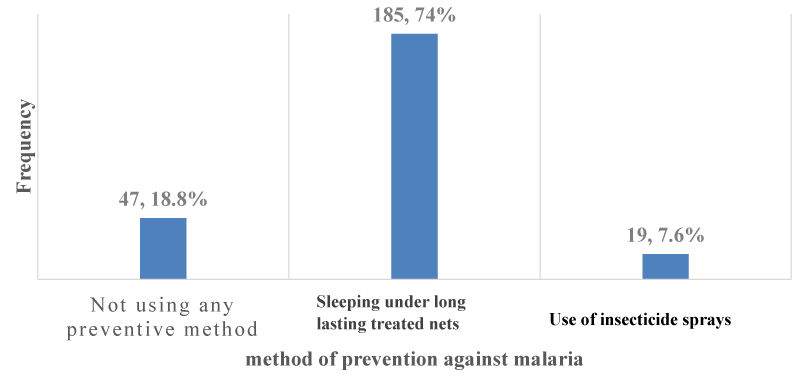
Use of preventive methods against malaria: The majority of 203 (81.2%); [95% CI: 75.8% – 85.8%] out of the 250 participants used preventive methods against malaria as shown in Figure 6. The preventive methods used by participants against malaria were sleeping under long-lasting treated nets 185 (74%); [95% CI: 68.1% – 79.3%] and use of insecticide sprays 19(7.6%); [95% CI: 4.6% – 11.6%] as shown in Figure 7.
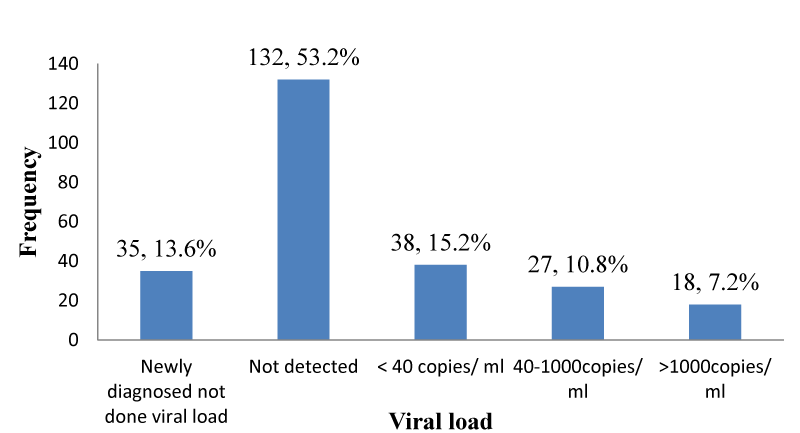
Recent viral load: As shown in Figure 8, 35 (13.6%); [95% CI: 9.9% – 18.9%] out of 250 participants were HAART naïve and had not yet done viral load. The viral load for the majority of the participants was not detected 132(53.2%) [95% CI: 46.4% – 59.1%]; while only 18 (7.2%); [95% CI: 4.3% – 11.1%] participants had a viral load >1000copies/ml.
The prevalence of malaria parasitemia in people living with hiv/aids in kumba, cameroon
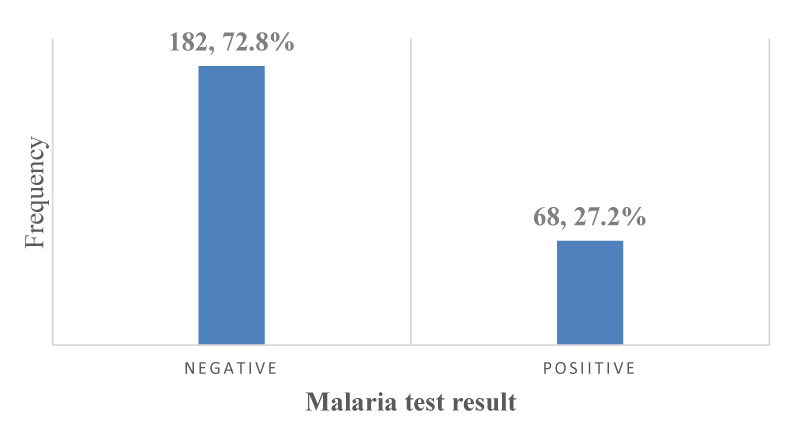
The overall prevalence of malaria parasitemia in PLWHA in Kumba: A total of 68 out of the 250 participants who enrolled in the study were positive for malaria parasitemia giving an overall prevalence of 27.2%; [95% CI: 21.8% – 33.2%] as shown in Figure 9.
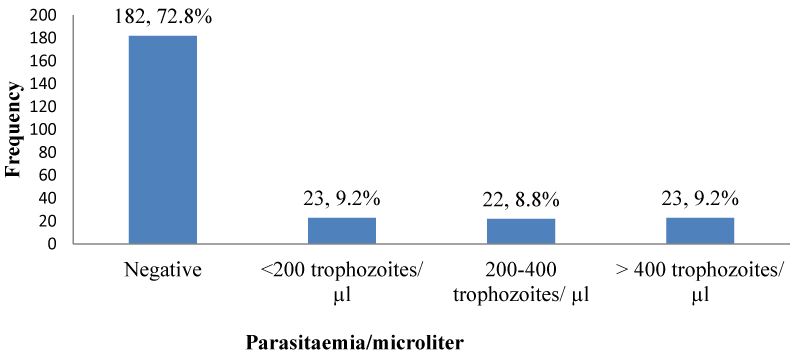
Malaria parasite density in PLWHA in Kumba: Less than a tenth of the participants 23 (9.2%); [95% CI: 5.9% – 13.5%] who were malaria positive had a parasite density of <200 and >400 trophozoites/ µL and few of them. 22 (8.8%); [95% CI: 5.6% – 13.0%] had parasite density 0f 200 - 400 trophozoites/µL as shown in Figure 10.
Comparing the prevalence of malaria parasitemia between HAART naïve and HAART-experienced participants in Kumba: Among 215 participants who were HAART-experienced, 49(22.79%); [95% CI: 17.6% – 27.9%] were found to be positive for malaria parasitemia while 19(54.29%); [95% CI: 48.1% – 60.5%] out of the 35 HAART naïve individuals were positive for malaria parasitemia.
Variation between HAART and malaria parasitemia outcome among PLWHA in Kumba: A chi-square test of independence was performed to examine the relationship between HAART and malaria parasitemia outcome. The relationship between the two variables was significant, X2 (1, N = 250) = 15.078, p < 0.000as shown in Table 3. Those who were HAART-experienced were 4 times more likely to be malaria negative than those who were HAART naïve.
Comparing parasite density for positive cases between HAART-experienced and HAART naïve individuals in Kumba, 2021: A chi-square test for homogeneity of proportions was performed to explore the differences in proportions of parasite density between HAART-experienced and HAART naïve participants. The proportion of parasite density between the two groups was significantly different(>400 Trophozoites/ µL), X2 (3, N = 68) = 16.715, p = 0.001 as shown in Table 4.
Variation between viral load and malaria parasite density for positive cases among PLWHA in Kumba: To test for the dependency of parasite density on viral load, a chi-square test for dependency was computed. The test statistic revealed that parasite density was significantly dependent on viral load, X2 (6, N = 49) = 61.065, p < 0.000 as shown in Table 5.
Association between malaria parasitemia prevalence and selected variables among plwha in kumba
Association between malaria parasitemia prevalence and selected variables based on univariate analysis among PLWHA in Kumba: A bivariate analysis was performed between malaria prevalence and all dependent variables. The selected predictors were entered into a multivariate analysis to reduce the effects of confounding factors.
After adjusting for confounders in the multivariate analysis, those who were in the age group of 41 years - 60 years were 0.09 times less likely to develop malaria than those who were in the age group of >60 years with their differences being significant (AOR 0.090, p = 0.045, [95% CI:0.009% - 0.951].
Being on a Bactrim treatment regimen was found to be significantly associated with malaria prevalence (AOR 0.112, p < 0.000, [95% CI: 0.035 - 0.361]. According to this study, those who practiced the use of preventive malaria methods were 0.0075times less likely to develop malaria than those who did not practice (AOR 0.075, p < 0.000, [95% CI: 0.23 - 0.246]. Those with undetected viral loads (AOR 0.006, p < 0.000, [95% CI:0.001 - 0.045] were 0.075 less likely to develop malaria than those who had never done a viral load test. Table 6 shows the relationship between malaria parasitemia prevalence and preselected variables based on univariate analysis among PLWHA in Kumba, 2021.
Discussion
Cameroon particularly the South West Region has been reported as one of the regions with the highest malaria and HIV prevalence [2]. Hence, there is a need to assess how these two infections interact in order to prevent serious complications resulting from coinfection. Thus, a cross-sectional hospital and community-based study were conducted between April and August 2021, involving 250 PLWHA in Kumba, Southwest region of Cameroon. Participants were recruited from five main support groups/CBO in Kumba. This study was aimed at determining the prevalence of malaria parasitemia in people living with HIV/AIDS in Kumba, comparing this prevalence between HAART naïve and HAART-experienced individuals as well as assessing the association between viral load and malaria parasite density in the study population.
The results showed that the overall prevalence of malaria parasitemia in PLWHA in Kumba was 27.2%. This prevalence is lower compared to the prevalence of 29.4% reported by Akenji, et al. [9] in the city of Douala in the Littoral Region of Cameroon [9]. This prevalence is however higher compared to the prevalence of 24.5% reported by Eyong, et al. [8] in Bamenda in the North West Region of Cameroon [8]. The prevalence is also higher compared to 7.3% reported by Njunda, et al. [10] in Yaounde in the Center Region of Cameroon [10]. Furthermore, the prevalence observed in this study is higher compared to the prevalence of 18.9% reported by Onyenekwe, et al. [16] in Southeastern Nigeria [16]. The prevalence is also higher compared to the 16.3% reported by Sandie, et al. [17] in Buea. This discrepancy in findings could be due to differences in climatic conditions and the level of malaria endemicity. The high prevalence compared to Bamenda, Buea, Yaounde and Southeastern Nigeria could be due to the fact that the city of Kumba has climatic and environmental factors that favor malaria vector survival such as the presence of tropical rain forest, high rainfall patterns, and an average annual temperature of 31 oC [13]. The low prevalence compared to the prevalence reported by Akenji, et al. [9] in Douala could be due to differences in study designs. They carried out a prospective cohort study as opposed to a cross-sectional study in our case. This means their data is likely to be more valid than ours. Also, this discrepancy could be most likely due to differences in study periods. The high prevalence compared to the prevalence reported by Sandie, et al. [17] in Buea could also be due to differences in study design. Sandie, et al. carried out a community-based retrospective cohort study as opposed to a cross-sectional study in our own case.
Furthermore, in this study, the prevalence of malaria parasitemia in HAART naïve participants (54.29%) in Kumba was significantly higher than in HAART-experienced participants (22.79%). Those who were HAART-experienced were 4 times more likely to be malaria negative than those who were HAART naïve. This finding suggests that the use of HAART could be protective against malaria. This is consistent with what Eyong, et al. [8] observed in Bamenda, North West region of Cameroon [8]. This finding is also similar to that of a study carried out of the country by Mermin, et al. in Uganda [18] where in a prospective cohort study, HAART-experienced individuals had a significantly lower prevalence of malaria parasitemia when compared to HAART naïve individuals. However, our findings were contrary to previous studies carried out in the Littoral Region of Cameroon [14] and other Sub-Saharan countries [19] which showed that the use of HAART was not significantly associated with the prevalence of malaria parasitemia. It is worth noting that though our findings were in line with those of Mermin, et al. in Uganda [18], there were differences in study designs as well as the HAART regimens used. We conducted a cross-sectional study while Mermin, et al. conducted a prospective cohort study. Also, many participants in our study were on a drug combination of Tenofovir, Lamivudine and Efavirenz while those of Mermin, et al. were on a drug combination of Stavudine, Lamivudine, and Nevirapine. From this finding, one could say that irrespective of study design and HAART regimens, the use of HAART could reduce the prevalence of malaria parasitemia in PLWHA. It was also observed in this study that HAART naïve participants who were positive for malaria parasitemia had significantly higher parasite density (>400trophozoites/µL) than HAART-experienced participants who were positive for malaria parasitemia. This could mean that malaria in HAART naïve individuals is more likely to be severe malaria than malaria in HAART-experienced individuals irrespective of the clinical presentation.
Moreover, it was observed in this study that parasite density was significantly dependent on viral load. HAART-experienced participants with high viral load (>1000copies/ml) had significantly higher parasite density (>400trophozoites/µL) than HAART-experienced participants with ‘not detectable’ viral load. This could also imply that malaria in HAART-experienced individuals with high viral load is more likely to be severe malaria than malaria in HAART-experienced individuals with low/’not detectable’ viral load irrespective of the clinical presentation. This finding was in line with those of Kublin, et al. in Malawi [20] and Tatfeng, et al. in Nigeria [21]. They observed that HIV-infected individuals with malaria had a significantly increased viral load which might enhance HIV transmission and accelerate disease progression [20]. It is worth noting that though our results were in line with those of Kublin, et al. [20] in Malawi, there were differences in study designs. They carried out a prospective cohort study as opposed to a cross-sectional study in our case. Thus, this could imply that irrespective of study designs, malaria parasite density is significantly dependent on viral load.
In addition, being on a Bactrim treatment regimen was found to be significantly associated with lower malaria parasitemia prevalence. These findings were in line with other studies carried out by Eyong, et al. [8] in Bamenda [9], Mermin, et al. in Uganda [18], Saracino, et al. [19], and Angaret, et al. [22] both in Mozambique, where the use of Bactrim was found to be significantly associated with a lower prevalence of malaria parasitemia as well as less severe malaria attacks. Thus this could mean that the active ingredients of Bactrim (Trimethoprim + sulfamethoxazole) may have some therapeutic effects against malaria parasites. However, this was contrary to the findings of Akenji, et al. [9] and Tchinda, et al. (2012) in Douala, Littoral Region of Cameroon. They did not observe any significant association between cotrimoxazole prophylaxis and the prevalence of malaria parasitemia. Although this study was in line with studies carried out by Mermin, et al. in Uganda [18], Saracino, et al. [19] and Angaret, et al. [22] both in Mozambique, there were differences in study designs. They carried out a prospective cohort study as opposed to a cross-sectional study in our case. Hence, this could imply that irrespective of the study design, co-trimoxazole could be protective against malaria.
Also, those who practiced the use of preventive malaria methods were 0.112 times less likely to develop malaria than those who did not practice. This indicates that the use of preventive methods against malaria vectors such as sleeping under long-lasting insecticide bed nets is essential in reducing the prevalence of malaria. This finding is in line with the study carried out by Kimbi, et al. [23] in Limbe. This shows that the physical barrier against malaria vectors is very essential in reducing the incidence of malaria.
Findings from this study also revealed that those who were in the age group of 41 years - 60 years were 0.09 times less likely to develop malaria than those who were in the age group of >60 years with their differences being significant. This could be due to the fact that those in the age group >60years have low immunity and thus are more prone to develop malaria compared to those in the age group of 41 years - 60years. Finally, this study revealed that participants with undetected viral loads were 0.075 times less likely to develop malaria than those who had never done a viral load test.
Carrying out this study in a malaria endemic area like Kumba(community-based), the use of accurate quantification of parasitemia using microscopy which is the gold standard test for malaria and the use of multivariate analysis controlling for age, gender, use of HAART, use of Bactrim, use of preventive methods against malaria and viral load were the strengths of this study.
A major limitation of this study was the few number of HAART naïve participants who had not done viral load. This was due to the fact that individuals were initiated on HAART immediately after they tested HIV positive and the first viral load test was done six months after initiation of HAART. The reliability of the findings would have increased if we had an equal number of HAART-experienced and HAART naïve participants.
Conclusion
From the findings of this study, we conclude that;
The overall prevalence of malaria parasitemia in people living with HIV/AIDS in Kumba, Southwestern region of Cameroon was 27.2%.
The prevalence of malaria parasitemia in HAART naïve participants in Kumba was significantly higher than HAART-experienced participants. HAART naïve participants who were positive for malaria parasitemia had significantly higher parasite density (>400trophozoites/µL) than HAART-experienced participants who were positive for malaria parasitemia.
Malaria parasite density was significantly dependent on viral load. HAART-experienced participants with high viral load (>1000copies/ml) had significantly higher parasite density(>400trophozoites/µL) than HAART-experienced participants with ‘not detectable’ viral load.
Author’s contributions
Nsagha DS, Fokunang CN, conceived and designed the survey and supervised data collection and analysis; Eyong CA and Adamu NN collected the data, analyzed it and wrote the manuscript. All authors made significant contributions to the manuscript and approved the final version for submission.
Data availability
The data used to support the findings of this study are available from the corresponding author and can be consulted upon request.
The authors are grateful to all the volunteer consented HIV/AIDS individual participants receiving treatment at the different treatment centers and support groups in Kumba. The Director and the entire Staff of District Hospital Kumba, especially the staff of the HIV/AIDS treatment center for their support and collaboration. The Dean, former Deans and the entire staff of the Faculty of Health Sciences of the University of Buea for the technical and material support.
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS report on the global AIDS epidemic 2018, Geneva, Switzerland. 2021. http://www.unaids.org/en/media/unaids/contentassets
- Ossono EN, Fosso MY, Missoko P. HIV AIDS in Cameroon-An update, 2013. The Denis and Lenora Foretia Foundation. 2021. https://www.foretiafoundation.org/hiv-aids-in-cameroon-an-update/ 2013.
- Okafor IM, Okoroiwu HU, Ekechi CA. Hemoglobin S and Glucose-6-Phosphate Dehydrogenase Deficiency Coinheritance in AS and SS Individuals in Malaria-Endemic Region: A Study in Calabar, Nigeria. J Glob Infect Dis. 2019 Jul-Sep;11(3):118-122. doi: 10.4103/jgid.jgid_154_18. PMID: 31543654; PMCID: PMC6733195.
- Ashley AE, PyaePhyo A, Woodrow CJ. Malaria. Lancet. 2018;391:1608-21. https://doi.org/10.1016/S01406736(18)30324-6
- World Health Organization (WHO). World Malaria Report 2017. Geneva, Switzerland: World Health Organization. 2017; 2021.
- Ekere EF, Mirabeau TY, Okoroiwu HU. Prevalence of asymptomatic malaria in HIV-infected subjects on cotrimoxazole antimalarial prophylaxis attending a tertiary health care center in southern Nigeria: a cross-sectional study. Germs. 2020 Mar 2;10(1):44-50. doi: 10.18683/germs.2020.1184. PMID: 32274359; PMCID: PMC7117883.
- World Health Organization (WHO). Malaria; Malaria in HIV/AIDS patients. 2021. https://www.who.int/malaria/areas/high_risk_groups /hiv_aids_patients/en/.
- Eyong CA, Kechia FA, TembeFokunang E, TatangCA,Wandum GC, Agbor MA, Mbanya DS, Fokunang CN: ‘Evaluation of malaria parasitemia among HIV/AIDS individuals attending the Bamenda Regional Hospital Treatment Center; Central African Journal of Public Health. 2019; 5(2): 65-76, doi: 10.11648/j.cajph.20190502.12.
- Nkuo-Akenji T, Tevoufouet EE, Nzang F, Ngufor N, Fon E. High prevalence of HIV and malaria co-infection in urban Douala, Cameroon. Afr J AIDS Res. 2008 Jul;7(2):229-35. doi: 10.2989/AJAR.2008.7.2.8.525. PMID: 25864399.
- Njunda AL, Njumkeng C, Nsagha SD, Assob JC, Kwenti TE. The prevalence of malaria in people living with HIV in Yaounde, Cameroon. BMC Public Health. 2016 Sep 13;16:964. doi: 10.1186/s12889-016-3647-z. PMID: 27619013; PMCID: PMC5376676.
- GPS coordinates of Kumba, Cameroon. Latitude: 4.6363 longitude: 9.4469. 2021.https://latitude.to/map/cm/cameroon/cities/kumba.
- Department of Town planning Kumba urban council. 2005.
- Data tables and charts monthly and yearly climate conditions in Kumba, Cameroon. 2021. http://hikersbay.com/climate-conditions/cameroon/kumba.
- Swinscow TDV, Campbell MJ. Statistics at square. 10th ed. London: BMJ books. 2002.
- Cheesbrough M. District Laboratory Practice in Tropical Countries. Part 1. Second Edition. Cambridge, UK. 2009.
- Onyenekwe CC, Ukibe N, Meludu SC, Ilika A, Aboh N, Ofiaeli N, Ezaeni M, Onochie A. Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria. J Vector Borne Dis. 2007 Dec;44(4):250-4. PMID: 18092531.
- Sandie SM, Sumbele IUN, Tasah MM, Kimbi HK. Malaria parasite prevalence and Haematological parameters in HIV seropositive patients attending the regional hospital Limbe, Cameroon: a hospital-based cross-sectional study. BMC Infect Dis. 2019 Nov 21;19(1):988. doi: 10.1186/s12879-019-4629-4. PMID: 31752719; PMCID: PMC6873725.
- Mermin J, Ekwaru JP, Liechty CA, Were W, Downing R, Ransom R, Weidle P, Lule J, Coutinho A, Solberg P. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006 Apr 15;367(9518):1256-61. doi: 10.1016/S0140-6736(06)68541-3. PMID: 16631881.
- Saracino A, Nacarapa EA, da Costa Massinga EA, Martinelli D, Scacchetti M, de Oliveira C, Antonich A, Galloni D, Ferro JJ, Macome CA. Prevalence and clinical features of HIV and malaria co-infection in hospitalized adults in Beira, Mozambique. Malar J. 2012 Jul 26;11:241. doi: 10.1186/1475-2875-11-241. PMID: 22835018; PMCID: PMC3439710.
- Kublin JG, Patnaik P, Jere CS, Miller WC, Hoffman IF, Chimbiya N, Pendame R, Taylor TE, Molyneux ME. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005 Jan 15-21;365(9455):233-40. doi: 10.1016/S0140-6736(05)17743-5. PMID: 15652606.
- Tatfeng YM, Ihongbe JC, Okodua M, Oviasogie E, Isibor J, Tchougang S, Tambo E, Otegbeye T. CD4 count, viral load and parasite density of HIV positive individuals undergoing malaria treatment with dihydroartemisinin in Benin City, Edo state, Nigeria. J Vector Borne Dis. 2007 Jun;44(2):111-5. PMID: 17722864.
- Anglaret X, Chêne G, Attia A, Toure S, Lafont S, Combe P, Manlan K, N'Dri-Yoman T, Salamon R. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999 May 1;353(9163):1463-8. doi: 10.1016/s0140-6736(98)07399-1. PMID: 10232311.
- Kimbi HK, Njoh DT, Ndamukong KJN, Lehman LG. ‘Malaria in HIV/AIDS patients at different CD4+ T cell levels in Limbe, Cameroon’. Journal of Bacteriology and Parasitology. 2013; 4: (164). doi: 10.4172/2155-9597.1000164.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
