Global Journal of Anesthesiology
Anesthetic Management of Critical COVID-19 Infection: A Narrative Review of Concepts and Evidence-Based Clinical Practices
Jameel Kassam* and Eapen Mathew
Cite this as
Kassam J, Mathew E (2022) Anesthetic Management of Critical COVID-19 Infection: A Narrative Review of Concepts and Evidence-Based Clinical Practices. Glob J Anesth 9(1): 001-011. DOI: 10.17352/2455-3476.000054Copyright
© 2022 Kassam J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction
Anesthesiologists are on the frontline in the war against the global COVID-19 pandemic, providing airway, ventilatory, and hemodynamic support to acute patients suffering from severe and critical COVID-19 infection. This is despite facing enhanced risk for cross-infection from patient respiratory secretions while performing intubations at high volume. The Chinese Society of Anesthesiology reports a 20% cross-infection rate among anesthesiologists who performed intubations on COVID-19 patients during the early phase of the pandemic [1]. Furthermore, 12% of New York anesthesiology residents reported presumed, suspected, or confirmed infection-higher than any other specialty including emergency medicine, ophthalmology, and otolaryngology [2]. In addition to providing life-saving intubation for severe COVID-19 patients, anesthesiologists have comforted critically ill patients in isolation from family due to IPC-mandated visitation restrictions. Many have dedicated their time and expertise in the critical care environment, serving as ancillary ICU staff or on hospital intubation teams. This review details these unique viral features and reviews the evidence base regarding infection prevention and control (IPC) in the anesthesia workspace. It also outlines the conceptual foundations behind airway, ventilatory, and hemodynamic management recommendations and the supplementary protocols for COVID-19 management. When necessary, critical appraisal of the evidence base surrounding these topics is offered, as well as suggested changes to current guidelines and topics for further investigation.
Clinical virology
Coronaviridae (CoV) is a zoonotic family of positive-sense single-stranded RNA viruses that exhibit strong genetic diversity and pandemic potential. This is likely due to the more error-prone RNA-dependent RNA polymerases causing frequent genomic recombination events during viral replication. This genomic diversity enables coronaviruses to evolve and periodically infect the respiratory tract of human hosts.
The precise pathogenesis from pneumonia to COVID-19 ARDS (CARDS) is unclear. Studies have reported significantly elevated levels of inflammatory signaling molecules IL-6, IL-10, G-CSF, and TNF-α among CARDS patients suggesting cytokine release syndrome (CRS), an inflammatory syndrome characterized by fever and multiple organ dysfunction, commonly in the setting of immunosuppressive drug therapy following transplantation [3]. Immune hyperreactivity paradoxically suppresses leukocyte production and attenuates cell-mediated immunity. The precipitous decline from baseline to critical status among patients with CARDS may be linked to underlying CRS causing depressed CD8+ T-Cell mediated viral clearance, prolonged neutrophil-mediated lung destruction, and upregulated macrophage-mediated fibroproliferative damage [4]. Autopsy studies also point to endothelial cell dysfunction, thrombotic microangiopathy, and virally-mediated lung damage in addition to hyperinflammatory injury [5].
Virulence and transmission dynamics
Virulence has been described as the degree to which a pathogen elicits disease in exposed individuals and is a measure of individual outcomes, such as the case fatality rate (CFR) and infection fatality rate (IFR), rather than population-level mortality. Though less virulent than its predecessors SARS-CoV-1 and MERS-CoV, which exhibited CFRs of 9.60% and 34.4% respectively [6,7]. SARS-CoV-2 exhibits higher overall virulence than influenza viruses [8-10]. Furthermore, COVID-19 demonstrates various degrees of attenuated or magnified virulence that make it clinically challenging and unpredictable. It is been posited that the heterogeneity and presentation among patients with COVID-19 are partially attributable to the differential interplay between individual susceptibility and the array of different viral species [11]. While some patients decompensate within a week, a large subset of patients remain asymptomatic throughout the course of infection (Table 1) [12] Children, in particular, are more likely to have few or no symptoms yet remain infectious carriers [13,14].
Perioperative implications
Surgical risk: Operating and performing anesthesia on COVID-19 positive patients, particularly those with comorbidities, pose significant health risks to both patients and providers. Anesthesia providers are at enhanced risk since endotracheal intubation exposes them to virally dense upper airway secretions. Perioperative assessment based on clinical screening is confounded by a significant portion of COVID-19 patients who portend little or no symptoms. As nations begin the transition towards pre-pandemic surgical volume, the potential for presymptomatic or asymptomatic transmission to healthcare providers may increase, especially if the predicted resurgence in COVID-19 cases coincides with this timeline.
Operating and performing anesthesia on COVID-19 positive patients also may also confer a significant risk to patients. The evidence-base regarding clinical outcomes for COVID-19 positive patients undergoing surgery is limited to one retrospective case series of 34 patients with comorbidities who were not symptomatic at the time of screening and underwent non-emergent vascular and GI surgeries [15]. An alarming proportion went on to develop ARDS (32%), shock or secondary infection (29%), arrhythmia (24%), acute cardiac injury (50%), and acute kidney injury (6%), with 21% ultimately dying [15] These patients endorsed comorbidities predictive of critical COVID-19 infection—specifically hypertension [13,16-19] cardiovascular disease [17,19] diabetes [13,17-20] malignancy [21,22] and age [23-25] > 65. Despite the dearth of other studies on surgical risks to infected patients, these findings are nevertheless concerning since early patient cohorts scheduled for medically necessary surgeries likely have similar chronic conditions and may have a similarly elevated risk for negative health outcomes.
Preoperative screening: Given both the dangers to patients and providers and the unreliability of clinical assessment alone for identifying presymptomatic or symptomatic carrier states, supplemental molecular-based screening is essential. Despite their utility in supplementing standard history and physical exam during preoperative evaluation, the precise utility of current RT-PCR requires accurate population prevalence estimates to help determine the post-test negative predictive value. Moreover, the true prevalence of infectious carriers will remain elusive unless governments institute population-level zero surveillance to determine the proportion of infectious presymptomatic, asymptomatic, mildly symptomatic carriers that go largely unreported [26]. While the current rate of confirmed infectious carriers worldwide is approximately 0.6%, rates of unconfirmed cases have been estimated at anywhere from 20-70% [27–29]. of Assuming an RT-PCR sensitivity of 70% and specificity of 90%, the negative predictive value of the test would drop from 99.27%, or 1 case in 137, to 98.41%, or 1 case in 63, as the true population prevalence increases from 1% to 5% [30] This constitutes a two-fold increase in the risk of performing surgery and administering anesthesia to the naïve COVID-19 positive patient.
Until representative prevalence rates on a regional level are accurately estimated, universal serial testing for those patients with higher pre/post-test probabilities represents the most risk-averse preoperative screening modality—depending on when the test is administered in the disease process, the sensitivity of RT-PCR may be only marginally superior to clinical assessment alone [31-37]. Molecular screening and clinical assessment should be used synergistically to enhance the overall sensitivity of the initial evaluation, and any subtle indication of infection or close contact from history and physical should be met with a high index of suspicion and a low threshold for retesting (PARIS score [38], is a pre-test probability assessment with demonstrated external validity). These recommendations are consistent with Anesthesia Patient Safety Foundation guidelines [39]. Additionally, if an emergent aerosol-generating medical procedure (AGMP) precludes testing or if RT-PCR is unavailable, donning full PPE with N95 should be considered prudent practice [40].
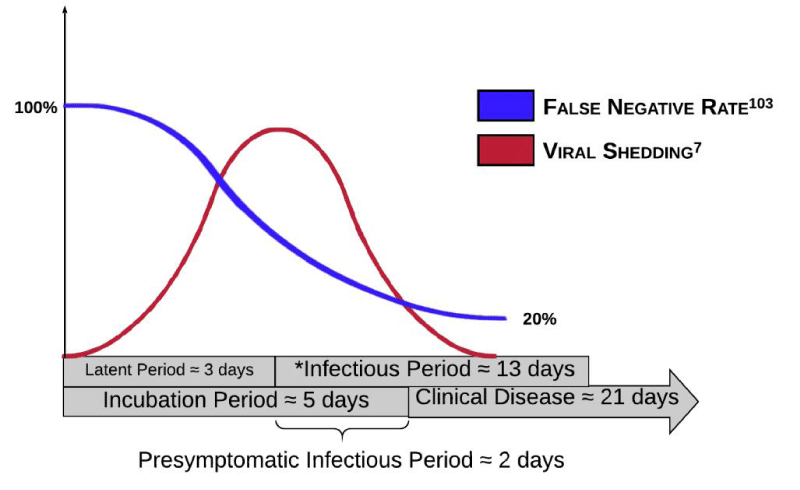
When retesting a negative screen in the background of high clinical suspicion for viral infection (i.e. sick contacts, viral symptoms), the WHO recommends repeat testing after 2-4 days to account for the latent period when the degree of viral shedding has not yet crossed the critical threshold for an adequate sample [41,42]. According to a robust meta-analysis of 1330 tests from 7 previously published studies, this window is represented by a steady decline in the false-negative rate of 100% on day one to a nadir of 20% on day eight post-exposure. This curve inversely mirrors the timeline of viral shedding described by He and colleagues (Figure 1) [43]. Another important consideration is the considerable variation in nasopharyngeal and oropharyngeal RT-PCR sensitivities depending on the initial location of viral inoculation [44]. Smaller viral aerosols preferentially colonize the LRT, while larger droplets colonize mucous membranes or the URT. The IDSA recommends that patients with negative URT samples but who have high post-test probabilities for infection undergo retesting of the LRT, either through sputum analysis [45].
Infection prevention and control
Preventing droplet transmission: Prevention measures against droplet transmission of SARS-CoV-2 focus on three sequential points of viral dissemination: (1) identifying infected patients (2) preventing patients presumed infectious from dispersing droplets and (3) maintaining adequate distance from droplet trajectory should dispersion occur. These first two measures invariably counter contact transmission as well. A type 1 surgical mask should be placed on the patient, and hospital staff should wear surgical masks at all times. This alone is sufficient to prevent direct inoculation by larger respiratory droplets (Figure 2) [46].
Preventing contact transmission: The two primary goals in IPC of indirect contact-transmission of SARS-CoV-2 are (1) prevent fomites via disposable physical barriers and (2) decontaminating surfaces that are likely to have fomites (such as monitors and anesthesia workstations) with viricidal agents. SARS-CoV-2 is a relatively bulky lipid-enveloped RNA virus that is exquisitely sensitive to low-and intermediate-level disinfectants, despite its generating resilient fomites on environmental surfaces [47-51]. Low-level disinfection results in significant decreases in pathogen density with more frequent and targeted cleaning of high-touch surfaces [52-54]. Furthermore, simulation studies found that double-gloving prevents cross-contamination of the anesthesia work environment after intubation [55,56]. Lastly, compliance with hand-hygiene protocols was greater in groups where disinfectants were readily available, with reported decreases in hourly noncompliance rates [57] from 50% to 20%.
Preventing airborne transmission: Noninvasive positive pressure ventilation presents more of a theoretical risk for aerosolization of pathogenic droplets because pressured air delivery can potentially compromise the device interface and leak if not firmly secured to the patient’s face. This risk is amplified if NIPPV causes dynamic hyperinflation of the noncompliant lung– high distending pressures are transferred across the patient-mask interface. If initiated, a full-face mask is preferred to minimize particle dispersion [58]. On average, the dispersal distance for NIPPV was reported as 90 cm versus only 10 cm for HFNC, with a greater reported risk of far-reaching aerosol dispersal if there was a compromise in the mask interface [58]. If possible, HFNC should be administered over NIPPV for initial treatment of acute hypoxic respiratory failure. Furthermore, since there is currently a global shortage of PPE, an N95 should be rationed to one per day and they should be kept in a “splash-proof” container for storage [59].
Epidemiology of critical infection
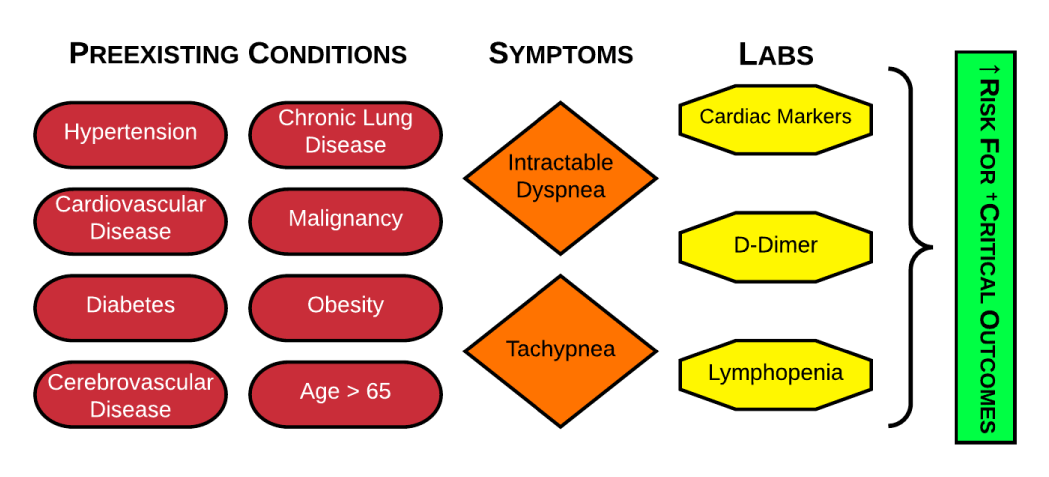
Predictors for critical outcomes: Comparative studies on hospitalized versus ICU cases consistently reported significantly higher rates of hypertension [13,16-19], cardiovascular disease [17,19], diabetes [13,17-20], cerebrovascular disease [17,60,61] chronic lung disease [19,62,63] malignancy [21,22] obesity [64,65] and age [23–25] > 65 among critically ill groups, though some of these studies did not adjust for baseline confounding factors between groups [66]. Additionally, intractable dyspnea and tachypnea were consistently reported in the literature as significantly associated with critical outcomes [13,17,19,67]. but not mortality [13]. A systematic review demonstrated that lymphopenia [18,68-71], on admission was associated with ARDS and death, especially in younger [71] patients. Studies also demonstrate that COVID-19 pneumonia can predispose patients to acute venous thromboembolism [5,72-77] and myocardial injury [17,19,67,78,79] even in the absence of risk factors with respectively elevated lab markers [76,80,81,81-83] notably, the SOFA [19,84] qSOFA [19] CURB65 [19], and APACHE II [84] scoring systems were all strong predictors of ICU mortality in patients with pneumonia from COVID-19 (Figure 3).
Critical outcomes: The most common critical outcome among patients admitted to the ICU for COVID-19 is ARDS. Chinese, US, and Italian studies demonstrate robust rates of ARDS progression among ICU admissions [16,63,85,86] (40-70%). An alarmingly high portion of patients who presented with ARDS decompensated to severe ARDS within 3-7 days [12,16,17,63,87]. Rates for shock [17,67,85,87] and cardiomyopathy [17,67,85] were 20-30% among ICU cohorts as well. High rates for these two concomitant pathologies should determine an appropriate induction agent. Mortality rates were consistent between studies, with around 50% of patients admitted to the ICU for COVID-19 infection.
Early interventions
Patient self-inflicted lung injury: Amplified respiratory drive during AHRF and ARDS is superimposed on NIPPV to generate large tidal volumes that, while normally tolerable in AHRF of extrapulmonary etiology, cause stress fractures and regional strains that result in intra-tidal shifts of gas to dependent areas of lung [88] with regional overdistension despite deceivingly modest elevations in global lung volume [89]. As lung aeration and oxygen saturation decline, more vigorous spontaneous efforts correspond with increasingly negative intrathoracic pressures that enhance venous return and augment transmural vascular hydrostatic pressures in the setting of increased capillary permeability from de novo ALI, worsening pulmonary edema [90] Lastly, excessive concentric shortening of the diaphragmatic muscles due to patient effort and insufficient mechanical support can cause muscle inflammation and myofibrillar damage [91].
NIPPV: Early initiation of NIPPV is associated with an approximately 50% reduction in the need for intubation in ARDS but does not affect mortality [92,93]. Studies on NIPPV outcomes in CARDS patients found similar failure rates of 13% [94] and 52% [60]. The inability to improve P/F after 1 hour of therapy is the greatest predictor of the need for endotracheal intubation and invasive mechanical ventilation [95]
HFNC: Conventional low-flow oxygen devices provide 100% FiO2 at a rate of 15 L/min less than normal physiologic inspiratory flow rates, resulting in dilution of oxygen concentration as room air mixes with pure oxygen to fulfill a respiratory demand [96]. This differential is exaggerated during respiratory distress, resulting in the entrainment of a much larger proportion of room air relative to pure oxygen [97]. HFNO overcomes these flow limitations by delivering up to 60 L/min of humidified gas which typically meets or exceeds the physiologic inspiratory flow of 30L/min to minimize room air entrainment and maximize FiO2 [98] Compared to NIPPV, HFNO is more comfortable for patients and facilitates self-proning [99-102] expectoration, oral suctioning, mucociliary clearance [103] and airflow conductance [104] Two studies reported HFNC failure rates for CARDS patients with a P/F < 200 mm Hg of 63% [105] and 100% [106]; conversely, CARDS patients with a P/F > 200 had a failure rate of 0% [105]. Additionally, a retrospective case-control study comparing HFNC with NIPPV in 73 patients with COVID-19 pneumonia found that failure rates for HFNC and NIPPV were 22% and 100% respectively [84].
Self-proning: A pilot study carried out in a single urban emergency department in New York City found that patients with moderate to severe hypoxemia related to COVID-19 lung injury demonstrated improvement in their SpO2 after being placed in the prone position for 30 to 120 minutes followed by 30 to 120 minutes in the left lateral decubitus position while on HFNC [101]. Other anecdotal studies have reported similar successes with self-proning of COVID-19 patients on HFNC [107,108].
Intubation and ventilatory management
The following guidelines have been founded on multiple literature reviews and expert recommendations regarding the minimization of aerosolization during intubation of patients with COVID-19:
Special considerations for COVID-19 patients: Critically ill patients with respiratory failure have the inadequate functional residual capacity (expiratory reserve volume + residual volume) to provide the necessary oxygen stores to comfortably endure the apneic phase of intubation [118]. Furthermore, as de novo lung injury progresses to ARDS, stress fractures along the alveolar-capillary interface compromise ventilation resulting in shunt physiology [119]. These patients are less amenable to preoxygenation than the typical “medically optimized” surgical patient. These patients can therefore not endure long apneic episodes, which can precipitate anoxic brain injury, cardiovascular collapse, and death [118]. Though manual ventilation is considered an AGMP, wearing appropriate airborne PPE and maintaining a tight mouth-mask seal will more effectively denitrogenate reserve lung volume for a shorter manual ventilation time [120] which, in turn, lowers viral aerosolization and transmission risk [121]. Supplemental HFNC while ventilating has the added benefit of delivering positive pharyngeal pressure (essentially low-grade PEEP ≈ 8 cm H2O) that prevents post-expiratory atelectasis common during denitrogenation of noncompliant lung [122].
Driving pressure: Ventilator-induced lung injury (VILI) is due to (1) excessive volume mechanically delivered to distal air spaces that produce stress-strain fractures at the capillary alveolar interface (volutrauma); (2) a systemic inflammatory response that increases pulmonary capillary permeability and pulmonary edema (biotrauma); and (3) shear injury from cyclical lung collapse during expiration (atelectrauma) [123-125]. The driving pressure is the difference between the plateau pressures and PEEP. Alternatively, it can be expressed as the ratio of tidal volume to respiratory system compliance, and is proportional to the ratio of the tidal volume to the FRC [126].
A study by the New England Journal of Medicine found that driving pressure was the greatest predictor of ventilator injury among patients with ARDS [127,128] which was later confirmed in two meta-analyses [129,130].
Ventilating the CARDS patient: As discussed in the section on P-SILI, volumetric information provided by global ventilatory readings (Vt) does not accurately reflect the degree of regional volutrauma and localized lung strain common in patients with ARDS. Gattinoni and colleagues speculate that 70% of patients with severe COVID-19 have only slightly decreased pulmonary compliance and consequently do not maintain the high-driving pressures typical of “traditional” ARDS [131-133]. This group of patients is termed the “L-“ or “Class I” phenotype of CARDS. Because compliance is only marginally reduced, delivering high PEEP in these patients can theoretically increase lung volume and precipitate VILI. The other 30% of patients are hypothesized to demonstrate the classic ARDS profile which consists of low compliance, high driving pressures, and higher recruit ability. These patients are said to possess the “H-phenotype” of CARDS. Nevertheless, most guidelines recommend using the same lung-protective ventilation strategy for both subtypes, i.e. low tidal volumes to prevent volutrauma and biotrauma and high PEEP to prevent atelectrauma.
Corticosteroids: The WHO offers weak best-practice recommendations [134] against the use of corticosteroids in patients with SARI from COVID-19 pneumonia, except in patients with concomitant shock. These recommendations are extrapolated from other viral studies which showed no survival benefit of corticosteroids among SARS patients [135] no reduction in mortality among influenza patients [136,137] and delayed viral clearance of the LRT in MERS-CoV patients [138]. In regards to the latter study, there is no evidence that delayed viral clearance in MERS-CoV is reflective of delayed clearance among patients with COVID-19 taking corticosteroids. Moreover, a more recent study on CARDS patients by Fang and colleagues found that low-dose corticosteroid therapy did not delay SARS-CoV-2 viral clearance [139]. Studies have shown reduced mortality and shorter length of stay among severe ARDS cases administered high dose methylprednisolone [87,140,141].
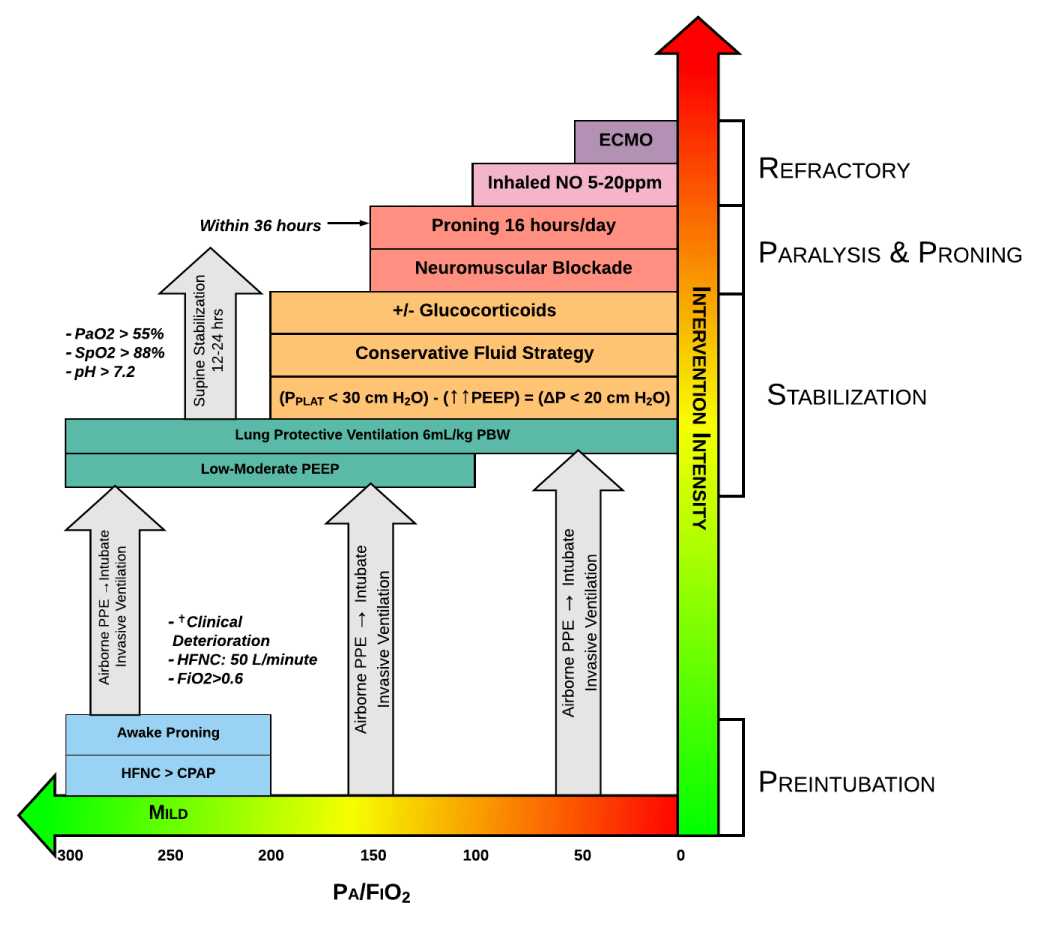
Proning: ARDS is associated with unevenly distributed inflammation, edema, and atelectasis that results in a greater transpulmonary pressure gradient that more sharply increases from dependent to nondependent areas of the lung. In the prone position, the pleural pressure is less negative in the nondependent lung and less positive in the dependent lung, reducing the pleural pressure gradient and by extension the transpulmonary pressure gradient. The result is more homogenized alveolar ventilation and less V/Q mismatch, decreasing the intra-pulmonary shunt fraction. Proning should be initiated within 12-24 hours if patients continue to demonstrate P: F ratios < 150 mmHg as reported in the PROSEVA trial (Figure 4) [142].
- Zhang J, Sun M, Zhang W. Predictive factors of transmission During endotracheal intubation for Coronavirus DISEASE 2019 (COVID-19. SSRN Electron J. Epub ahead of print 2020.
- Breazzano MP, Shen J, Abdelhakim AH. Resident physician exposure to novel coronavirus (2019-nCoV, SARS-CoV-2) within New York City during exponential phase of COVID-19 pandemic: Report of the New York City Residency Program Directors COVID-19 Research Group. Preprint, Epidemiology. Epub ahead of print 28 April 2020.
- Wang Q, Hu Z. Successful recovery of severe COVID-19 with cytokine storm treating with extracorporeal blood purification. Int J Infect Dis. 2020 Jul;96:618-620. doi: 10.1016/j.ijid.2020.05.065. Epub 2020 May 26. PMID: 32470601; PMCID: PMC7255259.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395(10229):1033-1034. doi: 10.1016/S0140-6736(20)30628-0. Epub 2020 Mar 16. PMID: 32192578; PMCID: PMC7270045.
- Poor HD, Ventetuolo CE, Tolbert T, Chun G, Serrao G, Zeidman A, Dangayach NS, Olin J, Kohli-Seth R, Powell CA. COVID-19 Critical Illness Pathophysiology Driven by Diffuse Pulmonary Thrombi and Pulmonary Endothelial Dysfunction Responsive to Thrombolysis. medRxiv [Preprint]. 2020 Apr 21:2020.04.17.20057125. doi: 10.1101/2020.04.17.20057125. Update in: Clin Transl Med. 2020 May 13;: PMID: 32511632; PMCID: PMC7276060.
- Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016 Jun;24(6):490-502. doi: 10.1016/j.tim.2016.03.003. Epub 2016 Mar 21. PMID: 27012512; PMCID: PMC7125511.
- Wilder-Smith A, Teleman MD, Heng BH, Earnest A, Ling AE, Leo YS. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis. 2005 Jul;11(7):1142-5. doi: 10.3201/eid1107.041165. PMID: 16022801; PMCID: PMC3371799.
- Basu A. Estimating The Infection Fatality Rate Among Symptomatic COVID-19 Cases In The United States. Health Aff (Millwood). 2020 Jul;39(7):1229-1236. doi: 10.1377/hlthaff.2020.00455. Epub 2020 May 7. PMID: 32379502.
- Jung SM, Akhmetzhanov AR, Hayashi K, Linton NM, Yang Y, Yuan B, Kobayashi T, Kinoshita R, Nishiura H. Real-Time Estimation of the Risk of Death from Novel Coronavirus (COVID-19) Infection: Inference Using Exported Cases. J Clin Med. 2020 Feb 14;9(2):523. doi: 10.3390/jcm9020523. PMID: 32075152; PMCID: PMC7074479.
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020 Feb 10;41(2):145-151. Chinese. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. PMID: 32064853.
- Gorbalenya AE, Baker SC, Baric RS. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group.
- Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Duu Wen S, Phua GC, Chan KS, Haja Mohideen S. Rapid Progression to Acute Respiratory Distress Syndrome: Review of Current Understanding of Critical Illness from Coronavirus Disease 2019 (COVID-19) Infection. Ann Acad Med Singap. 2020 Mar 16;49(3):108-118. PMID: 32200400.
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239-1242. doi: 10.1001/jama.2020.2648. PMID: 32091533.
- CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 10;69(14):422-426. doi: 10.15585/mmwr.mm6914e4. PMID: 32271728; PMCID: PMC7147903.
- Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, Zhan LY, Jia Y, Zhang L, Liu D, Xia ZY, Xia Z. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020 Apr 5;21:100331. doi: 10.1016/j.eclinm.2020.100331. PMID: 32292899; PMCID: PMC7128617.
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 Apr 28;323(16):1574-1581. doi: 10.1001/jama.2020.5394. Erratum in: JAMA. 2021 May 25;325(20):2120. PMID: 32250385; PMCID: PMC7136855.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. doi: 10.1001/jama.2020.1585. Erratum in: JAMA. 2021 Mar 16;325(11):1113. PMID: 32031570; PMCID: PMC7042881.
- Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, Yu X, Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020 Apr;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. PMID: 32345579; PMCID: PMC7222577.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. PMID: 32171076; PMCID: PMC7270627.
- Shenoy A, Ismaily M, Bajaj M. Diabetes and covid-19: a global health challenge. BMJ Open Diabetes Res Care. 2020 Apr;8(1):e001450. doi: 10.1136/bmjdrc-2020-001450. PMID: 32345580; PMCID: PMC7222578.
- Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Li Z, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020 Jun;10(6):783-791. doi: 10.1158/2159-8290.CD-20-0422. Epub 2020 Apr 28. PMID: 32345594; PMCID: PMC7309152.
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020 Mar;21(3):335-337. doi: 10.1016/S1470-2045(20)30096-6. Epub 2020 Feb 14. PMID: 32066541; PMCID: PMC7159000.
- Bajema KL, Oster AM, McGovern OL, Lindstrom S, Stenger MR, Anderson TC, Isenhour C, Clarke KR, Evans ME, Chu VT, Biggs HM, Kirking HL, Gerber SI, Hall AJ, Fry AM, Oliver SE; 2019-nCoV Persons Under Investigation Team; 2019-CoV Persons Under Investigation Team. Persons Evaluated for 2019 Novel Coronavirus - United States, January 2020. MMWR Morb Mortal Wkly Rep. 2020 Feb 14;69(6):166-170. doi: 10.15585/mmwr.mm6906e1. PMID: 32053579; PMCID: PMC7017962.
- CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 3;69(13):382-386. doi: 10.15585/mmwr.mm6913e2. PMID: 32240123; PMCID: PMC7119513.
- Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 17;69(15):458-464. doi: 10.15585/mmwr.mm6915e3. PMID: 32298251; PMCID: PMC7755063.
- Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, Ring L, Landau R, Purisch S, Friedman AM, Fuchs K, Sutton D, Andrikopoulou M, Rupley D, Sheen JJ, Aubey J, Zork N, Moroz L, Mourad M, Wapner R, Simpson LL, D'Alton ME, Goffman D. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 May;2(2):100118. doi: 10.1016/j.ajogmf.2020.100118. Epub 2020 Apr 9. PMID: 32292903; PMCID: PMC7144599.
- Kimball A, Hatfield KM, Arons M, James A, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Chisty Z, Bell JM, Methner M, Harney J, Jacobs JR, Carlson CM, McLaughlin HP, Stone N, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Russell D, Hiatt B, Gant J, Duchin JS, Clark TA, Honein MA, Reddy SC, Jernigan JA; Public Health – Seattle & King County; CDC COVID-19 Investigation Team. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 3;69(13):377-381. doi: 10.15585/mmwr.mm6913e1. PMID: 32240128; PMCID: PMC7119514.
- Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020 Mar;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. Erratum in: Euro Surveill. 2020 Jun;25(22): PMID: 32183930; PMCID: PMC7078829.
- Song H, Xiao J, Qiu J. A considerable proportion of individuals with asymptomatic SARS-CoV-2 infection in Tibetan population. Preprint, Infectious Diseases (except HIV/AIDS). Epub ahead of print 30 March 2020.
- Long DR, Sunshine JE, Van Cleve W. Considerations for Assessing Risk of Provider Exposure to SARS-CoV-2 after a Negative Test. Anesthesiology. 2020 Aug;133(2):483-485. doi: 10.1097/ALN.0000000000003392. PMID: 32404774; PMCID: PMC7255396.
- Yuan J, Kou S, Liang Y, Zeng J, Pan Y, Liu L. Polymerase Chain Reaction Assays Reverted to Positive in 25 Discharged Patients With COVID-19. Clin Infect Dis. 2020 Nov 19;71(16):2230-2232. doi: 10.1093/cid/ciaa398. PMID: 32266381; PMCID: PMC7184423.
- Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D. False-Negative Results of Initial RT-PCR Assays for COVID-19: A Systemic Review. Preprint, Infectious Diseases (except HIV/AIDS). Epub ahead of print 21 April 2020.
- Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020 Aug;296(2):E115-E117. doi: 10.1148/radiol.2020200432. Epub 2020 Feb 19. PMID: 32073353; PMCID: PMC7233365.
- Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020 May;20(5):453-454. doi: 10.1080/14737159.2020.1757437. Epub 2020 Apr 22. PMID: 32297805; PMCID: PMC7189409.
- Wikramaratna P, Paton RS, Ghafari M. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. Preprint, Epidemiology. Epub ahead of print 7 April 2020. DOI: 10.1101/2020.04.05.20053355.
- Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. Epub ahead of print 2020.
- Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020 Aug;296(2):E41-E45. doi: 10.1148/radiol.2020200343. Epub 2020 Feb 12. PMID: 32049601; PMCID: PMC7233363.
- Tordjman M, Mekki A, Mali RD, Saab I, Chassagnon G, Guillo E, Burns R, Eshagh D, Beaune S, Madelin G, Bessis S, Feydy A, Mihoubi F, Doumenc B, Mouthon L, Carlier RY, Drapé JL, Revel MP. Pre-test probability for SARS-Cov-2-related infection score: The PARIS score. PLoS One. 2020 Dec 17;15(12):e0243342. doi: 10.1371/journal.pone.0243342. PMID: 33332360; PMCID: PMC7745977.
- McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, Lewis J, Baer A, Kawakami V, Lukoff MD, Ferro J, Brostrom-Smith C, Rea TD, Sayre MR, Riedo FX, Russell D, Hiatt B, Montgomery P, Rao AK, Chow EJ, Tobolowsky F, Hughes MJ, Bardossy AC, Oakley LP, Jacobs JR, Stone ND, Reddy SC, Jernigan JA, Honein MA, Clark TA, Duchin JS; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020 May 21;382(21):2005-2011. doi: 10.1056/NEJMoa2005412. Epub 2020 Mar 27. PMID: 32220208; PMCID: PMC7121761.
- Lee TH, Junhao Lin R, Lin RTP, Barkham T, Rao P, Leo YS, Chien Lye D, Young B; National Centre for Infectious Diseases COVID-19 Outbreak Research Team. Testing for SARS-CoV-2: Can We Stop at 2? Clin Infect Dis. 2020 Nov 19;71(16):2246-2248. doi: 10.1093/cid/ciaa459. PMID: 32306042; PMCID: PMC7188180.
- Ashokka B, Loh MH, Tan CH, Su LL, Young BE, Lye DC, Biswas A, Illanes SE, Choolani M. Care of the pregnant woman with coronavirus disease 2019 in labor and delivery: anesthesia, emergency cesarean delivery, differential diagnosis in the acutely ill parturient, care of the newborn, and protection of the healthcare personnel. Am J Obstet Gynecol. 2020 Jul;223(1):66-74.e3. doi: 10.1016/j.ajog.2020.04.005. Epub 2020 Apr 10. PMID: 32283073; PMCID: PMC7151436.
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020 May 26;323(20):2052-2059. doi: 10.1001/jama.2020.6775. Erratum in: JAMA. 2020 May 26;323(20):2098. PMID: 32320003; PMCID: PMC7177629.
- Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann Intern Med. 2020 Aug 18;173(4):262-267. doi: 10.7326/M20-1495. Epub 2020 May 13. PMID: 32422057; PMCID: PMC7240870.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020 Mar 19;382(12):1177-1179. doi: 10.1056/NEJMc2001737. Epub 2020 Feb 19. PMID: 32074444; PMCID: PMC7121626.
- Hanson KE, Caliendo AM, Arias CA. Infectious Diseases Society of America G8uidelines on the Diagnosis of COVID-19. 2020; 70.
- Au Yong P, Chen X. Reducing droplet spread during airway manipulation: lessons from the COVID-19 pandemic in Singapore. Br J Anaesth.
- Xiangdong C, You S, Shanglong Y. Perioperative Care Provider’s Considerations in Managing Patients with the COVID-19 Infections. Transl Perioper Pain Med; 7. Epub ahead of print 15 February 2020.
- Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020 Apr 28;323(16):1610-1612. doi: 10.1001/jama.2020.3227. PMID: 32129805; PMCID: PMC7057172.
- Kaye K, Paprottka F, Escudero R, Casabona G, Montes J, Fakin R, Moke L, Stasch T, Richter D, Benito-Ruiz J. Elective, Non-urgent Procedures and Aesthetic Surgery in the Wake of SARS-COVID-19: Considerations Regarding Safety, Feasibility and Impact on Clinical Management. Aesthetic Plast Surg. 2020 Jun;44(3):1014-1042. doi: 10.1007/s00266-020-01752-9. Epub 2020 May 14. PMID: 32410196; PMCID: PMC7224128.
- Dexter F, Parra MC, Brown JR, Loftus RW. Perioperative COVID-19 Defense: An Evidence-Based Approach for Optimization of Infection Control and Operating Room Management. Anesth Analg. 2020 Jul;131(1):37-42. doi: 10.1213/ANE.0000000000004829. PMID: 32217947; PMCID: PMC7172574.
- Bowdle A, Munoz-Price LS. Preventing Infection of Patients and Healthcare Workers Should Be the New Normal in the Era of Novel Coronavirus Epidemics. Anesthesiology. 2020 Jun;132(6):1292-1295. doi: 10.1097/ALN.0000000000003295. PMID: 32195701; PMCID: PMC7155906.
- Loftus RW, Koff MD, Brown JR, Patel HM, Jensen JT, Reddy S, Ruoff KL, Heard SO, Yeager MP, Dodds TM. The epidemiology of Staphylococcus aureus transmission in the anesthesia work area. Anesth Analg. 2015 Apr;120(4):807-18. doi: 10.1213/ANE.0b013e3182a8c16a. PMID: 24937345.
- Munoz-Price LS, Fajardo-Aquino Y, Arheart KL. Ultraviolet powder versus ultraviolet gel for assessing environmental cleaning. Infect Control Hosp Epidemiol. 2012 Feb;33(2):192-5. doi: 10.1086/663713. Epub 2011 Dec 27. PMID: 22227990.
- Biddle C, Robinson K, Pike B, Kammerman M, Gay B, Verhulst B. Quantifying the rambunctious journey of the anesthesia provider's hands during simulated, routine care. Am J Infect Control. 2016 Aug 1;44(8):873-8. doi: 10.1016/j.ajic.2016.02.014. Epub 2016 Mar 31. PMID: 27040571.
- Jaffe G, Moriber N. Use of a Double Gloving Technique to Decrease Cross-Contamination by Anesthesia Providers. AANA J. 2019 Aug;87(4):307-312. PMID: 31587715.
- Biddle CJ, George-Gay B, Prasanna P, Hill EM, Davis TC, Verhulst B. Assessing a Novel Method to Reduce Anesthesia Machine Contamination: A Prospective, Observational Trial. Can J Infect Dis Med Microbiol. 2018 Feb 4;2018:1905360. doi: 10.1155/2018/1905360. PMID: 29623137; PMCID: PMC5829426.
- Munoz-Price LS, Bowdle A, Johnston BL, Bearman G, Camins BC, Dellinger EP, Geisz-Everson MA, Holzmann-Pazgal G, Murthy R, Pegues D, Prielipp RC, Rubin ZA, Schaffzin J, Yokoe D, Birnbach DJ. Infection prevention in the operating room anesthesia work area. Infect Control Hosp Epidemiol. 2019 Jan;40(1):1-17. doi: 10.1017/ice.2018.303. Epub 2018 Dec 11. Erratum in: Infect Control Hosp Epidemiol. 2019 Apr;40(4):500. PMID: 30526699.
- Hui DS, Chow BK, Chu L, Ng SS, Lee N, Gin T, Chan MT. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7(12):e50845. doi: 10.1371/journal.pone.0050845. Epub 2012 Dec 5. PMID: 23239991; PMCID: PMC3516468.
- Lee CCM, Thampi S, Lewin B, Lim TJD, Rippin B, Wong WH, Agrawal RV. Battling COVID-19: critical care and peri-operative healthcare resource management strategies in a tertiary academic medical centre in Singapore. Anaesthesia. 2020 Jul;75(7):861-871. doi: 10.1111/anae.15074. Epub 2020 May 3. PMID: 32267963; PMCID: PMC7262214.
- Guan W, Ni Z, Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020; 382: 1708–1720.
- Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. Epub ahead of print 2020.
- Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PloS One 2020; 15: e0233147.
- Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020 Apr 28;323(16):1612-1614. doi: 10.1001/jama.2020.4326. PMID: 32191259; PMCID: PMC7082763.
- Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, Stachel A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis. 2020 Jul 28;71(15):896-897. doi: 10.1093/cid/ciaa415. PMID: 32271368; PMCID: PMC7184372.
- Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020 Jul;108:154244. doi: 10.1016/j.metabol.2020.154244. Epub 2020 Apr 19. PMID: 32320741; PMCID: PMC7166301.
- Li G, Li H, Lu J. No adequate evidence indicating hypertension as an independent risk factor for COVID-19 severity. Clin Res Cardiol. Epub ahead of print 23 April 2020.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30;: PMID: 31986264; PMCID: PMC7159299.
- Bermejo-Martin JF, Almansa R, Menéndez R, Mendez R, Kelvin DJ, Torres A. Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection. J Infect. 2020 May;80(5):e23-e24. doi: 10.1016/j.jinf.2020.02.029. Epub 2020 Mar 5. PMID: 32145214; PMCID: PMC7133663.
- Liu M, Han S, Liao Q. Outcomes and Prognostic Factors in 70 Non-Survivors and 595 Survivors with COVID-19 in Wuhan, China. SSRN Electron J. Epub ahead of print 2020.
- Mei Q, Wang AY, Bryant A, Yang Y, Li M, Wang F, Du S, Kurts C, Wu P, Ma K, Wu L, Chen H, Luo J, Li Y, Hu G, Yuan X, Li J. Survival Factors and Metabolic Pathogenesis in Elderly Patients (≥65) With COVID-19: A Multi-Center Study. Front Med (Lausanne). 2021 Jan 7;7:595503. doi: 10.3389/fmed.2020.595503. PMID: 33585504; PMCID: PMC7873923.
- Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020 May 24;8:36. doi: 10.1186/s40560-020-00453-4. PMID: 32483488; PMCID: PMC7245646.
- Danzi GB, Loffi M, Galeazzi G. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. Epub ahead of print 2020.
- Llitjos JF, Leclerc M, Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. Epub ahead of print 2020.
- Robba C, Battaglini D, Ball L, Patroniti N, Loconte M, Brunetti I, Vena A, Giacobbe DR, Bassetti M, Rocco PRM, Pelosi P. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir Physiol Neurobiol. 2020 Aug;279:103455. doi: 10.1016/j.resp.2020.103455. Epub 2020 May 11. PMID: 32437877; PMCID: PMC7211757.
- Remy KE, Verhoef PA, Malone JR, Ruppe MD, Kaselitz TB, Lodeserto F, Hirshberg EL, Slonim A, Dezfulian C. Caring for Critically Ill Adults With Coronavirus Disease 2019 in a PICU: Recommendations by Dual Trained Intensivists. Pediatr Crit Care Med. 2020 Jul;21(7):607-619. doi: 10.1097/PCC.0000000000002429. PMID: 32420720; PMCID: PMC7331597.
- Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020 Apr 23;382(17):e38. doi: 10.1056/NEJMc2007575. Epub 2020 Apr 8. PMID: 32268022; PMCID: PMC7161262.
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 May;18(5):1094-1099. doi: 10.1111/jth.14817. Epub 2020 Apr 27. PMID: 32220112.
- Shi S, Qin M, Shen B. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. Epub ahead of print 2020.
- Chen T, Wu D, Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368 1091.
- Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 May;18(5):1023-1026. doi: 10.1111/jth.14810. Epub 2020 Apr 27. PMID: 32338827.
- Arachchillage DRJ, Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 May;18(5):1233-1234. doi: 10.1111/jth.14820. PMID: 32291954; PMCID: PMC7262191.
- Cardiology AC. Troponin and BNP Use in COVID-19. Cardiology.
- Chapman AR, Bularga A, Mills NL. High-Sensitivity Cardiac Troponin Can Be an Ally in the Fight Against COVID-19. Circulation. 2020 Jun 2;141(22):1733-1735. doi: 10.1161/CIRCULATIONAHA.120.047008. Epub 2020 Apr 6. PMID: 32251612.
- Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ, Zhu Q, Hu M, Li XY, Li Y, Liang LR, Tong ZH, Sun B, Peng P, Shi HZ. Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1. Chest. 2020 Jul;158(1):195-205. doi: 10.1016/j.chest.2020.03.032. Epub 2020 Mar 26. PMID: 32224074; PMCID: PMC7151343.
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8(5):475-481. doi: 10.1016/S2213-2600(20)30079-5. Epub 2020 Feb 24. Erratum in: Lancet Respir Med. 2020 Apr;8(4):e26. PMID: 32105632; PMCID: PMC7102538.
- CDC COVID-19 Response Team. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020 Mar 27;69(12):343-346. doi: 10.15585/mmwr.mm6912e2. PMID: 32214079; PMCID: PMC7725513.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934-943. doi: 10.1001/jamainternmed.2020.0994. Erratum in: JAMA Intern Med. 2020 Jul 1;180(7):1031. PMID: 32167524; PMCID: PMC7070509.
- Yoshida Y, Takeda S, Akada S, Hongo T, Tanaka K, Sakamoto A. Factors predicting successful noninvasive ventilation in acute lung injury. J Anesth. 2008;22(3):201-6. doi: 10.1007/s00540-008-0637-z. Epub 2008 Aug 7. PMID: 18685924.
- Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019 Sep;85(9):1014-1023. doi: 10.23736/S0375-9393.19.13418-9. Epub 2019 Mar 12. PMID: 30871304.
- Kallet RH, Alonso JA, Luce JM, Matthay MA. Exacerbation of acute pulmonary edema during assisted mechanical ventilation using a low-tidal volume, lung-protective ventilator strategy. Chest. 1999 Dec;116(6):1826-32. doi: 10.1378/chest.116.6.1826. PMID: 10593817.
- Jiang TX, Reid WD, Belcastro A, Road JD. Load dependence of secondary diaphragm inflammation and injury after acute inspiratory loading. Am J Respir Crit Care Med. 1998 Jan;157(1):230-6. doi: 10.1164/ajrccm.157.1.9702051. PMID: 9445304.
- Ye L, Wang J, Xu X, Song Y, Jiang J. Noninvasive ventilation on mortality of acute respiratory distress syndrome. J Phys Ther Sci. 2016 Aug;28(8):2284-8. doi: 10.1589/jpts.28.2284. Epub 2016 Aug 31. PMID: 27630415; PMCID: PMC5011579.
- Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care. 2010 Dec;55(12):1653-60. PMID: 21122173.
- Liao X, Chen H, Wang B. Critical Care for Severe COVID-19: A Population-based Study from a Province with Low Case-fatality Rate in China. Preprint, Intensive Care and Critical Care Medicine. Epub ahead of print 27 March 2020. DOI: 10.1101/2020.03.22.20041277.
- Antonelli M, Conti G, Esquinas A, Montini L, Maggiore SM, Bello G, Rocco M, Maviglia R, Pennisi MA, Gonzalez-Diaz G, Meduri GU. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007 Jan;35(1):18-25. doi: 10.1097/01.CCM.0000251821.44259.F3. PMID: 17133177.
- Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, Tubiolo D, Tagliabue P, Zanella A, Grasselli G, Pesenti A. Potential for Lung Recruitment and Ventilation-Perfusion Mismatch in Patients With the Acute Respiratory Distress Syndrome From Coronavirus Disease 2019. Crit Care Med. 2020 Aug;48(8):1129-1134. doi: 10.1097/CCM.0000000000004386. PMID: 32697482; PMCID: PMC7188034.
- Sim MA, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008 Sep;63(9):938-40. doi: 10.1111/j.1365-2044.2008.05536.x. Epub 2008 Jun 6. PMID: 18540928.
- Drake MG. High-Flow Nasal Cannula Oxygen in Adults: An Evidence-based Assessment. Ann Am Thorac Soc. 2018 Feb;15(2):145-155. doi: 10.1513/AnnalsATS.201707-548FR. PMID: 29144160.
- Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010 Apr;55(4):408-13. PMID: 20406507.
- Sasaki H, Yamakage M, Iwasaki S, Mizuuchi M, Namiki A. Design of oxygen delivery systems influences both effectiveness and comfort in adult volunteers. Can J Anaesth. 2003 Dec;50(10):1052-5. doi: 10.1007/BF03018373. PMID: 14656787.
- Caputo ND, Strayer RJ, Levitan R. Early Self-Proning in Awake, Non-intubated Patients in the Emergency Department: A Single ED's Experience During the COVID-19 Pandemic. Acad Emerg Med. 2020 May;27(5):375-378. doi: 10.1111/acem.13994. PMID: 32320506; PMCID: PMC7264594.
- Schwabbauer N, Berg B, Blumenstock G, Haap M, Hetzel J, Riessen R. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol. 2014 Aug 7;14:66. doi: 10.1186/1471-2253-14-66. PMID: 25110463; PMCID: PMC4126617.
- Salah B, Dinh Xuan AT, Fouilladieu JL, Lockhart A, Regnard J. Nasal mucociliary transport in healthy subjects is slower when breathing dry air. Eur Respir J. 1988 Oct;1(9):852-5. PMID: 3229484.
- Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009 Oct;103(10):1400-5. doi: 10.1016/j.rmed.2009.04.007. Epub 2009 May 21. PMID: 19467849.
- Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020 Mar 30;10(1):37. doi: 10.1186/s13613-020-00653-z. PMID: 32232685; PMCID: PMC7104710.
- Zheng Y, Sun LJ, Xu M, Pan J, Zhang YT, Fang XL, Fang Q, Cai HL. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B. 2020 May;21(5):378-387. doi: 10.1631/jzus.B2000174. PMID: 32425003; PMCID: PMC7238397.
- Jiang LG, LeBaron J, Bodnar D, Caputo ND, Chang BP, Chiricolo G, Flores S, Kenny J, Melville L, Sayan OR, Sharma M, Shemesh A, Suh E, Farmer B. Conscious Proning: An Introduction of a Proning Protocol for Nonintubated, Awake, Hypoxic Emergency Department COVID-19 Patients. Acad Emerg Med. 2020 Jul;27(7):566-569. doi: 10.1111/acem.14035. Epub 2020 Jun 23. PMID: 32462708; PMCID: PMC7283629.
- Ng Z, Tay WC, Ho CHB. Awake prone positioning for non-intubated oxygen dependent COVID-19 pneumonia patients. Eur Respir J. 2020 Jul 23;56(1):2001198. doi: 10.1183/13993003.01198-2020. PMID: 32457195; PMCID: PMC7251246.
- Kearsley R. Intubation boxes for managing the airway in patients with COVID-19. Anaesthesia. 2020 Jul;75(7):969. doi: 10.1111/anae.15081. Epub 2020 Apr 24. PMID: 32311772; PMCID: PMC7264779.
- Asenjo JF. Safer intubation and extubation of patients with COVID-19. Can J Anaesth. 2020 Sep;67(9):1276-1278. doi: 10.1007/s12630-020-01666-9. Epub 2020 Apr 22. PMID: 32323101; PMCID: PMC7175818.
- Brewster DJ, Chrimes N, Do TB, Fraser K, Groombridge CJ, Higgs A, Humar MJ, Leeuwenburg TJ, McGloughlin S, Newman FG, Nickson CP, Rehak A, Vokes D, Gatward JJ. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020 Jun;212(10):472-481. doi: 10.5694/mja2.50598. Epub 2020 May 1. Erratum in: Med J Aust. 2020 Oct;213(7):312. PMID: 32356900; PMCID: PMC7267410.
- Zuo MZ, Huang YG, Ma WH, Xue ZG, Zhang JQ, Gong YH, Che L; Chinese Society of Anesthesiology Task Force on Airway Management, Airway Management Chinese Society of Anesthesiology Task Force on. Expert Recommendations for Tracheal Intubation in Critically ill Patients with Noval Coronavirus Disease 2019. Chin Med Sci J. 2020 Feb 27;35(2):105–9. doi: 10.24920/003724. Epub ahead of print. PMID: 32102726; PMCID: PMC7367670.
- Yao W, Wang T, Jiang B, Gao F, Wang L, Zheng H, Xiao W, Yao S, Mei W, Chen X, Luo A, Sun L, Cook T, Behringer E, Huitink JM, Wong DT, Lane-Fall M, McNarry AF, McGuire B, Higgs A, Shah A, Patel A, Zuo M, Ma W, Xue Z, Zhang LM, Li W, Wang Y, Hagberg C, O'Sullivan EP, Fleisher LA, Wei H; collaborators. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020 Jul;125(1):e28-e37. doi: 10.1016/j.bja.2020.03.026. Epub 2020 Apr 10. PMID: 32312571; PMCID: PMC7151238.
- Brown S, Verma S, Lean A, Patrao F. One Size Does Not Fit All: How to Rapidly Deploy Intubation Practice Changes in a Pediatric Hospital During the COVID-19 Pandemic. Anesth Analg. 2020 Jul;131(1):e48-e50. doi: 10.1213/ANE.0000000000004907. PMID: 32332294; PMCID: PMC7188051.
- Mayo PH, Hegde A, Eisen LA, Kory P, Doelken P. A program to improve the quality of emergency endotracheal intubation. J Intensive Care Med. 2011 Jan-Feb;26(1):50-6. doi: 10.1177/0885066610384070. PMID: 21262753.
- Meng L, Qiu H, Wan L. Intubation and Ventilation amid the COVID-19 Outbreak. Anesthesiology. Epub ahead of print 8 April 2020.
- Orser BA. Recommendations for Endotracheal Intubation of COVID-19 Patients. Anesth Analg. 2020 May;130(5):1109-1110. doi: 10.1213/ANE.0000000000004803. PMID: 32209810; PMCID: PMC7172572.
- Mosier JM, Hypes CD, Sakles JC. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med. 2017 Feb;43(2):226-228. doi: 10.1007/s00134-016-4426-0. Epub 2016 Jun 24. PMID: 27342820.
- Oliveira J E Silva L, Cabrera D, Barrionuevo P, Johnson RL, Erwin PJ, Murad MH, Bellolio MF. Effectiveness of Apneic Oxygenation During Intubation: A Systematic Review and Meta-Analysis. Ann Emerg Med. 2017 Oct;70(4):483-494.e11. doi: 10.1016/j.annemergmed.2017.05.001. Epub 2017 Jul 14. PMID: 28712606.
- Papazian L, Corley A, Hess D, Fraser JF, Frat JP, Guitton C, Jaber S, Maggiore SM, Nava S, Rello J, Ricard JD, Stephan F, Trisolini R, Azoulay E. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016 Sep;42(9):1336-49. doi: 10.1007/s00134-016-4277-8. Epub 2016 Mar 11. PMID: 26969671.
- Whittle JS, Pavlov I, Sacchetti AD, Atwood C, Rosenberg MS. Respiratory support for adult patients with COVID-19. J Am Coll Emerg Physicians Open. 2020 Apr 13;1(2):95–101. doi: 10.1002/emp2.12071. Epub ahead of print. PMID: 32427171; PMCID: PMC7228246.
- Vourc'h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau PY, Asehnoune K, Mercat A, Reignier J, Jaber S, Prat G, Roquilly A, Brule N, Villers D, Bretonniere C, Guitton C. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015 Sep;41(9):1538-48. doi: 10.1007/s00134-015-3796-z. Epub 2015 Apr 14. PMID: 25869405.
- Gattinoni L, Marini JJ, Collino F, Maiolo G, Rapetti F, Tonetti T, Vasques F, Quintel M. The future of mechanical ventilation: lessons from the present and the past. Crit Care. 2017 Jul 12;21(1):183. doi: 10.1186/s13054-017-1750-x. PMID: 28701178; PMCID: PMC5508674.
- Al-Qadi MO, Erb CT. Hyperventilation (Not Ventilator)-induced Lung Injury. Am J Respir Crit Care Med. 2017 Oct 1;196(7):936-937. doi: 10.1164/rccm.201703-0594LE. PMID: 28460178.
- Gattinoni L, Protti A, Caironi P, Carlesso E. Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med. 2010 Oct;38(10 Suppl):S539-48. doi: 10.1097/CCM.0b013e3181f1fcf7. PMID: 21164395.
- Williams EC, Motta-Ribeiro GC, Vidal Melo MF. Driving Pressure and Transpulmonary Pressure: How Do We Guide Safe Mechanical Ventilation? Anesthesiology. 2019 Jul;131(1):155-163. doi: 10.1097/ALN.0000000000002731. PMID: 31094753; PMCID: PMC6639048.
- Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015 Feb 19;372(8):747-55. doi: 10.1056/NEJMsa1410639. PMID: 25693014.
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998 Feb 5;338(6):347-54. doi: 10.1056/NEJM199802053380602. PMID: 9449727.
- Aoyama H, Yamada Y, Fan E. The future of driving pressure: a primary goal for mechanical ventilation? J Intensive Care. 2018 Oct 4;6:64. doi: 10.1186/s40560-018-0334-4. PMID: 30305906; PMCID: PMC6172758.
- Aoyama H, Pettenuzzo T, Aoyama K, Pinto R, Englesakis M, Fan E. Association of Driving Pressure With Mortality Among Ventilated Patients With Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Crit Care Med. 2018 Feb;46(2):300-306. doi: 10.1097/CCM.0000000000002838. PMID: 29135500.
- Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020 Apr 16;24(1):154. doi: 10.1186/s13054-020-02880-z. PMID: 32299472; PMCID: PMC7160817.
- Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 Jun;46(6):1099-1102. doi: 10.1007/s00134-020-06033-2. Epub 2020 Apr 14. PMID: 32291463; PMCID: PMC7154064.
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020 May 15;201(10):1299-1300. doi: 10.1164/rccm.202003-0817LE. PMID: 32228035; PMCID: PMC7233352.
- Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020 May;46(5):854-887. doi: 10.1007/s00134-020-06022-5. Epub 2020 Mar 28. PMID: 32222812; PMCID: PMC7101866.
- Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006 Sep;3(9):e343. doi: 10.1371/journal.pmed.0030343. PMID: 16968120; PMCID: PMC1564166.
- Lansbury L, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2019 Feb 24;2(2):CD010406. doi: 10.1002/14651858.CD010406.pub3. PMID: 30798570; PMCID: PMC6387789.
- Delaney JW, Pinto R, Long J, Lamontagne F, Adhikari NK, Kumar A, Marshall JC, Cook DJ, Jouvet P, Ferguson ND, Griesdale D, Burry LD, Burns KE, Hutchison J, Mehta S, Menon K, Fowler RA; Canadian Critical Care Trials Group H1N1 Collaborative. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care. 2016 Mar 30;20:75. doi: 10.1186/s13054-016-1230-8. PMID: 27036638; PMCID: PMC4818504.
- Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA; Saudi Critical Care Trial Group. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018 Mar 15;197(6):757-767. doi: 10.1164/rccm.201706-1172OC. PMID: 29161116.
- Fang X, Mei Q, Yang T. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 2020; S0163445320301687.
- Qin YY, Zhou YH, Lu YQ, Sun F, Yang S, Harypursat V, Chen YK. Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial. Chin Med J (Engl). 2020 May 5;133(9):1080-1086. doi: 10.1097/CM9.0000000000000791. PMID: 32149773; PMCID: PMC7147272.
- Veronese N, Demurtas J, Yang L, Tonelli R, Barbagallo M, Lopalco P, Lagolio E, Celotto S, Pizzol D, Zou L, Tully MA, Ilie PC, Trott M, López-Sánchez GF, Smith L. Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature. Front Med (Lausanne). 2020 Apr 24;7:170. doi: 10.3389/fmed.2020.00170. PMID: 32391369; PMCID: PMC7193030.
- Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 Jun 6;368(23):2159-68. doi: 10.1056/NEJMoa1214103. Epub 2013 May 20. PMID: 23688302.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
