Open Journal of Analytical and Bioanalytical Chemistry
Assessment of diastase levels in different floral honey from Oromia region, Ethiopia
Deressa Kebebe Meskele*, Teferi Damto, Meseret Gemeda and Gemechis Laggase
Cite this as
Meskele DK, Damto T, Gemeda M, Laggase G (2022) Assessment of diastase levels in different floral honey from Oromia region, Ethiopia. Open J Anal Bioanal Chem 6(1): 024-029. DOI: 10.17352/ojabc.000027Copyright License
© 2022 Meskele DK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Diastase is an enzyme that is found naturally in honey and degrades over time, especially when exposed to heat. Diastase can be used to indicate the age and exposure of honey to heat. However, they play a very significant role in honey quality. This study aimed to assess the diastase levels of different floral honey in potential areas of the Oromia region. In this study diastase activity, free acidity, and pH contents of different floral kinds of honey of potential areas in the Oromia region of Ethiopia were investigated based on the IHC- 2009- method. The honey pollen spectra were also characterized. When compared to other flora honey collected from the study area, the diastase activity of Erica arborea honey revealed significantly different (p < 0.05). It ranged from 4.61 ± 1.50 to 12.75 ± 4.78 Schade unit. The results of the pH value and free acidity analyses test demonstrated that the honey samples used in this investigation were fresh which ranged from 3.22 ± 0.13 to 4.17 ± 0.97 and 22.43 ± 6.37 to 35.10 ± 10.51 meq/kg honey, respectively.
Honey bees’ geographical race, location and harvesting time affected diastase activities. However, it was not possible to identify the real factor influencing its diastase activities which needs further study at different agroecologies for different floral kinds of honey of the country.
Introduction
Enzymes are the most essential and also the most exciting honey components. They are responsible for the conversion of nectar and honeydew to honey and serve as a sensitive indicator of the honey treatment. In some countries, the specification of enzymes is a binding legal indicator [1]. Honey naturally contains small amounts of enzymes. Enzymes play an important role in honey and contribute to its functional properties. Honey holds small amounts of enzymes of which diastase and invertase are the most important because they are carefully chosen for the validation of honey quality. However, the predominant ones are diastase (Vorlova & Pridal, 2002). The higher the content of this enzyme, the higher the quality of honey. Diastase is one of the major enzymes found in honey. Diastase activity and HMF content are well used as criteria to assess the quality of the product (Thrasyvoulou, 1986).
Diastase is found in nectar and is also added by honeybees during the collection and ripening of nectar. Like HMF, the diastase activity of honey can be used as an indicator of aging, overheating, and the degree of preservation /storage (Kędzierska-Matysek, et al. 2016). The activity of diastase in honey is affected by storage and is sensitive to temperature increase and can thus be used as an indicator of storage time/freshness. Numerous studies indicated that honey shows significant variation in amylase content based on composition, pH value and floral source (White, 1992; Babacan and Rand, 2007). Additionally, free acidity and pH are important parameters for the evaluation of honey quality, and indicators of freshness and adulteration of the honey (Silva, et al. 2016).
Ethiopia produces different types of floral honey (Adgaba, 2007) and the majority of the identified multi-floral and mono-floral honey types produced in the country is from Oromia (Gemechis Legesse, 2013). So far different studies have been conducted on the physical and chemical characterization of the honey types produced in different parts of the country (Adgaba, 1996; Belay, et al. 2013; Gebremedhin, et al. 2013; Legesse, 2014).
However, a few studies conducted on the enzyme level of different honey types collected from different parts of the country focusing only on the diastase level indicated that the average diastase level of honey samples from the whole country was lower than the EU and world standard. Ethiopia currently exports honey to EU member countries, Middle Eastern countries and African countries (Legesse, 2014). The enzyme content especially the diastase level of various honey types exported from Ethiopia is below the generally accepted average set for international trade, according to comments from their international buyers, which honey processing and exporting companies have been complaining about (personal communications). However, the enzyme levels of honey vary depending on composition, pH value, and floral source (White, 1992; Babacan and Rand, 2007). So far, in Ethiopia, all the available information showing the low enzyme level indicated that the honey samples were originally from different market sources in the country (Adgaba, 1996). Due to their botanical origin, some monofloral honey naturally has a lower diastase value and is accepted on the global market, according to these standards [1]. The honey from virgin intact forest areas, however, was the subject of repeated complaints in the European market due to a reduced level of enzymes. Therefore, it is necessary to investigate the diastase level of honey from different plant sources and other important factors like pH and acidity level to assess the freshness of the honey. As a result, this research study aims to evaluate the diastase levels of major monofloral and multi-floral honey in potential Oromia areas of the region.
Materials and methods
Honey sample collection sites
Honey samples were collected from major honey-producing zones of the Oromia region to represent the major mono-floral and multi-floral honey types of the region. Accordingly, Gera and Goma Districts from Jimma, Bacho, Didu, Mettu and Yayo Districts of Illu Abbabora, Ejere, Ada’s
Berga and Tokke-kuttaye, Districts of West Shoa and Ammaya, Waliso and Wenchi Districts of Southwest Shoa zones were selected. These zones were selected based on the identified monofloral honey and multiflora and the potentiality of honey in the study areas.
Honey samples collection
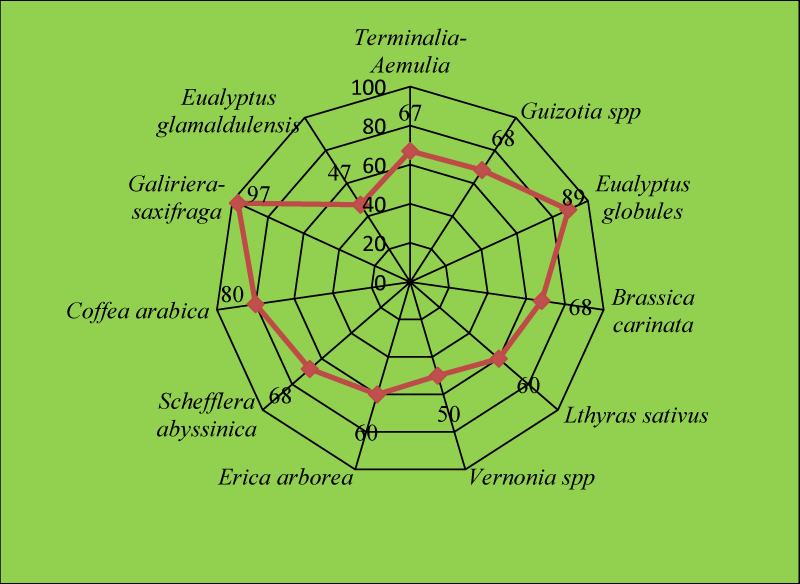
For this study, major mono-floral and multiflora honey types were considered and samples were purchased from their areas of production. Each honey type was represented based on its availability. A total of sixty-nine crude honey samples each weighing 1 kg from beekeepers were collected and brought to Holeta Bee Research Center, Bee Product Quality Analysis Laboratory. Multiflora honey was obtained after processing crude honey samples and identifying honey types into monofloral and multiflora honey based on the pollen count (Figure 1).
Straining
Firstly, the honey samples were strained to separate the pure honey from the wax using a drainage system with moderate warming when it is necessary to heat. Then, each purified honey sample was stored in uncontaminated containers until analysis at 4 oC in the freezer.
Botanical sources of each honey type
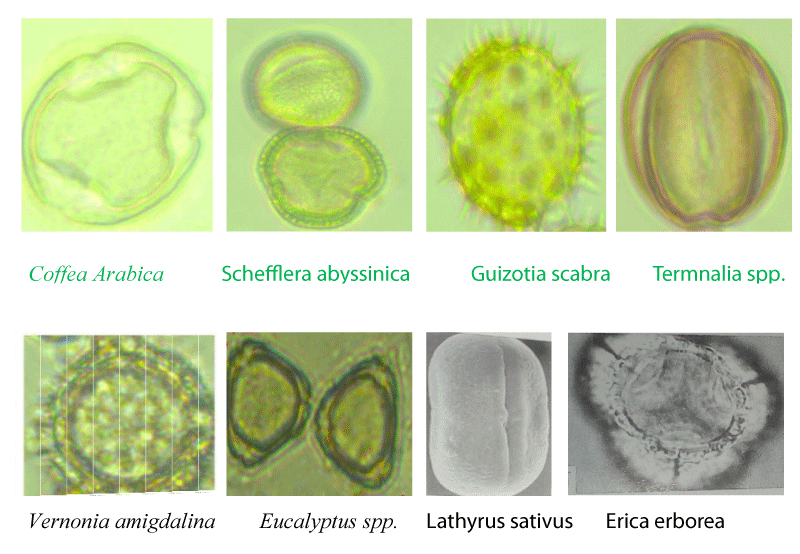
The pollen spectrum of the honey was analyzed using procedures for honey pollen analysis recommended by Louveaux, et al. [2]. For this, ten grams of honey was dissolved in 20 ml of warm distilled water and the sediment was concentrated by repeated centrifuging at 3800 rpm for 10 minutes and the supernatant was decanted. The distilled water of 20 ml again was added to completely dissolve the remaining sugar crystals for 5 minutes and the supernatant was removed completely. The sediment was spread evenly using a sterile micro spatula on a microscope slide and the sample was dried for a while. Thereafter, a drop of glycerin jelly was added to the coverslip and the pollen grains were identified using a pollen atlas [3]. The percentage of pollen types in each honey sample was calculated based on the total number of different types of pollen grains counted in each sample. If pollen grains counted were greater than 45 %, used as predominant pollen (monofloral honey), while honey with no predominant pollen was used as multifloral honey [2]. The pollen count was done under the light microscope (Swift instrument international, Japan, high power 400 x) linked to a computer.
Diastase activity
Diastase activity was evaluated spectrophotometrically (UV-visible Spectrometer) using the Schade method in the Holeta Bee Research Center bee product quality laboratory. The diastase activity is calculated as diastase number (DN). DN expresses units of diastase activity (Gothe unit). The diastase activity was determined using 10 g of honey weighed in a 50 mL beaker and 5 mL of acetate buffer was added, together with 15 mL of distilled water. When the sample was dissolved, 3 mL of sodium chloride (NaCl) prepared by dissolving 2.9 g of NaCl in water and diluting to 100 ml were added and the solution was diluted to 50 mL with distilled water. Additionally, a starch solution was standardized using an iodine solution. Both solutions were heated at 40 oC. Five milliliters of starch solution were added into 10 mL of honey solution and the stop-watch was started. An aliquot was taken every 5 min and was added to 5 mL of iodine solution. The absorbance was recorded and a calibration curve was obtained. According to the IHC, 2009 method the number 300 was divided by the time needed to reach the absorbance value of 0.235 and expressed as DN or diastase number (IHC, 2009). The diastase activity was calculated as diastase number (DN) as follows:
Where tx is the reaction time in minutes obtained as absorbance values of the test sample solutions plotting against the corresponding reaction times in minutes after subtracting the absorbance of the blank value control.
Free acidity and pH values
Free acidity and pH values of honey samples were analyzed according to the International Honey Commission (2009) by pH meter (METTLER TOLEDO, China). To determine pH and free acidity from each honey sample ten grams of honey was dissolved in 75 ml of distilled water in a 250 ml beaker and stirred using a magnetic stirrer. The electrode of the pH meter was immersed in the solution and the pH of the honey was recorded. For measurement of free acidity, the solution was further titrated with 0.1 M NaOH solution to pH 8.30. FA is expressed as milli equivalents per kilogram of honey and is equal to ml of 0.1M NaOH times ten.
FA =10 x VNaOH consumed, Where: V = the volume of 0.1N NaOH in 10 g of honey used and FA is free acidity
Data management and analysis
All the experiments were performed in triplicate (n = 3) and the results were expressed as mean standard deviation. The data were analyzed by One-way ANOVA followed by the Tukey test. All the statistical analyses were carried out using SPSS Software version 20 adopting a significance level of 5% (p < 0.05).
Results and discussion
Botanical sources of each honey type
In this result, the botanical origin of the honey samples dominantly originated from nine different nectar source plant species (Guizotia scabra, Eucalyptus globulus, Eucalyptus camaldulensis, Sheffleria abyssinica, Vernonia spp, Erica arborea, Coffea arabica, Lathyrus sativus and Terminalia schimperiana (Table 1). These plant species are also known as major and minor honey plants for honey bees (A. mellifera). The relative pollen frequency of honey samples indicated that Schefflera abyssinica was a predominant honey plant in honey samples collected from the Bacho and Didu districts of I/A/bora and Gera and Gomma districts of Jimma Zones. Schefflera abyssinica is an abundant bee forage plant in moist highlands of southwestern and southeastern parts of Ethiopia and it provides monofloral honey in these areas [4]. It was also reported that Schefflera abyssinica is a major monofloral honey source tree in the Gera area (Degaga, 2017) which is similar to the current study results. Guizotia species was predominant in honey samples collected from the Ada Barga and Toke-Kuttaye districts of the west, and Waliso of Southwest Shoa Zones, while Eucalyptus globules were predominant in honey samples collected from the Amaya district of Southwest and Ejere and Chalia of West Shoa zones. Admasu, et al. (2018) reported that most of the Ethiopian highlands are covered with golden yellow flowers of Guizotia spp. with many different colors. On the other hand, the majority of honey samples from the Gera districts of the Jimma Zone were dominated by Coffea arabica which was identified as monofloral honey (Table 1) (Figure 2). Coffea arabica is a common cash crop in western Oromia and contributed much to monofloral honey samples. Admasu, et al. (2014) stated that Coffea arabica flowers provide abundant pollen and nectar in January for honeybees.
In addition, Erica arborea was a predominant honey plant in the honey samples collected from the Wenchi district of the Southwest Shoa Zone which is supported by literature of Admasu and Tura [4] studies, that Erica arborea honey is commonly found in Wenchi district in West Shoa Zone.
Diastase activity, pH and acidity
Diastase activity of the honey samples ranged from 4.61 ± 1.50 (Lathyrus sativus) to 12.75 ± 4.78 Schade unit (Erica arborea). The diastase activity of Erica arborea honey showed significant differences (p < 0.05) from that of other honey collected from the study areas. Abera, et al. (2017) reported that the diastase activity of Eucalyptus globulus honey was 5.86 ± 0.890 Schade Unit which is lower than the present study resulting. This showed that honey from the same botanical origin can probably differ in diastase activity, due to the influence of the environment, in which the honey plant species grow and the occurrence of different geographical races of bees, which are mostly governed by biotic and abiotic factors [5]. Similar values for diastase content were also reported in Argentina honey which averaged 19.7 Schade units [6]. The diastase activity of multifloral honey for the current study was 6.84 ± 1.75 which is comparable with the finding of Al-Farsi, et al. (2018) who found the diastase activity ranged from 0.78 to 5.55 Schade unit for multiflora samples.
According to Oddo, et al. [1], the level of diastase activity in honey varies depending on its sugar content, its floral and geographic origins, the length of time it was collected, the age of the bees, and the bee colony. In addition, the variation in reported diastase values could be due to different factors, such as processing, storage conditions, bee species, and harvesting time (Belay, et al. 2017; Al-Farsi, et al. 2018; Seraglio, et al. 2018). The diastase activity is a very interesting enzyme to know the freshness of honey (Oddo, et al. 1990). The location also affects the diastase value. Waykar, et al. (2022) found that the enzyme values in honey varied from location to location.
The diastase levels of Coffee Arabica, Eucalyptus globulus, Terminalia Schumpeterian and Erica arborea honey were almost found to be within the Codex Alimentarius and European union standard requirements [1]. Based on international legislation (Codex Alimentarius Commission, 2001), the permitted minimum diastase activity limit for honey is ≥ 8 Schade units of diastase per gram of honey.
The other honey samples showed lower diastase levels than the acceptable range. As a result, the obtained values can be regarded as characteristics of the examined honey and not dependent on external factors. The lower diastase level of some of the honey samples in the current study may be related to the internal characteristics of the honey and their botanical origin. Low levels of diastase activity may not be indicative of adulteration or a lack of quality, according to Cortopassi-Laurino & Gelli (1991), it is specific characteristics of certain types of honey that supported the findings of the current study. The current study was also supported by Wang and Li’s [7] findings that fresh honey from different plant origins that was not heated had lower diastase activity. According to White [8-22] and Bogdanov, et al. [1], honey is also used as a quality parameter even though some have a lower level of enzymes intrinsically. Diastase, a heat-sensitive enzyme found in honey, is a parameter that shows the freshness of honey and inappropriate heat treatment and storage conditions (Anklam, 1998; Marquele-Oliveira et al., 2017).
Even though the current study focused on the diastase content of monofloral and multiflora honey purchased from beekeepers in the study areas, the honey samples may not be heated or aged. But from the current study results, it could be interpreted that honey diastase contents can vary due to different factors such as the location of sampling, sampling time and botanical origin. This indicated that fresh honey with the permitted level of diastase level may be found by following the specific floral honey. The enzyme level in different floral honey may differ due to heat, age, geographical origin, processing, honey origin and etc. Therefore, honey diastase levels may be affected starting from apiary site management to the consumption of bee products. Controlling all factors affecting the quality of honey may improve the diastase levels in the floral honey types.
Free acidity and pH values
Free acid and pH values for monofloral and multiflora honey are presented in Table 2. The free acidity ranged from 22.43 ± 6.37 (Schefflera abyssinica) to 35.10 ± 10.51 meq/kg (Coffee arabica honey) taken from Gera Districts (Table 2). On the other hand, the free acidity of Schefflera abyssinica honey was significantly different from all honey types (p < 0.05) which is the lowest free acidity recorded in this study. According to Belay, et al. (2017), Schefflera abyssinica honey had a free acidity of 23.90 ± 1.85 meq/kg, which is comparable to the current study. Even though enzymes are present in very small amounts, they have a significant effect on the quality of honey. This is because the enzymes would significantly affect the protein content, free amino acid profile and acidity of honey samples. A variation in free acidity among the monofloral honey types might be because of the differences in honey harvesting conditions, plan origin, location and seasons. All of the investigated samples (monofloral and multifloral) met the demands imposed by the regulations, which require in general not more than 50 meq/kg (Codex, 2001). This indicates the absence of unwanted fermentations and honey sample freshness.
The pH values for the current study ranged from 4.17 ± 0.97 (Eucalyptus globulus) to 3.22 ± 0.13 (Terminalia schimperiana) honey. The pH of Terminalia schimperiana honey was significantly different from that of other honey types at (p < 0.05). According to previous reports, the pH of honey is between 3.2 and 5.5 (Bogdanov, et al. 2004; Karabagias, et al. 2014). The variation in the pH might be due to the effect of extraction, storage conditions, and floral types.
The pH of the honey was reported by other scholars and is in line with the current investigation. Belay, et al. (2017c) and Adgaba, et al. (2020) reported that the pH value of 3.61 – 3.77 and 3.5– 3.7, respectively for Ethiopian monofloral honey. In addition, Saxena, et al. (2010) stated the pH of monofloral honey ranged from 3.7 – 4.4.
The low level of diastase found in the current results may be attributable to an inherent characteristic of the honey samples, as both the pH and free acidity of honey indicates the freshness of the honey sample examined in this study. Besides affecting the pH value, the activity of enzymes might change the flavor and aroma of honey after fermentation (Chua, et al. 2014). Thus, the diastase levels of monoflaral and multifloral honey types can be altered due to many factors affecting honey quality if not managed to start from the production areas to the consumers or users.
Conclusion and recommendation
Diastase is found in nectar and is also added by honeybees during the gathering and maturing of nectar. Different pollen source plant species were identified to classify honey into various monofloral and multifloral honey types. The highest diastase level was found in Erica arborea honey from the Wenchi district in the South West Shoa zone, while the lowest diastase level was found in Lathyrus sativus honey. Some of the monofloral and multifloral honey types did not meet the international standard for diastase level. It may be concluded that variations in the diastase level may be caused by various uncontrollable intrinsic and external factors as well as by the botanical origin of honey. Although the honey sample examined in this study was fresh and unheated, the results of the pH and free acidity test also demonstrated the honey samples’ freshness. However, some floral honey has lower diastase levels than are normally required. The lower levels of diastase could be inherent characteristics of monofloral and multiflora honey. Thus, monitoring the factors affecting the diastase levels such as heating, using after long time storage, time of harvesting, floral type and location is important. In addition, it should be recommended to conduct further research on the diastase activity of various floral honey types gathered from different agroecologies in the country to identify and control the factors affecting the diastase of floral honey types.
- Bogdanov S, Lullman C, Martin P, Von Der Ohe W, Russmann H, Vorwohl G, Persano Oddo L, Sabatini AG, Marcazzan GL, Piro R, Flamini C, Morlot M, Heritier J, Borneck R, MarioleasP, Tsigouri A, Kerkvliet J, Ortiz A, Ivanov T, D’arcy B, Mossel B, VIT P. Honey quality and international regulatory standards: review by the international honey commission. Bee World. 1999; 80(2): 61–69.
- Louveaux J, Maurizio A, Vorwohl G, Methods of melissopalynology. Bee World. 1978; 59 (4):139-157.
- Adgaba N. Geographical races of the Honeybees (Apis mellifera L) of the Northern Regions of Ethiopia (Doctoral dissertation, Rhodes University). 2002.
- Adamasu A, Bareke T. Floral resources diversity and vegetation types important for honeybees in Ethiopia. Journal of Forestry. 2019; 3:2; 64-68
- Adgaba N, Al-Ghamdi A, Tadesse Y, Getachew A, Awad AM, Ansari MJ, Owayss AA, Mohammed SE, Alqarni AS. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J Biol Sci. 2017 Jan;24(1):180-191. doi: 10.1016/j.sjbs.2016.05.002. Epub 2016 May 10. PMID: 28053589; PMCID: PMC5198994.
- Cantarelli MA, Pellerano RG, Marchevsky EJ, Camina JM. Quality of honey from Argentina: Study of chemical composition and trace elements. 2008.
- Guo W, Liu Y, Zhu X, Wang S. Dielectric properties of honey adulterated with sucrose syrup. J Food Eng. 2011; 107(1): 1-7. doi: 10.1016/j. jfoodeng.2011.06.013
- White JW. The role of HMF and diastase assays in honey quality evaluation. Bee world. 1994; 75(3):104-117. https://doi.org/10.1080/0005772X.1994.1109921
- Schade JE, Marsh GL, EckertJE. Diastase activity and hydroxymethylfurfural in honey and their usefulness in detecting heat adulteration. Food Research. 1958; 23, 446-463.
- Hadorn H, Zürcher K. Eine einfache kinetische Methode zur Bestimmung der Diastasezahl in Honig. Deutsche Lebensmittel Rundschau. 1972; 68:209-216.
- White JW, PairentFW. Report on the analysis of honey. J.Assoc. Off.Agric.Chemists. 1959; 42:341-348.
- Estevinho LM, Feás X, Seijas JA, Pilar Vázquez-Tato M. Organic honey from Trás-Os-Montes region (Portugal): chemical, palynological, microbiological and bioactive compounds characterization. Food Chem Toxicol. 2012 Feb;50(2):258-64. doi: 10.1016/j.fct.2011.10.034. Epub 2011 Oct 13. PMID: 22019893.
- Von Der Ohe W, Oddo LP, Piana ML, Morlot M, Martin P. Harmonized methods of melissopalynology. Apidologie. 2004; 35 (Suppl. 1), S18-S25.
- Bogdanov S, Lu¨llmann C, Martin P. Honey quality, methods of analysis and international regulatory standards: Review of the work of the international honey commission.
- Switzerland: Swiss Bee Centre. 2000.
- White JW. Comparison of honey. Comprehensive Survey, by C. Eva. Heinemann, London. 1975; 157-206
- Journal of the Argentine Chemical Society. 96(1–2): 33-41.
- Maria Josiane Sereia, Paulo Henrique Março, Marcia Regina Geraldo Perdoncini, Rejane Stubs
- Parpinelli, Erica Gomes de Lima and Fernando Antônio Anjo. Techniques for the Evaluation of Physicochemical Quality and Bioactive Compounds in Honey Hive and honey Apiary (2020). Apiary Digestive Enzymes and The Role Of Raw Honey. 2016. https://www.hiveandhoneyapiary.com/Digestive-Enzymes-And-The-Role-Of-Raw- honey.html
- Joanna K, Adam G, Marianna K. Determination of the diastase activity in honeys. 2017. https://repozytorium.biblos.pk.edu.pl/redo/resources/28435/file/suwFiles/KucJ_Det erminationDiastase.pdf
- Beespoke Info. Enzymes in Honey. 2013. |beespoke.info/11/05/enzymes-in-honey
- Persano Oddo L, Baldi E, Accorti M, Diastatic Activity of Some Unifloral Honeys, Apidologie. 1990; 21:17-24.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
