Open Journal of Plant Science
Antimicrobial activities and phytochemical profile of the leaf extracts of Barringtonia racemosa L. (Putat) against selected pathogens: An ethnomedicinal plant species
Jeffry M Saro1*, Jacob E Josue1, Noel P Sastrillas1, Almay Rose Joy C Gundaya2, Mary Grace A Valencia3, Rommel R Rimando1, John Mark C Sarongon4 and Leila A Allado-Ombat5
2Department of Education, Gingoog City (Teacher), Philippines
3Teacher III, San Vicente National High School, Prosperidad District V., Philippines
4College Instructor, St. Peter’s College of Misamis Oriental Inc, Philippines
5Department of Biology, College of Arts and Sciences, Caraga State University - (CSU), Ampayon, Butuan City, Philippines
Cite this as
Saro JM, Josue JE, Sastrillas NP, Joy C Gundaya AR, Valencia MGA, et al. (2022) Antimicrobial activities and phytochemical profile of the leaf extracts of Barringtonia racemosa L. (Putat) against selected pathogens: An ethnomedicinal plant species. Open J Plant Sci 7(1): 020-024. DOI: 10.17352/ojps.000047Copyright
© 2022 Saro JM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Barringtonia racemosa L. (Putat) was mostly utilized as a treatment for wound infections and other types of skin diseases locally. The leaves of B. racemosa are traditionally used to treat high blood pressure and as a depurative. This study aimed to assess the antimicrobial activities and phytochemical profile of the leaf extracts of Barringtonia racemosa L. (Putat) against selected pathogens. The ethanolic extracts of the leaves were explicitly prepared and applied to two Gram-positive bacteria (Staphylococcus aureus and Bacillus cereus), whereas the Gram-negative bacteria (Escherichia coli) used the disc diffusion method based on the standard procedure. Moreover, among the test microorganisms, only the Gram-positive bacteria were sensitive to the ethanolic extracts of Barringtonia racemosa L. with the diameter of zone of inhibition ranging from 13.81±0.96 mm to 14.85±0.57 mm. The findings of this study suggested that the tribes could explicitly continue utilizing this ethnomedicinal plant as a source of treatment. Thus, the ethanol extracts of the leaves of B. racemosa were found to be effective against several pathogens used in this study, which certainly highlights the potential extremity of herbal drugs and their possible use as local medicine. Hence, there has been a continuing search for new and more potent antibiotics.
Introduction
The interactivity between plants and humans has long been established since ancient times. The plant’s medicinal properties have been acknowledged and conceded very well. Also, considered humans’ living pharmacy for thousands of years [1]. The knowledge of traditional medicine and ethnobotanical uses of plant species in every tribe might act as a starting point for extensive pharmacological studies to be carried out on medicinal plant species. Barringtonia racemosa L. which is also known as Putat, a fish poison tree, or powder puff tree is a type of highly valuable plant species due to its medicinal values and components (Bhat, et al. 2012). The plant extracts are essential antibacterial agents due to the presence of phytochemicals [2]. Herein, phytochemicals with known antimicrobial properties have explicit prominent importance in therapeutic treatments [3]. Antibiotics have been produced by pharmacological industries in the last 30 years, wherein the resistance and aversion to these drugs by microorganisms has been widely increased day by day. The problem of bacterial resistance is growing and the outlook and viewpoint for the use of antimicrobial drugs in the future are still uncertain [4]. Thus, actions should be taken appropriately to reduce this kind of problem, for example, administering the use of antibiotics, must develop research to better understand the genetic mechanisms of resistance, and taking up studies to recognize new drugs, either synthetic or natural upshot [5].
For a long period of time, plants have been a valuable source of natural products for maintaining a good condition of human health, withal passed down from generation to generation in several parts of the world, plants have significantly contributed to the different traditional and new plan systems of medicine [6]. There are thousands of natural, semi-synthetic, and synthetic antibiotics that are well-produced to treat various microbial infections and complications that plainly saved countless lives yet, several of these substances are toxic preeminent and have negative side effects on the consumers or an individual. Accordingly, the current study has been focused on finding out effective natural plant species with antimicrobial properties against pathogens. Aqueous, methanol, petroleum ether, and ethanolic extracts of P. hydropiper L., B. acutangular L., A. nilotica L., and A. precatorious L. were tested against four carbapenem-resistant [7]. Additionally, the fact that various known pathogens are sensitive and tactful to certain antibiotics however numerous are very resistant or greatly developed resistance after subsequent exposure to antibiotics. Therefore, the search for antibacterial agents that are accurately safe and with an inclusive spectrum is still uncertain.
This study aimed to screen the ethanolic leaves extract from the Philippine sakoo, Barringtonia racemosa (L.) for antimicrobial analysis against Staphylococcus aureus, Escherichia coli, and Bacillus cereus. The powdered leaves and all other parts of the plant were exploited as a fish poison, also the extracts might be used as an insecticide. Various ethnic groups in Agusan del Sur used this plant as tanning agents as they contain high levels of tannin. Moreover, the seed of Barringtonia racemosa (L.) is used to treat eye inflammation and by midwives for parturition. In the Philippines and several countries, the leaves of B. racemosa are traditionally used to treat high blood pressure and as a depurative. The pounded leaves are said to treat chickenpox. Lastly, it is occasionally cultivated as an ornamental tree along roadsides.
Materials and methods
Plant material
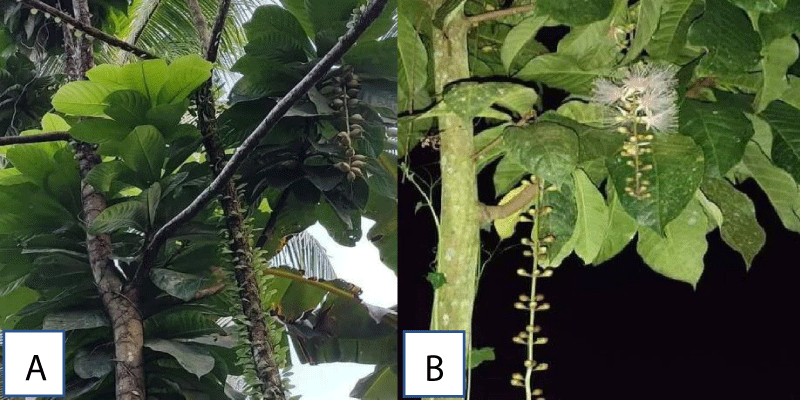
The fresh leaves of Barringtonia racemosa L. (common name: Common Putat, Fish-Killer Tree, Fish-Poison Tree, Freshwater Mangroove, Powder-Puff Tree, Putat Ayam, Putat Kampong, and Small-Leaved Barringtonia), were collected at 8.4909039’ N and 125.7432843’ E, respectively in San Luis, Agusan del Sur, CARAGA Region, Philippines. The fresh sample of the plant was submitted and conducted at the laboratory area of the Department of Biology, Caraga State University (CSU), Ampayon, Butuan City, Agusan del Norte for the plant species to be examined for some procedural activities Figure 1.
Preparation of plant crude extracts
The collected leaves of Barringtonia racemosa L. were cleaned with deionized water and slashed into small pieces and ground into a wispy powder using a scissor and blender after air-drying for 7 days (1 week). Additionally, the powdered leaves sample were placed into a tube and spouted with 80% ethanol to obtain ethanolic extracts.
Ethanol extraction
Forty-eight (48) grams of air-dried powder was added to 450 mL 80% ethanol in an Erlenmeyer flask. Later, the Erlenmeyer flask was covered with aluminum foil for three (3) days. With that, 285 mL of ethanolic extracts were congregated from the leaves sample of Barringtonia racemosa L. after the filtration procedure. The 285 mL of ethanolic extracts were placed into the two-evaporating dish. And, concentrated to 80 mL at less than 500C in a steam/water bath. The extract was placed in a small Erlenmeyer flask and stored at 40C.
Qualitative phytochemical screening
Phytochemical analysis of the leaf extracts of Barringtonia racemosa L. has been carried out according to standard protocols. Additionally, the plant extracts were performed to determine the presence of alkaloids, flavonoids, saponins, steroids, and tannins using the method described [8].
Screening for alkaloids
An equivalent of 6.8 mL of the extract was evaporated into syrupy consistency through a steam bath. Then, 1 mL of 2M HCl was added and heated for 5 minutes with constant stirring. When cool, 0.1 gram of NaCl was explicitly mixed and filtered. The residue was washed with enough 2 M HCl to bring filtrate to 1 mL. Two to three drops of Mayer’s reagent were added. The white to cream precipitate indicates the positive presence of alkaloids.
Screening for flavonoids
A 0.6g of the extract (1.7 mL) was added with 5 mL of 80% ethanol. The solution was plainly divided into two parts (3.55 mL per test tube). The first part served as control while the other part of the solution was mixed with 0.5 mL concentrated HCL (12 M). The solutions were properly warmed for 15 minutes in a water bath. The appearance of strong red or violet color indicates a positive result.
Screening for saponins
A 3.4 mL (2 grams) of the extract was diluted with 10 mL distilled water and shaken vigorously for 2 minutes. A stable persistent froth indicated the presence of saponins.
Screening for steroids
An equivalent of 0.6g of the extract (1.7 mL) from the stock solutions was added with a 1 mL Ferric chloride (FeCl3) reagent. Also, 1 mL of concentrated sulfuric acid was plainly added slowly to the side of the test tube. Wherein, the two layers formed, aqueous and sulfuric acid in the upper and lower portion. The appearance of reddish-brown to purple in the interface of the two layers indicates the positive presence of steroids.
Screening for tannins
A 0.36 mL solution of extract was added to 1.66 mL distilled water (H2O) in a 20 mL test tube to make a 2 mL solution. Then, 10% ferric chloride solution was added to the mixed solution. The appearance of blue or green color signifies the positive presence of tannin. However, a blue or black indicates the presence of hydrolyzable tannins, while a brownish to green may indicate the presence of condensed tannins.
Test microorganisms preparation
Bacterial cultures of Staphylococcus aureus and Escherichia coli were collected from the Biology Department – Laboratory, Caraga State University, Ampayon, Butuan City. The microorganism cultures were directly maintained in slants at 370C. Preceding the activity test, the bacteria were pre-cultured in nutrient broth for 24 hours and precisely transferred into a potato-glucose broth. Herein, the cultures’ density was adjusted based on 0.5 McFarland standards (1.5x108 CFU/mL).
Antimicrobial assay
The methodology for antimicrobial analysis of the extracts was adapted from the work of Guevara [8]. A 0.6g of the extracts (1.7 mL) were clearly taken from the sample stocks and evaporated to incipient dryness through a steam bath at less than 500C. Furthermore, a sterile cotton applicator was properly dipped into a potato-glucose broth medium and the screening of the test microorganisms were straightened out into their respective sterile culture medium (nutrient Agar for the bacteria). The sterile paper discs (6 mm in diameter) were impregnated with the leaf extracts, ethanol (negative controls), and penicillin (commercial antimicrobial or positive control) were placed on the test- microorganism seeded plates in triplicate. The diameter (mm) of the zone of inhibition was taken after 24-hours incubation at 370C of bacteria, respectively.
Preparation of disk
The sterile paper discs will be utilized in preparing around 6 mm in diameter disk which will be set in hot air for disinfection. Subsequent to being cleaned, the disk will be hampered with various concentrations of a sweeping range of antibiotic penicillin (positive control) and 80% ethanol (negative control), the readied ethanolic leaf extracts (treatment) of Barringtonia racemosa L. will be held under refrigeration for 24 hours.
Reading and measuring of zone of inhibition
The zone of inhibition is the point where there is no growth development evident to the naked eyes. The appearance of individual colonies and an irresolute zone inside the zone of inhibition will be recorded. However, the indistinct portion will be disregarded while evaluating the zone of inhibition due to measuring only the zone of normal growth. A Caliper (0.5 mm) will be wrought in measuring the zone of inhibition and will then be rounded at the nearest mm.
Statistical analysis
The collected data on antimicrobial assay were presented in means and the standard error of the mean was properly calculated for the zones of inhibition measured for the experiment. These means were statistically compared based on the ranged introduced by Guevara (2005) [7].
Results and discussion
The present study revealed that the ethanolic leaf extracts of Barringtonia racemosa L. contained flavonoids, saponins, steroids, and tannins. However, the alkaloids are not present (no presence of a white to cream-colored precipitate indicating a negative result of the alkaloids) based on the conducted experimental assessment by the researchers, shown in Table 1. Accordingly, in various ethnomedicinal plant species, the parts of the plant such as leaf ethanol extracts have an ability to prevent the growth of bacteria and are caused by the existence of compounds that have a role as antibacterial, the flavonoids, saponins, steroids, alkaloids, and tannins (Meutia, 2017). The phytochemical analysis result of the leaf extracts of B. racemosa leaves has these secondary metabolite compounds namely, flavonoids, saponins, steroids, and tannins. Furthermore, most of these phytochemicals are mainly the basis for plant medicinal properties and the starting point of materials for the production of new medicinal drugs today.
The high antibacterial activity in the ethanolic leaf extracts of B. racemosa might be the presence of a high amount of tannins and flavonoids that possess a similar procedure. The extracts were found to be effective against the pathogens used in this study, which certainly highlights the potential of herbal drugs and their possible use as local medicine. Various studies have been done to assess and evaluate the phytochemical compositions and antibacterial activities of different parts of diverse plant species. The main purpose of utilizing these plants for the treatment of microbial diseases and infections as possible alternatives to synthetic drugs to which several infectious bacteria have been developed resistance. Accordingly, the findings indicated that the tannins are present, however, the flavonoids are not detected in the crude extract of Musa sapientum L., it was likely considered that the antibacterial activity of the banana peel is something related to tannins and not to flavonoids compound [9].
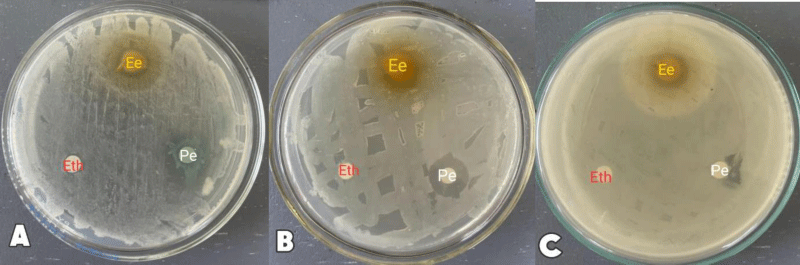
In Table 2, the ethanolic extracts of Barringtonia racemosa L. exhibited the highest activities against the selected pathogens namely, Staphylococcus aureus (13.81±0.96) and Bacillus cereus (14.85±0.57), whereas the Gram-negative microorganism (Escherichia coli) 9.89±0.13. The observed zones of inhibition for the negative control (80% ethanol) were 7.96±0.88, 7.53±0.83, and 6.00±0 (mm) for S. aureus, E. Coli, and B. cereus respectively. While, the positive control (penicillin) exhibited relatively higher inhibitory activity against the bacterial strains, with zones of inhibition for S. aureus (20.08±0.33) and E. Coli (16.13±1.13) respectively.
The concentration of the crude extracts of Barringtonia racemosa L. was raised to 3 grams (5.1 mL) for inhibitory activity against Staphylococcus aureus and Escherichia coli was approximately the same as the inhibitory activity obtained by Bacillus cereus. The phytochemicals namely, tannins, saponins, steroids, and flavonoids were all detected in the ethanolic leaf extracts of Barringtonia racemosa L. Thus, the high antibacterial activity in the extracts might be due to the presence of a high number of tannins and the said secondary metabolites compound. The two compounds such as tannins and flavonoids possess a similar mechanism by providing a source of stable free radicals and also forming a complex with nucleophilic amino acids in protein leading to the inactivation of the protein and loss of function. Their potential antimicrobial effect and properties are great as they probably target microbial cells of surface-exposed adhesins, cell wall polypeptides, and membrane-bound enzymes [10].
In connection, the differences observed in the antimicrobial activities suggest the susceptibility of the test microorganisms to several secondary metabolites compound present in this plant sample (Barringtonia racemosa L.). Therein, the composition of these secondary metabolites in turn varies from one species to another plant species, climatic conditions, and the physiological state of developments of the plant species [11]. Nonetheless, the antibacterial substance within the Barringtonia racemosa L. seemed to be most prominent in the leaves, and the inhibitory activity was observed for Gram-positive bacteria (S. aureus and B. cereus). This might be attributed to the fact that the cell wall in Gram-negative bacteria synthesizes a thin single peptidoglycan layer covered by an outer membrane, whereas the Gram-positive bacteria produce a thick multi-layer peptidoglycan.
This study’s findings are greatly consistent with those of Zuvairea, et al. (2014), they precisely determine the standard antibiotics and antibacterial used, tetracycline, chloramphenicol, and nystatin showed a zone of inhibition ranging from 8 to 35 mm against all test microorganisms while the negative controls did not plainly show any antimicrobial activity on the screening process. In the same study, the researchers evaluated the discovery of new types of antibiotic treatment like substances that could serve as selective and has a low-cost source of natural antibacterial agents and might also help to conserve the environment.
In this study, the leaves of Barringtonia racemosa L. were used, this is because the leaves of the plant species are mostly utilized as a treatment for wound infections and other types of skin diseases locally. The ethanol extracts were found to be effective against several pathogens used in this study, which certainly highlights the potential extremity of herbal drugs and their possible use as local medicine Figure 2.
The results of this study inferred that the people tribe particularly from San Luis, Agusan del Sur could utilize this Philippine sakoo, Barringtonia racemosa L. (Putat) as ethnomedicine. Accordingly, the Barringtonia racemosa is a medicinal plant belonging to the Lecythidaceae family. Herein, the water extract of B. racemosa leaf has been shown to be rich in polyphenols and has diverse medicinal properties [12-19]. Therefore, antibiotics provide the main premise for the therapy of bacterial infections. Yet, the high genetic variability of microorganisms enables them to rapidly evade the action of antibiotics by promoting and developing antibiotic resistance. Hence, there has been a continuing search for new and more potent antibiotics.
Conclusion
The result of this experimental study showed that Barringtonia racemosa L. (Putat) has antibacterial properties against the selected pathogens namely, Staphylococcus aureus and Bacillus cereus. The other test organism, Escherichia coli is mainly resistant to the ethanolic extracts. Thus, this proposed that the tribes could explicitly continue utilizing this ethnomedicinal plant as a source of treatment. Ethnomedicinal plant’s uses and promising phytomedicinal values and components of this species, the Barringtonia racemosa L. which had been proven through scientific studies have indeed verified it's worth exploring properties and may serve as a potential candidate for future drug development.
The authors are grateful to the Department of Biology for having an advanced plant physiology subject course in the prospectus of Masters of Science Education with specialization in Biological Science (MSciEd-Biology). The authors extended their profound appreciation to Dr. Leila A. Ombat for the assistance during plant samples determination, support, and guidance during the laboratory activities.
- Nurul-Maryam H, Radzali M, Johari R, Syahida A, Maziah M. Malaysian Journal of Biochemistry and Molecular Biology. J Pharm Sci Res 2015; 7(4): 185-188.
- Hermant Ranjan AL. Plant Stress Tolerance Physiological & Molecular Strategies. Scientific publishers 2016.
- Nascimento GG, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J Microbial. 2010; 31:247-256.
- Karuppiah P, Mustaffa M. Antibacterial and antioxidant activities of Musa sp. leaf extracts against multidrug resistant clinical pathogens causing nosocomial infection. Asian Pac J Trop Biomed. 2013 Sep;3(9):737-42. doi: 10.1016/S2221-1691(13)60148-3. PMID: 23998016; PMCID: PMC3757284.
- Doughari JH, El-mahmood AM, Tyoyina I. Antimicrobial activity of leaf extracts of Senna obtusifolia (L). Afr J Pharm Pharmacol. 2008; 2:7-13.
- Hussain S, Malik F, Khalid N, Muhammad AQ, Riaz H. Alternative and traditional medicines systems in Pakistan: History, regulation, trends, usefulness, challenges, prospects and limitations, a compendium of essays on alternative therapy. In: Bhattacharya Arup., editor. Shanghai: InTech; 2012.
- Matin MM, Bhattacharjee SC, Hoque Md S, Ahamed F. Antibacterial activity of some medicinal plants against cardapenem-resistant Acinetobacter baumannii isolated from patients. Eur J Pharm Med Res. 2019; 6:111-116.
- Guevara BQ. A Guidebook to Plant Screening: Phytochemical and Biological. University of Santo Tomas, Research Center for the Natural Sciences, Manila, Philippines. 2005;
- Lino PB, Correa CF, Archondo ME, Dellovann DC. Evaluation of post-surgical healing in rats using a topical preparation based on extract of Musa sapientum epicarp. Braz J Pharmacogon 2013; 21:491.
- Hernandez NE, Tereschuk ML, Abdala LR. Antimicrobial activity of flavonoids in medicinal plants from Tafi 0301; del Valle (Tucuman, Argentina). J Ethnopharmacol. 2016; 73 (1):317-322.
- Mohamad FM, Ameenah GF, Anwar HS. Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. 2008; 43.
- Kong KW, Abdul Aziz A, Razali N, Aminuddin N, Mat Junit S. Antioxidant-rich leaf extract of Barringtonia racemosa significantly alters the in vitro expression of genes encoding enzymes that are involved in methylglyoxal degradation III. PeerJ. 2016 Aug 25;4:e2379. doi: 10.7717/peerj.2379. PMID: 27635343; PMCID: PMC5012310.
- Islam N, Islam MdD, Rahman MdR, Matin MM. Octyl 6-O-hexanoyl-B-D- glucopyranosides: Synthesis, PASS, antibacterial, in silico ADMET, and DFT studies. CCL. 10: 413-426.
- Khan AV, Ahmed QU, Shukla I, Khan AA. Antibacterial activity of leaves extracts of Trifolium alexandrium Linn. Against pathogenic bacteria causing tropical diseases. Asian Pac J Trop Biomed. 2012; 2:189-194.
- Muddathir AM, Mitsunaga T. Evaluation of anti-acne activity of selected Sudanese medicinal plants. J Wood Sci. 2013; 59(1): 73-79.
- Ogila KO, Waihenya RW, Ochola AO. Phytochemical Profile and Antimicrobial Activities of Edible Mushroom Termitomyces striatus. 2021: 3025848.
- Sanaullah AFM, Bhuiyah MdMH, Matin MM. Stearoyl Glucopyranosides: Selective Synthesis, PASS Analysis, In Vitro Antimicrobial, and SAR Study. E J of Chemistry. 2022.
- Sobia MZ, Nasir R, Asim M, Fozia A, Munawar I, Muhammad M, Muhammad S. Antioxidant, antibacterial, antifungal activities and phytochemical analysis of dagger (Yucca aloifolia) leaves extracts. Journal of Medicinal Plants Research. 2013; 7:243-249.
- Zainab AGC, Al-Charrakh AH, Nada KH, Shatha KKH. Antimicrobial effect of aqueous banana peel extract, Iraq. Pharmaceutical Science. 2013; 73-75.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
