Journal of Cardiovascular Medicine and Cardiology
A Twin-Herbal Combination was found to have Cardio-Vascular Protection Effects after repeated RCTs on four groups of patients with Different Disease Background A Cross-Biostatical Study
William KF Cheng1, Ping Chook1, KS Woo2, Timothy CY Kwok3, Bryan PY Yan2, Johnny CM Koon1 and Ping-Chung Leung1,4*
2Division of Cardiology, Department of Medicine and Therapeutics, the Chinese University of Hong Kong, Hong Kong
3Division of Geriatrics, Department of Medicine and Therapeutics, the Chinese University of Hong Kong, Hong Kong
4State Key Laboratory of Research on Bioactivities and Clinical Applications of Medicinal Plants (The Chinese University of Hong Kong), Hong Kong
Cite this as
KF Cheng W, Chook P, Woo KS, CY Kwok T, Leung PC, et al. (2021) A Twin-Herbal Combination was found to have Cardio-Vascular Protection Effects after repeated RCTs on four groups of patients with Different Disease Background A Cross-Biostatical Study. J Cardiovasc Med Cardiol 8(3): 063-068. DOI: 10.17352/2455-2976.000172Background and Objective: To perform an integrated Cross-Biostatical analysis of four randomized controlled trials (RCTs) to assess the efficacy and safety of oral administration of D&G capsule (D&G) for the maintenance of cardiovascular health.
Methods: Data from the populations of four completed RCTs were pooled for this integrated Cross-Biostatical analysis. Mean changes from baseline in IMT and lipid profile were the main lines of attention; treatment differences were compared between D&G and Placebo groups. Primary outcome was Carotid artery intima-media thickness (IMT). Secondary outcome was the lipid profile. Analyses included primary and secondary outcome measures repeated at individual time points.
Results: The pooled RCT data involved 453 subjects (245 D&G; 208 Placebo); demographic characteristics were similar between treatment groups. Taking all 4 groups together, the mean improvements in IMT from baseline to week 24 were 0.9163 to 0.8939 in D&G group, and 0.8997 to 0.8964 in Placebo group. A statistically significant improvement after treatment was observed (P < 0.001) in D&G group, not in Placebo group.
Conclusion: The integrated analysis of the pooled biostatic data further confirmed the efficacy and safety of D&G in the vascular protection effects.
Introduction
Aging has become a worldwide public health problem. One of the most threatening concern is related to cardio-vascular health, as age related deteriorations of the heart and peripheral arteries progressively lead to circulatory hazards of varying severities [1]. In the most serious situations, coronary or cerebral obstruction could lead to cardiac or cerebral ischemic disasters. In more gradual affections, selective and regional morbidities could result [2,3].
As the risk factors leading to cardiovascular hazards have become better known and well defined, energetic explorations, including surgical and pharmaceutical means to prevent the hazards have started. Successful surgical stentings of obstructed arteries have led to preventive enlargements for even early obstructions. On the pharmaceutical side, it seems that statin control of vascular endothelial cholesterol deposits has remained the only reliable means.
Marzilli in 2012 discussed about the causative factors of atherosclerosis, which involves the complicated microenvironment of the endothelium of the artery. Apart from simple cholesterol deposits, other conditions like the inflammatory state; antioxidant and muscular relaxation states etc. are all contributing towards the progressive deterioration. A logical deduction from Marzilli’s “solar system theory” might be that the ideal form of prevention of arteriosclerosis is one that would maintain an multiple targets, all-round favorable microenvironment [4]. Marzilli’s logical deduction should have invited serious exploration on the use of medicinal agents that possibly provide the multiple cardiovascular protective effects [5].
Herbal medicine of traditional chinese medicine origin
Many medicinal herbs have been used in Traditional Chinese Medicine (TCM) as supportive agents to maintain cardiovascular health, which has been interpreted as “circulatory strength”. We had a thorough study of relevant literature and decided to select one simple twin-herb formula to be used as a standard supplement to maintain cardiovascular health. One component of the formula viz. salvia miltiorrhiza belongs to a most frequently used herb for the same purpose and in the past decades, have been studied extensively in the laboratory with proven effects on endothelial deposits, smooth muscle relaxation and anti-inflammation etc [6]. To match this most popular cardiovascular protective agent, a less popular medicinal herb best known for its anti-inflammatory effects was selected to make the twin formulae [7].
This innovative twin preparation was repeatedly tested in the laboratory to confirm its basic vascular protective effects before its application in clinical trials. Four groups of patients with different orientations of cardiovascular deterioration had been selected for separate clinical trials, using the same twin formula since 2001, at intervals of 3-4 years. The four selected groups of patients with different clinical problems included:
✓ Coronary artery disease [8,9]
✓ Diabetics’ with hypertension [10,11]
✓ Postmenopausal and syndrome [12] and
✓ Peripheral vascular disease [13].
Methods
Integrated analysis of the four clinical trials using pooled biostatics data
The design of the trials was kept standard and the only one serving team remained uniform throughout. The twin-herb formula in the fixed herbal ratio was prepared as capsules by a reputable manufacturer and the essential features of the Clinical Trials are presented as follows:
Design of trials: double-blind, randomized, placebo-controlled trials (RCTs) [14,15].
Objectives: to assess the efficacy and safety of a Herbal Formula D&G Capsule (twin-herb formula) for the maintenance of cardiovascular health.
Method: Trials were similar in design. Patient populations are shown in Table 1. Individual patient data were pooled; a repeated analysis of covariance was performed in the intent-to-treat (ITT) population.
Data illustrated that the four separate clinical trials on four different group of patients with different clinical backgounds did show good uniformity in age, dosage and duration of treatment.
All RCTs were approved by the Clinical Research Ethics Committee (CREC) and all patients provided written informed consent.
Study herbal formula
The raw herbs (ratio 7:3 by weight) of D&G capsule were prepared, authenticated, and extracted according to the standards of good manufacturing practice (GMP) at the Hong Kong Institute of Biotechnology (HKIB). The prepared D&G herbs were aqueous-extracted (herb–water ratio of 1:10) at 1000C two times for 60 minutes, and once for 30 minutes, sprayed dried at -660mm Hg and 50–600C, and the dried powder was encapsulated (500mg/capsule).
Patient populations
In total, 453 male and female patients, aged 36 to 85 years, were included in the integrated analysis; 100 patients age 40–70 years, with angiographically documented coronary artery disease (>50% reduction in luminal diameter CAD) in at least one vessel and stable angina status participated in Trial I; 90 patients with essential hypertension (SBP 160/90mmHg before treatment)participated in Trial II; 165 patients aged 45—65 years, menstruation stopped for more than 12 months, and fasting serum LDL ≥ 3.5 mmol/L, without taking hormones, statins ornutritional supplements participated in Trial III and 98 patients with known Peripheral Arterial Disease (PAD) participated in Trial IV.
Inclusion criteria
Angiographically documented coronary artery disease (Trial I)
Established essential hypertension (Trial II)
Postmenopausal women (Trial III)
Symptoms of intermittent claudications (Trial IV)
Exclusion criteria (for all groups)
Unstable angina
Renal insufficiency
Bleeding disorders
Hepatic or gastrointestinal diseases
Severe ischaemic ECG changes,
Fasting serum glucose > 7.0 mmol/L,
Creatinine > 100 umol/L, or
Triglyceride > 11.3 mmol/L
Maintenance on Warfarin
All patients were informed of the details of the entire study, including the purpose, procedures, data collection methods, test articles, risks, potential adverse effects, rescue measures, and confidentiality.
Outcome measures
Carotid artery Intima-Media Thickness (IMT) was taken as the primary outcome. Secondary outcome included the lipid profile (TC, TG, LDL and HDL).
Statistical analysis
Data were analyzed using the SPSS software (version 25.00, SPSS Inc.). IMT was the primary outcome measure of treatment efficacy and used for the pooled analysis. The efficacy analysiswas based on changes in IMT in the pooled population, using longitudinal analysis to incorporate information in the 4 trials. The Integrated Change from Baseline in IMT and lipid profiles were analyzed with an ANCOVA, with factor of age as a covariate. Changes within one treatment group were assessed by using paired t-test. Significance level was defined as ɑ= 0.05.
Results of pooled data analysis
Results from the four different clinical trials had been separately analyzed and every trial had been shown significant improvements in the primary and secondary outcomes. In order to gain more credibility of the trial results, we believe pooling the data in a cross biostatistical analysis would be indicated.
Analyses were based on individual patient data from Case Report Forms (CRFs), assembled into a data set for the pooled population. This provides a comparison that avoids some limitations inherent to clinical trials. Importantly, these analyses provide an overview of the clinical evidence related to the safety and effectiveness of the Herbal Formula D&G Capsule.
Demographics and disposition
The baseline (before treatment) status of the 4 different groups of patients showed their great similarity which supports the cross-data biostatistical analysis Table 2.
Analysis of overall efficacy
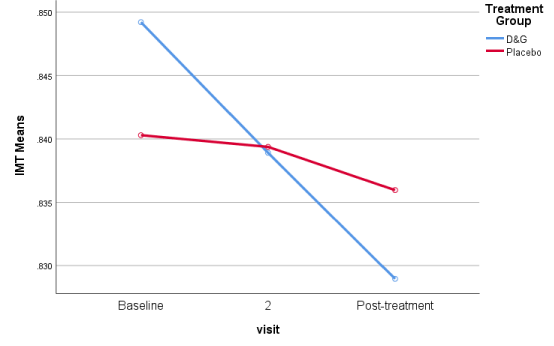
Results indicated that IMT was significantly improved in D&G group after treatment (p< 0.001), while, there no significant changes were observed in Placebo group after treatment (Table 3, Figure 1). ITM could be conveniently pooled to illustrate different groups with uniform responses.
Table 3 gives detailed data of changes in IMT in the pooled D&G and placebo groups. TC, HDL and LDL were also significantly decreased in both groups (p< 0.05), but TG in the placebo group was significantly increased at the end of study (p< 0.05).
There was no change of the blood sugar level in both groups throughout the study period.
Summation of IMT results of treatment (D&G) group and placebo group showing obvious superiority of treatment group.
Safety analysis
D&G herbal capsules were well tolerated in all groups. No significant adverse events were reported. Liver function and hematological profiles remained normal.
Discussion
Now that the risk factors leading to cardio-vascular hazards are thoroughly known, preventive measures accordingly have developed along a comprehensive direction. “Primary Level” prevention is the basic arrangement for patients known to be at high risk when cholesterol lowering therapy is the routine. “Secondary Prevention” refers to more targeted prophylaxis when a specific pathology like another coronary or cerebrovascular attack is to be expected. A more proactive preventive direction aims at the prevention of occurrence of the risk factors viz. hypertension, atherosclerosis, cholesterol and sugar levels, and is understood as “Primordial Prevention” [16-18].
Accumulation of statistical results in the past decades does indicate the successes of different forms of prevention [19,20]. However, it is clear that individual differences prevail: there are plenty of affected people not responding well to the preventive measures. More embarrassing disappointments occur when not only are the preventive measures against blood cholesterol not working perfectly but undesirable outcomes of coronary or cerebrovascular accidents still occur. Apart from more careful observations, apparently little can be offered to the disappointed group [21].
Genetic predisposition is widely accepted as the explanation to the failure of preventive measures with wide variations [22]. On the other hand, looking at the complexity of microenvironment leading to obstructive pathology of cardiovascular structures, one could also question: could the single target type of preventive treatment be over specific, good for one pathology (e.g. Cholesterol deposits) and yet not taking care of inflammation, spastic tendencies etc? [23,24].
Herbal medicine started in the old days as “Folk-forces”. The thousands of years of practice has offered no scientific explanations apart from clinical observations. The “satisfactory” clinical applications have not been target (pathology) orientated but instead, aim at the acquisition of general well-being through holistic considerations. Since cardiovascular problems must have been affecting all human beings ever since history started, it is easy to identify favourable choices of supportive agents for cardiovascular problems.
When we started this research project in 2001, we carefully selected two widely used medicinal herbs in Traditional Chinese Medicine to form the twin herbs formula, with the aim of giving additional support to the prevention of cardiovascular hazards. We assumed that orthodox preventive therapies might be deficient and not very effective for some patients because the specific orientations might not be able to give comprehensive preventive effects. We assumed that the twin-herb formula could be taking care of more mis-matching problems leading to the cardiovascular hazards [25,26].
While we started planning the clinical trials for the efficacy of the twin-formula, we performed a series of laboratory experiments with the aim of showing multiple bioactivities of the formula, which should indirectly indicate its pharmacological effects.
The bioactivity studies included (i) Anti-inflammatory and anti-oxidative tests [27,28]; ii) Vascular protection tests [29,30]; iii) Myocardial effects [31,32]; and Genomic studies. Results of the bioactivity studies allowed us to speculate that the twin formula should be able to create a favorable microenvironment with good control of inflammatory activities in the promotion of an effective prevention program in a holistic direction [33, 34].
We chose four areas of major concern related to cardiovascular hazards viz. coronary obstruction, hypertension, perimenopause stress and peripheral arterial disease, for separate clinical trials under randomized control directions. The objectives were uniform and the major outcome measure was Intimal Medium Thickness (IMT), which is a well-accepted surrogate marker for the study of vascular deterioration. Uniformity of trial results could be expected. The blood lipid profile was selected as supportive outcome measures.
The individual clinical trials all showed positive effects of the twin formula. The pooled data of the four trials well supported the individual trials, thus further strengthening the data and it gives additional encouragement to the further development of the twin formula.
In the completed trials, patients on specific anti coagulants like Warfarin were excluded for fear of interference and safety. To continue a proper development of the twin formula, whether its application has significant disturbances to the routine vascular therapies needs careful studies [35].
We have performed limited investigation in the “herb-drug” interaction of the twin formula and common anti coagulants. A brief summary is given as follows.
The simultaneous administration of the twin formula and aspirin/ Warfarin to experimental rats showed that the metabolism of aspirin was not affected whereas the Warfarin pharmacokinetic and pharmacodynamics states showed significant interferences. The preliminary studies indicated that patients on Warfarin might avoid using this twin formula until more information is obtained [36,37].
Conclusion
We used a simple twin herb formula D&G as an evidence-based supplement to maintain the cardiovascular health of 4 groups of patients with different primary clinical problems in 4 separate randomized control trials. The results in the individual trials already showed very positive results as were exemplified in the IMT data and lipid profiles. We venture to pool the data together in a cross-biostatistical analysis with the openness to further testify with a bigger population. To our satisfaction, this exercise showed a consistent improvement with the D&G formula as had been shown in the previous individual clinical studies. Earlier, we have separately reported the treatment results of the four clinical trials together with extensive laboratory studies [38]. The present cross-biostatistical analysis would provide additional evidence.
D&G can be recommended as a safe supplement for the maintenance of cardiovascular health. Further studies to work out possible herb-drug interactions with many standard cardiac therapeutic agents would further guarantee its safety, efficacy and wider circulation.
This work was supported by Area of Excellence Grant from the University Grants Committee of the Hong Kong Special Administration Region (2001-2011), Health and Medical Research Fund (HMRF) (2015-2017) and the State Key Laboratory Fund provided by the Innovation and Technology Commission of Hong Kong (2011-2021).
- Nabel EG (2003) Cardiovascular disease. N Engl J Med 349: 60‐72. Link: https://bit.ly/36g2IKm
- Ross R (1986) The pathogenesis of atherosclerosis – an update. N Engl J Med 314: 488‐500. Link: https://bit.ly/3jO2Dp1
- Kearney M, Whelton K, Reynolds K, Muntner P, Whelton PK, et al. (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365: 217-223. Link: https://bit.ly/2Um6ZJw
- Marizilli M, Merz N, Boden WE, Bonow RO, Capozza PG, et al. (2012) Obstructive Coronary Atherosclerosis and Ischemic Heart Disease. J Am Coll Cardiol 60: 951-956. Link: https://bit.ly/3qMv5JE
- Almgren T , Wilhelmsen L, Samuelsson O, Himmelmann A, Rosengren A, et al. (2007) Diabetes in treated hypertension is common and carries a high cardiovascular risk: results from a 28-year follow-up. J Hypertens 25: 1311-1317. Link: https://bit.ly/36hX4at
- Leung PC (2005) A critical look at traditional Chinese Medicine – Recommendation in the line of Research Approach. In PC Leung & Xue CL (Eds), Chinese Medicine – Modern Practice. World Scientific Publisher, Singapore. Link: https://bit.ly/3yqvT9x
- Chan YL, Woo KS, Leung PC, Fung KP (2006) Traditional Chinese medicine Danshen and Gegen combination formula improves Atherogenic pathophysiology: an in‐vitro and ex‐vivo study. J HK Coll Cardiol 14: 68. Link: https://bit.ly/3AzOotW
- Chook P, Tam WY, Chan LT, Qiao M, Cheng KF, et al. (2011) Efficacy and safety of Danshen and Gegen as adjunctive secondary prevention therapy in coronary artery disease. South China Journal of Cardiovascular Diseases 17: 48‐52. Link: https://bit.ly/2Vb8tXw
- Chook P, Tam WY, Poon YK, Qiao M, Chan LT, et al. (2009) Danshen and Gegen as cardiovascular tonic in coronary patients: a novel strategy for secondary atherosclerosis prevention. South China Journal of Cardiovascular Diseases 15: 56‐64. Link: https://bit.ly/3wqEJmw
- Yip TWC, Chook P, Kwong SK, Wong EML, Cheng W, et al. (2009) Adjunctive Danshen and Gegen therapy improves atherogenic process: a final report of double‐blind placebo control trial in high risk hypertension. J HK Coll Cardiol 17: 12. Link: https://bit.ly/36iVjd8
- Woo KS, Yip TWC, Chook P, Kwong SK, Szeto CC, et al. (2013) Cardiovascular protective effects of adjunctive alternative medicine (Salvia miltiorrhiza and Pueraria lobata) in high‐risk hypertension. Evid Based Complement Alternat Med 2013: 132912. Link: https://bit.ly/36gXL40
- Kwok TCY, Leung PC, Lam C, Ho S, Wong CK, et al. (2014) A randomized placebo controlled trial of an Innovative Herbal Formula in the prevention of atherosclerosis in postmenopausal women with Borderline hypercholesterolemia. Complement Ther Med 22: 473-480. Link: https://bit.ly/3wirSCM
- Yan PY, Woo KS, Kwok CY, Leung PC (2020) Symptoms of Intermittent Claudication and Walking tolerance in patients suffering from Peripheral arterial disease can be improved with a simple herbal formula. J Integrative Cardiology. Link: https://bit.ly/3qLCzws
- Leung PC, Koon CM, Lau CBS, Chook P, Cheng KF, et al. (2013) Ten years’ research on a cardiovascular tonic: A comprehensive approach – from quality control and mechanisms of action to clinical trial. Evid Based Complement Alternat Med 2013: 319703. Link: https://bit.ly/3wjsZ57
- Leung PC, Koon CM, Kwok T, Woo KS, Lau CBS, et al. (2014) Development of an effective cardiovascular protective agent using evidence-based research platforms. Experimental & Clinical Cardiology 20: 4235-4250. Link: https://bit.ly/3qOKRn4
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, et al. (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 333: 1301‐1308. Link: https://bit.ly/3yrEHfw
- Down JR, Glearfield M, Weis S, Whnitney E, Shapiro DR, et al. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279: 1615‐1622. Link: https://bit.ly/3jJjqK4
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Cobbe SM, et al. (2002) Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomized controlled trial. Lancet 360: 1623‐1630. Link: https://bit.ly/3qSzFpQ
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, et al. (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐ average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial ‐ Lipid Lowering Arm (ASCOT‐LLA): a multicenter randomized controlled trial. Lancet 361: 1149‐1158. Link: https://bit.ly/3jMKmIT
- Antithrombotic Trialists’ (ATT) Collaboratioin, Baigent C, Blackwell L, Collins R, Emberson J, et al. (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet 373: 1849‐1860. Link: https://bit.ly/3dMRDVr
- Nabel EG, Braunwald E (2012) A tale of coronary artery disease and myocardial Infarction. N Engl J Med 366: 54‐63. Link: https://bit.ly/3qQE3Fu
- O’Donnell CJ, Nabel EG (2011) Genomics of cardiovascular disease. N Engl J Med 365: 2098‐2109. Link: https://bit.ly/3ypWm73
- European Society of Hypertension‐European Society of Cardiology Guidelines Committee (2003) European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 21: 1011‐1053.
- Cucchiara BL, Messe SR (2008) Antiplatelet therapy for secondary prevention of Stroke. Link:: https://bit.ly/3dN5Gdt
- Ji XY, Tan BK, Zhu YZ (2000) Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin 21: 1089‐1094. Link: https://bit.ly/3hkccdT
- Sieveking DP, Woo KS, Fung KP, Lundman P, Nakhla S, et al. (2005) Chinese herbs Danshen and Gegen modulate key early atherogenic events in vitro. Int J Cardiol 105: 40‐45. Link: https://bit.ly/36fK853
- Chiu PY, Leung HY, Leong PK, Chen N, Zhou L, et al. (2012) Danshen‐Gegen decoction protects against hypoxia/reoxygenation‐induced apoptosis by inhibiting mitochondrial permeability transition via the redox‐sensitive ERK/Nrf2 and PKC ɛ/mKATP pathways in H9c2 cardiomyocytes. Phytomedicine 19: 99‐110. Link: https://bit.ly/3hF1Ynl
- Lam HM, Yam WS, Lau LK, Leung LK, Koon CM, et al. (2005) Antioxidative and vasodilative effects of Danshen and Gegen. J Mol Cell Cardiol 38: 840. Link: https://bit.ly/3qMyAzM
- Ng CF, Koon CM, Cheung DW, Lam MY, Leung PC, et al. (2011) The anti‐hypertensive effect of Danshen (Salvia miltiorrhiza) and Gegen (Pueraria lobata) formula in rats and its underlying mechanisms of vasorelaxation. J Ethnopharmacol 137: 1366‐1372. Link: https://bit.ly/2Uvpb3C
- Koon CM, Woo KS, Leung PC, Fung KP (2011) Salviae Miltiorrhizae Radix and Puerariae Lobatae Radix herbal formula mediates anti‐atherosclerosis by modulating key artherogenic events both in vascular smooth muscle cells and endothelial cells. J Ethnopharmacol 138: 175‐183. Link: https://bit.ly/36fGSGN
- Chiu PY, Wong SM, Leung HY, Leong PK, Chen N, et al. (2011) Acute treatment with Danshen‐Gegen decoction protects the myocardium against ischemia/reperfusion injury via the redox‐sensitive PKCε/mKATP pathway in rats. Phytomedicine 18: 916‐925. Link: https://bit.ly/36eHhsW
- Deng Y, Ng ES, Yeung JH, Kwan YW, Lau CB, et al. (2012) Mechanisms of the cerebral vasodilator actions of isoflavonoids of Gegen on rat isolated basilar artery. J Ethnopharmacol 139: 294‐304. Link: https://bit.ly/3weCRwY
- Wong SM, Chiu PY, Leung HY, Zhou L, Zuo Z, et al. (2011) Myocardial post‐conditioning with Danshen‐Gegen decoction protects against isoproterenol‐induced myocardial injury via a PKCɛ/mKATP‐mediated pathway in rats. Chin Med 6: 7. Link: https://bit.ly/3hheNW0
- Zhang Q, Yang MM (2010) DNA microarray technology and traditional Chinese medicines. Progress Nutrition 12: 6‐12. Link: https://bit.ly/3wjY8VW
- Zhou L, Zuo Z, Chow MSS (2005) Danshen: an overview of its chemistry, pharmacology,pharmacokinetics, and clinical use. J Clin Pharmacol 45: 1345‐1359. Link: https://bit.ly/3wmpomC
- Zhou L, Chow MS, Zuo Z (2009) Effect of sodium caprate on the oral absorptions of danshensu and salvianolic acid B. Int J Pharm 379: 109‐118. Link: https://bit.ly/36jB3Ix
- Zhou L, Chow M, Zuo Z (2006) Improved quality control method for Danshen products ‐consideration of both hydrophilic and lipophilic active components. J Pharm Biomed Anal 41: 744‐750. Link: https://bit.ly/3woRJJ8
- Yan PY, Woo KS, Kwok CY, Leung PC (2021) A twin herbal formula has gained sufficient clinical and laboratory evidences to be accepted as an effective cardio-vascular protective supplement. Int J Cardiol Cardiovasc Dis 1: 24-30. Link: https://bit.ly/3dPn9Sv

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
