Open Journal of Pain Medicine
A new threat is on the horizon: The monkeypox virus. Should we worry about it or just keep an eye on it?
Gastón Sanglier Contreras*
Cite this as
Contreras GS (2022) A new threat is on the horizon: The monkeypox virus. Should we worry about it or just keep an eye on it? Open J Pain Med 6(1): 001-006. DOI: 10.17352/ojpm.000027Copyright License
© 2022 Contreras GS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Monkeypox is a rare viral disease that is endemic in many African countries. However, within days, dozens of cases of monkeypox were confirmed in at least 12 non-African countries. It first appeared in the UK and was reported on 7 May 2022. The discovery of the virus in many patients and in different populations around the world within a few days raised the issue of scientific, medical, managerial, and social logic. The cases reported to date have no established travel links to endemic areas, but a recent mass event may have served as a focus for amplification.
Epidemiological investigations are ongoing. With increased surveillance in non-endemic countries, it is likely that more cases of monkeypox will be identified and confirmed. With all this, we must ask ourselves, as we did with the advent of COVID-19, whether the population is seriously threatened and whether or not we should be concerned about the new virus in the future.
Introduction
Although not as contagious and deadly as traditional smallpox, monkeypox is a disease that can become complicated, experts say. Monkeypox, also called monkeypox, is a rare zoonotic disease caused by a virus endemic to Africa, which has recently been reported in 1,285 people in 28 countries outside the continent, according to the World Health Organisation (WHO) [1].
The first of these cases appeared in May in individuals who had traveled to Africa, but the virus then began to spread within other nations and international and local health authorities are analyzing its modes of transmission and the danger it may pose to the population.
The WHO describes monkeypox as a viral zoonosis (a disease caused by a virus transmitted from animals to humans) that produces symptoms similar to those seen in smallpox patients in the past, although less severe [2].
Monkeypox occurs mainly in central and western Africa, often near tropical rainforests, although its presence is increasing in urban areas.
It was first identified by scientists in 1958 when there were two outbreaks of a “smallpox-like” disease in research monkeys, hence the name “monkeypox”.
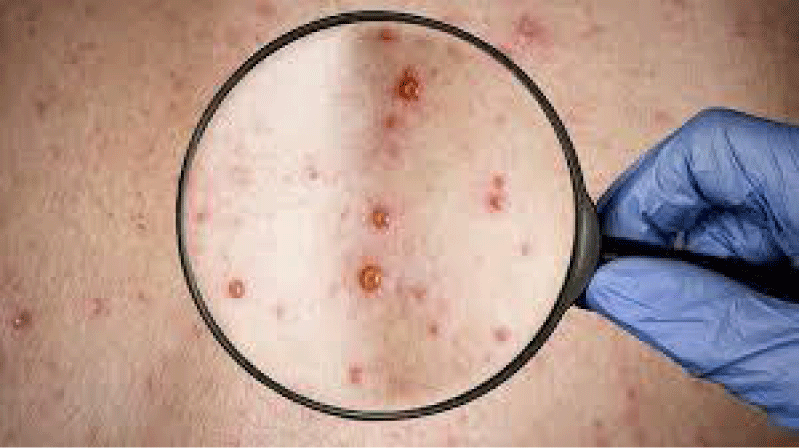
The African continent has been facing a resurgence of monkeypox in the last decade. More confirmed cases of simian smallpox have been diagnosed since 2016 than in the previous forty years [3]. The symptoms of this disease appear on the skin as shown in Figure 1.
To shed light on the situation, four explanations, possibly simultaneous, are put forward for the increase in the incidence of monkeypox:
- The first is the cessation, in 1980, of vaccination against smallpox. The first is the cessation of smallpox vaccination in 1980 and the consequent decline in immunity to orthopoxviruses.
- The second is the increased or more frequent exposure to animal species that act as reservoirs for the disease.
- The third is the increased rate of human-to-human transmission, particularly in immunocompromised hosts.
- The fourth is the advancement in diagnostic capacity and the development of health education programs.
At present, there are no specific treatments available to combat monkeypox infection, but outbreaks of the disease can be controlled. Some data indicate that the smallpox vaccine is at least 85% effective in preventing monkeypox. The antivirals cidofovir and ST-246, as well as specific immunoglobulins, can also be used to control an outbreak of monkeypox.
In Africa, the case fatality rate ranges from 4% to 22%. The majority of patients are children. In addition, the disease is also a global health security problem, as the 2003 US outbreak demonstrated [4,5]. Investigators determined that a shipment of animals from Ghana, imported into Texas in April 2003, introduced the simian pox virus into the United States. The shipment contained approximately 800 small mammals representing nine different species, including six types of rodents. Laboratory testing by the Centers for Disease Control and Prevention (CDC) showed that two African giant rats, nine dormice, and three squirrels were infected with the monkeypox virus [6].
After importation into the United States, some of the infected animals were housed near prairie dogs at an Illinois animal dealer’s facility. These prairie dogs were sold as pets before they developed signs of infection. Seventy-two people became ill with monkeypox, all after contact with the infected prairie dogs.
It is certainly pertinent to monitor the occurrence of new cases or outbreaks of monkeypox and to take appropriate preventive measures because the disease poses a significant threat to human health [7,8].
Methodology and materials
So far in May, several cases of monkeypox have been reported in the UK, Spain, Portugal, Italy, and the US, raising alarm bells.
Monkeypox is a zoonosis (a disease transmitted between vertebrate animals and humans) caused by a DNA virus. Although it produces symptoms and has a similar presentation to smallpox, which has been eradicated since 1980, it is less severe, less transmissible, and less fatal.
It is endemic mainly in Central and West Africa, and in recent years there has been a clear increase in both frequency and distribution. Sporadic imported cases have been reported, mainly in the UK. There are two variants: the West African variant and the Central African variant [9].
Transmission is thought to be through saliva or respiratory excretions, or by contact with lesion exudate or scab material. The possibility of transmission during sexual intercourse is also being considered and that viral shedding through faeces may represent another source of exposure, although more data on this is needed.
Sustained human-to-human transmission is limited and is much less efficient than animal-to-human transmission.
The incubation period of the disease (time from infection to onset of symptoms) is usually 7 to 14 days but can vary from 5 to 21 days.
The disease starts with flu-like symptoms (fever, headache, muscle aches, back pain, chills, and exhaustion), associated with significant swelling of the lymph nodes. This is followed by a rash, which often starts on the face and then spreads to other parts of the body, particularly the hands and feet [10]. These lesions go through different stages before crusting over and finally falling off. The course of the disease usually lasts 2-4 weeks.
Monkeypox does not appear to be contagious during its incubation period, but only once symptoms appear and the possibility of contagion persists until the scabs fall off.
To reach a diagnosis, samples can be taken from skin lesions, scabs, and the oropharynx. Molecular PCR techniques are often used for diagnosis.
The most frequent complications are bacterial superinfection of lesions, keratitis, bronchopneumonia, corneal superinfection, or encephalitis.
Mortality reported in outbreaks in Africa varies from 1% to 10%. Cases identified in the UK are of the West African variant, which has a mortality of approximately 1%, compared to the Central African variant which has higher mortality (10%).
The highest mortality rates occur in children, young people, and the immunocompromised. A more severe course of infection has been observed in people not vaccinated against smallpox [11].
There is no specific antiviral treatment. Some antiviral drugs have been used experimentally. The treatment administered is supportive, to control symptoms and prevent bacterial over-infection.
As this is a new virus in Spain, protocols for its specific management are currently being drawn up.
According to the definition of the UK Health Safety Agency (UKHSA) and recently provisionally adopted by the CCAES and the Spanish Autonomous Communities, monkeypox should be suspected in subjects with unexplained vesicular rash anywhere on the body associated with at least one of the classical symptoms of monkeypox infection (acute illness with fever, severe headache, muscle and joint pain, back pain, lymphadenopathy) from 15 March 2022.
In addition, you must meet other requirements: have an epidemiological link to a confirmed or probable case of monkeypox in the 21 days prior to the onset of symptoms, be a man who has sex with men (MSM), or have been traveling in West or Central Africa in the 21 days prior to the onset of symptoms [12,13].
At present, with the information available, the epidemiological evolution of monkeypox is unknown. Therefore, anyone who is in doubt that they may be infected is advised to wear a mask and to keep lesions covered.
In addition, it is advisable to contact the health center of reference or the local public health authorities to make the most appropriate diagnostic and therapeutic decisions in each case.
Discussion
In recent weeks we have seen, both in the scientific community and in the media, a growing interest in the outbreaks of monkeypox (MPX) that have appeared in different countries.
It is notable that, for the first time, most of the cases have been detected in young men who have not recently traveled to Africa (where it is endemic) and who have reported having sex with men. But be careful, because this does not mean that the virus is only transmitted in sex between men. And by miscommunicating it, we run the risk of stigmatizing a section of the population, as was the case with the AIDS virus.
The monkeypox situation has caused us to look back to about two and a half years ago when we started to hear about SARS-COV2 without giving it much importance. However, the situation generated by MPX is quite different.
To begin with, the MPX virus (MPXV) is an enveloped double-stranded DNA virus belonging to the genus Orthopoxvirus of the family Poxviridae, first identified in humans in 1970 in the Republic of Congo. Transmission can be zoonotic (animal-to-human) or human-to-human.
Cases outside Africa are rare. However, since 7 May 2022, there has been a steady trickle of cases in different European countries and in the USA. In Spain, a health alert for monkeypox was activated after the confirmation of seven cases and the suspicion of 24 others.
This is the first time that chains of MPXV transmission have been detected in Europe in which no epidemiological links with West or Central Africa (where the disease is endemic) have been identified. It is also unique in that, for the first time, the majority of cases have been detected in young men who have not recently traveled to Africa and who have reported having sex with men. Moreover, this is the first time that cases of PMX have been described in men who have sex with men (MSM).
The treatment of this information needs to be handled with great care. Firstly, it does not imply that the virus is only transmitted in sex between men. Sex is, by definition, physical proximity and prolonged direct contact. Transmission within a sexual network (direct and second-degree contacts) followed by travel could easily explain why clusters of cases are appearing in different countries within such extended networks. This transmission through close contact during sex is not unique to MSM.
As the US Centers for Disease Control and Prevention (CDC) has pointed out, “anyone, regardless of sexual orientation, can become infected with MPX through contact with body fluids, sores from a person with MPX, or shared items (such as clothing and bedding) that have been contaminated with fluids or sores from a person with MPX”. They add that “it can also spread from person to person through respiratory droplets, usually in a close environment, such as the home or a healthcare setting”.
The European Center for Disease Control recommends that “public health and community organizations take steps to raise awareness of the potential spread of PMX in communities of people who identify as MSM or who have casual sex or multiple sexual partners”.
Secondly, while being aware that certain groups are more susceptible to certain practices may be useful in establishing preventive and public health measures, it also carries the risk of creating a stigma for these very groups that it is intended to protect. In other words, creating prejudice and labeling individuals as part of a group that is considered socially unacceptable.
Precisely, MSM was one of the groups that suffered - and suffer - from the stigma related to the Human Immunodeficiency Virus (HIV), linked to the images and early news of the early 1980s. Even today, there is still a belief that HIV only affects certain groups, moral judgments are made about infected people and they are blamed for the infection.
It is therefore important to remember the responsibility of the media not to contribute to this potential stigmatization.
Likewise, in view of the frenetic work that lies ahead in the coming weeks, we must work to ensure that the protocols that are being drawn up by different bodies are not stigmatizing.
Conclusion
There is no doubt that the situation suggests that we are at the beginning of a possible epidemic and that it is important to raise social awareness, provide technical guidance, strengthen and support surveillance and diagnostic systems, prevent the development of the outbreak, protect health workers and provide information on the characteristics of the disease.
There is a need for vigilance and caution. But fortunately, it is not all bad news. This new infectious outbreak has some good news:
We are not dealing with a new unknown pathogen. The virus was discovered in 1958 when two outbreaks of a smallpox-like disease occurred in colonies of monkeys kept for research purposes. The first human case of monkeypox was reported in August 1970 in the Democratic Republic of Congo. Since then, the virus has been studied and cases and outbreaks have been tracked.
Monkeypox virus is a relatively large DNA virus that mutates at a slower rate than RNA viruses such as coronaviruses or influenzaviruses (26). DNA viruses have better systems for detecting and repairing mutations than RNA viruses, which means that the simian pox virus is unlikely to have mutated suddenly or to mutate at a high rate to achieve excellent human transmission or to exhibit high variability.
This situation results in the individual acquiring long-term immunity to the virus once the disease is overcome. To date, two genetic clades of the monkeypox virus have been characterized, the West African clade and the Central African clade. Both are geographically separated and have distinct epidemiological and clinical differences. DNA sequencing shows that the virus causing the current outbreak is of the mild type circulating in West Africa and is closely related to the monkeypox viruses detected in the UK, Singapore, and Israel in 2018 and 2019.
Monkeypox virus, human smallpox virus, and vaccinia virus are closely related orthopoxviruses [14]. The successful vaccination campaign against human smallpox led to the disease being declared eradicated in 1980. Historical data suggest that the human smallpox vaccine is about 85% protective against monkeypox, so that people who have been vaccinated against smallpox, which is a large proportion of those over 45 years of age, are less vulnerable to the virus [15].
Zoonotic transmission from animal to human can occur through direct contact with the blood, body fluids, mucous membranes, or skin lesions of infected animals [16]. Eating raw or undercooked meat from infected animals is a risk factor. Animal-to-human transmission can also occur through bites or scratches.
Person-to-person transmission can occur through close contact with droplets of respiratory particles and secretions, skin lesions of an infected person, or recently contaminated objects. Transmission can also occur through the placenta from mother to foetus or during close contact during and after birth. The virus enters the body through wounds on the skin, (even if unnoticeable), respiratory tract, or mucous membranes. Knowing the routes of transmission allows effective prevention measures to be put in place.
This is the first time that chains of transmission of the disease have been reported in Europe with no known epidemiological links to West or Central Africa [17,18]. The most likely channels of transmission of the disease are via droplets and/or contact with infected lesions.
Most cases in Europe have occurred in young men, many of whom self-identify as men who have sex with men. Transmission between sexual partners is increased due to intimate contact during sex with infectious skin lesions, but the likelihood of transmission between individuals without close contact is considered low.
Monkeypox is usually a self-limiting disease with symptoms lasting 2-4 weeks. Historically, the case fatality rate of monkeypox in the African context has ranged from 0%-11% in the general population and has been highest among young children.
The West African clade, the type seen so far in Europe, has a case fatality rate of about 3.6% (estimated from studies in African countries). Mortality is highest in children, young adults, and immunocompromised people. Severe cases can occur, but most people recover from the disease within a few weeks [19].
The virus is easy to track because, unlike SARS-CoV-2, which can spread asymptomatically, monkeypox does not usually go unnoticed. This is largely due to the skin lesions it causes.
In addition, the symptomatology of monkeypox (fever, severe headache, swollen lymph nodes, back pain, muscle aches, and weakness) makes it easier to diagnose the disease and detect infected persons [20].
On the first to third day after the onset of fever, a characteristic rash appears. The rash affects the face (in 95% of cases), and the palms of the hands and soles of the feet (in 75% of cases) [21]. Oral mucous membranes, genitalia, and conjunctivae are also affected, as well as the cornea.
The rash evolves sequentially from macules (lesions with a flat base) to papules (slightly raised), vesicles (filled with clear fluid), pustules (filled with yellowish fluid), and crusts that dry and fall off. The number of lesions can vary from a few to several thousand.
In numerous laboratories in Europe, America, and Africa, the detection of simian pox virus DNA from suspected skin lesions by real-time polymerase chain reaction is well established [22]. Crusts, swabs, and aspirated lesion fluid are preferable to blood samples.
Recent real-time PCR protocols can discriminate not only simian smallpox virus from other orthopoxviruses but also the two clades described.
The original human smallpox vaccines (first generation) are no longer available worldwide, but new second and third-generation vaccines based on the vaccinia virus have been developed. These vaccines have activity against human smallpox and monkeypox [23].
The ACAM2000 and Aventis Pasteur Smallpox Vaccine (APSV) vaccines are based on attenuated, replication-competent vaccinia virus and are administered by the multi-drop technique [24]. The Jynneos vaccine, named Imvanex in the EU and Imvamune in Canada, is a third-generation vaccine containing a modified vaccinia Ankara virus (MVA-BN) that is unable to replicate in the human body but is capable of eliciting a potent immune response against human smallpox and monkeypox. Jynneos is the only simian and non-replicating smallpox vaccine approved by the FDA for non-military use [25].
Cidofovir and Brincidofovir have proven activity against poxviruses in vitro and in animal studies. Brincidofovir is a potent DNA polymerase inhibitor of a variety of double-stranded DNA viruses such as the simian pox virus.
Tecovirimat (ST-246) is also effective in the treatment of orthopoxvirus-induced diseases and human clinical trials indicate that the drug is safe and tolerable with only minor side effects. Tecovirimat is indicated for the treatment of bovine smallpox, monkeypox, and human smallpox in adults and children with a body weight of at least 13 kg.
Despite the good news, we must be cautious and vigilant because there are still unanswered questions. Some relate to the possibility that the sudden increase in cases is due to a mutation that allows this monkeypox virus to be transmitted more easily than those of the past, that the virus may have spread silently and that each of the outbreaks can be traced back to a single origin or several simultaneous ones.
Even so, it is to be hoped that the current outbreak does not require containment strategies beyond that involving ring vaccination.
A responsible attitude will make it possible to tackle a public health problem. Let us remember that the protocols “exist and are there to be complied with”, and an effort must be made to continue to vaccinate all those who need it in the fight against this new virus.
We acknowledge the support of Dentists who have participated in this study.
- Brock E. Biología de los microorganismos (13a ed), 2015. Pearson.
- Leroy EM, Rouquet P, Formenty P, Souquière S, Kilbourne A, Froment JM, Bermejo M, Smit S, Karesh W, Swanepoel R, Zaki SR, Rollin PE. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004 Jan 16;303(5656):387-90. doi: 10.1126/science.1092528. Erratum in: Science. 2004 Jan 20;303(5658):628. PMID: 14726594.
- Centers for Disease Control and Prevention (CDC). Update: multistate outbreak of monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003 Jul 11;52(27):642-6. PMID: 12855947.
- Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011 Mar 5;377(9768):849-62. doi: 10.1016/S0140-6736(10)60667-8. PMID: 21084112; PMCID: PMC3406178.
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003 Oct 10;302(5643):276-8. doi: 10.1126/science.1087139. Epub 2003 Sep 4. PMID: 12958366.
- Hjelle B, Glass GE. Outbreak of hantavirus infection in the Four Corners region of the United States in the wake of the 1997-1998 El Nino-southern oscillation. J Infect Dis. 2000 May;181(5):1569-73. doi: 10.1086/315467. Epub 2000 May 15. PMID: 10823755.
- Coker R, Rushton J, Mounier-Jack S, Karimuribo E, Lutumba P, Kambarage D, Pfeiffer DU, Stärk K, Rweyemamu M. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect Dis. 2011 Apr;11(4):326-31. doi: 10.1016/S1473-3099(10)70312-1. Epub 2011 Mar 2. PMID: 21376670; PMCID: PMC7129889.
- Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg Infect Dis. 2002 Dec;8(12):1468-73. doi: 10.3201/eid0812.010317. PMID: 12498665; PMCID: PMC2738515.
- Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, Szczeniowski MV, Esposito JJ. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001 May-Jun;7(3):434-8. doi: 10.3201/eid0703.010311. PMID: 11384521; PMCID: PMC2631782.
- Callaway TR, Carr MA, Edrington TS, Anderson RC, Nisbet DJ. Diet, Escherichia coli O157:H7, and cattle: a review after 10 years. Curr Issues Mol Biol. 2009;11(2):67-79. PMID: 19351974.
- Ford J. The Role of Trypanosomiasis in African Ecology. Oxford, U.K.: Clarendon Press, 1971.
- Henderson DA, Moss B. Smallpox and vaccinia. En: Plotkin SA, Orenstein WA, Eds. Vaccines (3ra. Edición), Philadelphia, PA: WB. Saunders. 1999; 74-97.
- Karesh E. Wildlife Trade and Global Disease Emergence»; chimpancés, de Beatrice H. Hahn et al., «AIDS as a Zoonosis: Scientifi c and Public Health Implications, Science. 2000; 287:607-614.
- Jezek Z, Marennikova SS, Mutumbo M, Nakano JH, Paluku KM, Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986 Oct;154(4):551-5. doi: 10.1093/infdis/154.4.551. PMID: 3018091.
- CDC. Monkeypox Virus Infection in the United States and Other Non-endemic Countries-2022. CDCHAN-00466; CDC; 2022. https://emergency.cdc.gov/han/2022/han00466.asp.
- Hutin YJ, Williams RJ, Malfait P, Pebody R, Loparev VN, Ropp SL, Rodriguez M, Knight JC, Tshioko FK, Khan AS, Szczeniowski MV, Esposito JJ. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001 May-Jun;7(3):434-8. doi: 10.3201/eid0703.010311. PMID: 11384521; PMCID: PMC2631782.
- Delgado C. Livestock to 2020: The Next Food Revolution, Food, Agriculture, and the Environment Discussion Paper, Washington, DC: International Food Policy Research Institute. 1999.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008 Feb 21;451(7181):990-3. doi: 10.1038/nature06536. PMID: 18288193; PMCID: PMC5960580.
- Mukinda VB, Mwema G, Kilundu M, Heymann DL, Khan AS, Esposito JJ. Re-emergence of human monkeypox in Zaire in 1996. Monkeypox Epidemiologic Working Group. Lancet. 1997 May 17;349(9063):1449-50. doi: 10.1016/s0140-6736(05)63725-7. PMID: 9164323.
- Bergna A, Ventura CD, Marzo R, Ciccozzi M, Galli M, Zehender G, Lai A. Phylogeographical and evolutionary history of variola major virus; a question of timescales? Infez Med. 2022 Mar 1;30(1):109-118. doi: 10.53854/liim-3001-13. PMID: 35350249; PMCID: PMC8929734.
- Relman DA. Microbial genomics and infectious diseases. N Engl J Med. 2011 Jul 28;365(4):347-57. doi: 10.1056/NEJMra1003071. PMID: 21793746; PMCID: PMC3412127.
- World Health Organization (WHO), Situation Reports: Ebola Response Roadmap. 2014. www.who.int/csr/disease/ebola/situation-reports.en/.
- WHO. Multicouty monkeypox outbreak. WHO; 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385.
- Sims LD, Domenech J, Benigno C, Kahn S, Kamata A, Lubroth J, Martin V, Roeder P. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet Rec. 2005 Aug 6;157(6):159-64. doi: 10.1136/vr.157.6.159. PMID: 16085721.
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY; Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005 Sep 29;353(13):1374-85. doi: 10.1056/NEJMra052211. Erratum in: N Engl J Med. 2006 Feb 23;354(8):884. PMID: 16192482.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
