Open Journal of Pain Medicine
A comparison between major chemical markers of the cultivated and wild harvested Siphonochilus aethiopicus, African ginger, from Mpumalanga, South Africa, using Liquid Chromatography-Mass Spectrometry
Tina Chunga1, Sechaba Bareetseng2*, Jeremiah Senabe3 and Ebrahim Wadiwala4
2Indigenous Knowledge Systems Programme Manager, IKS Research Area Advanced Agriculture and Food Cluster, South Africa
3Researcher, Agroprocessing Research Area Advanced Agriculture and Food Cluster, South Africa
4Senior Enterprise Development Specialist, Enterprise Creation and Development Advanced Agriculture and Food Cluster, South Africa
Cite this as
Chunga T, Bareetseng S, Senabe J, Wadiwala E (2022) A comparison between major chemical markers of the cultivated and wild harvested Siphonochilus aethiopicus, African ginger, from Mpumalanga, South Africa, using Liquid Chromatography-Mass Spectrometry. Open J Pain Med 6(1): 007-011. DOI: 10.17352/ojpm.000028Copyright License
© 2022 Chunga T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Siphonochillus aethiopicus, known as African ginger, is indigenous to South Africa and has multiple traditional uses in health to treat human diseases. The multiple traditional uses of African ginger have exacerbated the over-harvesting of the plant species from the wild for trade on the traditional medicine market. The wild populations of African ginger have almost completely depleted from the wild and a few African ginger cultivation sites have been established in South Africa, to conserve the plant species.
The aim of the study was to compare the major chemical markers of the cultivated and wild harvested African ginger from Mpumalanga using Liquid Chromatography-Mass Spectrometry (LC-MS). The Council for Scientific and Industrial Research (CSIR) wild-harvested African ginger dated 2010 was used as a reference sample for comparison purposes. The LC-MS data generated from the ethanol extracts of the cultivated African ginger detected the presence of 4,4a,5,8a,9-tetrahydro-3,5,8a-trimethylnaptho[2,3-b]furan-8. This chemical marker was also detected in the wild harvested African ginger as compared to a previous study, which auto oxidised in the referenced sample over time. This study supports the efforts to conserve African ginger through cultivation for further development in commercialisation.
Introduction
Siphonochilus aethiopicus (Schweinf.) B.L. Burtt is a member of the family Zingiberaceae, as described in the “Medicinal Plants of South Africa (2nd Edition)” book of Van Wyk and co-authors [1]. The plant species are commonly known as African ginger or Wild ginger and occur relatively widely on the African continent, in countries such as Mozambique, Zimbabwe, Malawi, Angola, Senegal, Benin, and Ethiopia (Gordongray, Cunningham and Nicholus, 1989; Noudogbessi, 2013). There are reports which suggest that the African ginger is only indigenous to South Africa [1] and has been naturalized on the African continent.
The plant species bears elongated leaves, distinctive cone-shaped rhizomes, and purple flowers. The communities including traditional health practitioners use rhizomes and roots to prepare a traditional medicine, to treat a variety of human diseases which have been widely reported in the literature. For instance, the plant species are used to treat coughs, colds, and asthma [2], as well as menstrual pains, navel pains, toothache, rheumatism, neuralgia, sexually transmitted infections, and stomach-ache [3,4]. The plant species is also traditionally used to provide children health care in rural areas which often have limited access to western healthcare facilities (Seile, et al. 2022).
African ginger is reported as a source of food used as a spice in dish flavouring in Nigeria [5]. It is not clear if the communities including traditional health practitioners use the leaves of the African ginger in traditional medicines to treat human diseases, as there is limited scientific knowledge about the chemical fingerprinting of the leaves of the plant species.
An optimised LC-MS method has been developed for the diethyl ether, ethanol, and water extracts of the wild harvested African ginger in South Africa by the study of Fouche et al., [6], which investigated the chemical profile and biological activity of the wild harvested African ginger against traditional uses to treat asthma. The major chemical marker was detected and identified as 4,4a,5,8a,9-tetrahydro-3,5,8a-trimethylnaptho[2,3-b]furan-8-one using LC-MS ( [6], as shown in Figure 1. The chemical marker belongs to a class of chemical compounds known as furanoterpenoids, which have the potential to treat asthma as shown in vitro and in vivo studies [6]. The furanoterpenoids have also been shown to have anti-plasmodial activity in other studies [7].
In 2021, the study of Al-Tannaka isolated another class of chemical compounds known as sesquiterpenoid, characterized as 5-acetoxy-9aβ-hydroxy-4aαH-3,5α, 8aβ-trimethyl-4, 4a, 6, 7, 8a, 9-hexahydronaphtho-([2,3 b]-dihydrofuran-2-one)-8-one in hexane and ethyl acetate extracts prepared from the wild harvested African ginger rhizomes obtained from the traditional medicine market in South Africa, after following a series of column and gel filtration chromatography [8]. This chemical marker is believed to have potential bioactivity of the plant species to treat some of the reported traditional uses.
The natural distribution of African ginger is restricted to Mpumalanga, Northern Province, and Kwa-Zulu Natal Provinces of South Africa [1,2]. The wild populations of African ginger have already depleted in KwaZulu Natal [9] and rapidly declining in the wild in Mpumalanga and Limpopo due to over-harvesting to trade on the informal traditional medicine market. In a survey of the marketing of indigenous medicinal plants in South Africa, African ginger was reported the ninth most frequently bought plant in the Durban market [10]. The trade in African ginger is probably attributed to multiple traditional uses to treat human health problems, as well as various socioeconomic reasons such as an increase in human population, increase in the cost of living, and unemployment [11-13]. Unfortunately, African ginger is highly endangered in South Africa due to overharvesting [14] and wild populations are probably unavailable in the wild.
Some studies have brought to light that African ginger can be cultivated on a small scale, thus these plants have been saved in the past from the verge of extinction [15,16]; Kunene, et al. [17]. Cultivation is generally one of the strategies for the conservation of medicinal plant species, thus considered an alternative to wild harvesting to alleviate pressure on wild harvesting of the medicinal plants [18-21]. Moreover, the cultivation of medicinal plant species can create opportunities for communities including traditional health practitioners, who no longer have easy access to wild populations of medicinal plant species, to continue practicing their traditions and cultures. However, there is limited scientific knowledge about the acceptability of cultivated medicinal plants by traditional health practitioners and concerns about the activity or potency of cultivated medicinal plant species. Nonetheless, the traditional health practitioners in the Mpumalanga province of South Africa are practicing the cultivation of African ginger in their back yards as part of the sustainable use of the plant species in traditional practices [22].
Aim of the study
The aim of the study was to compare the chemical markers of the cultivated African ginger with wild harvested African ginger from Mpumalanga using LC-MS.
Materials and methods
African ginger sample collection and pre-treatment
About 3 kg of the wet weight of the cultivated African ginger rhizomes and roots were collected from a private farm in Mpumalanga and supplied to the CSIR through a courier in August 2021. Since African ginger is the only indigenous plant species in the Zingiberaceae family in South Africa and has such a unique and distinctive morphology, no voucher specimen was collected.
The rhizomes and roots were washed with pure running tap water to remove soil particles, followed by slicing and then drying in an oven at 60oC for three days. The final moisture of the dried rhizomes and roots after three days of drying was determined at 7% of water, using a moisture analyser. The dried rhizomes and roots were milled into a grounded material using a hammer mill through a 6mm screen. Figure 2 indicates the sequence followed for the primary processing of African ginger.
Extraction
The Standard Operating Procedures (SOPs) developed by the CSIR in 2011 for the extraction of chemical compounds from the rhizomes and roots of African ginger using ethanol were followed, according to the method of Fouche, et al. 2011. In short, in the first cycle of extraction, 10g of grounded African ginger rhizomes and roots was mixed in 60ml of absolute ethanol and stirred at room temperature for one hour with occasional stirring and filtered. The ethanol-biomass mixture was filtered through grade 2 Filter Papers. The second cycle of extraction involved the filter cake or pulp extracted again with 60ml of fresh and absolute ethanol for one hour. The third extraction of the filter cake or pulp was left overnight in 60ml ethanol. The filter cake or pulp was finally discarded, and the extracts were dried using a Buchi rotavapor to give a dried ethanol extract of 0.26 g (0.086%, w/w).
This extraction process was repeated on the wild harvested African ginger of 2010 that is still available at the CSIR research facility and stored in a freezer at 4°C in a powdery form, to prevent microbial contamination.
LC-MS analysis for the detection of major chemical marker
The method development for chemical marker identification by Fouche et al. 2011 was followed. In brief, each extract (25 mg) from both the cultivated and wild harvested African ginger rhizomes and roots extracts was dissolved in ethanol in an ultrasonic sonicator bath for 30 minutes in the dark. The extraction solvent was removed and filtered through a 0.2 μm syringe filter before analysis.
A pooled sample was created by mixing equal amounts of each extract. The blank sample was the ethanol used as a solvent for the samples. The samples were analyzed with the Waters Acquity UPLC coupled to a Waters G1 HDMS mass spectrometer and ESIPos (ESI+) and ESINeg (ESI-) ionization mode covering the mass range of 50 – 1200 Dalton (Da). The samples were analyzed in a randomized order and each sample was analyzed four (4) times to allow for statistical processing of the raw data.
Results and discussion
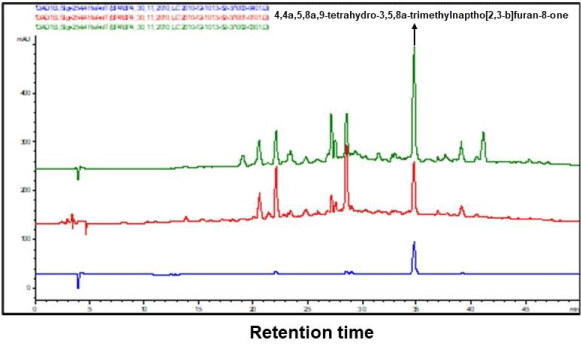
The ethanol extracts prepared from the cultivated and wild harvested African ginger rhizomes and roots were analyzed for major chemical marker identification using LC-MS and are shown in Figure 3.
Good separations of the ethanol extracts were obtained, and the chemical marker could be detected and observed in the cultivated African ginger extract, showing the presence of the furanoterpenoids, signifying the chemical marker as 4,4a,5,8a,9-tetrahydro-3,5,8a-trimethylnaptho[2,3-b]furan-8-one (Figure 3A). A similar chemical marker compound was detected using the LC-MS in the study of Fouche et al. 2011 on the wild African ginger rhizomes and roots, which was shown to have asthmatic effects on African ginger in vitro and in vivo.
The marker compound was however not detected in 2010 in the wild harvested African ginger rhizomes and roots (Figure 3B). This is probably due to the process of auto-oxidation of the marker compound [23-26] since the sample has been stored in open-air contained in cold rooms to prevent possible microbial contamination. The auto-oxidation process implies the stability and therapeutic effects of the formulated African ginger formulated products over time.
Conclusion
The aim of the study was to compare the chemical markers of the cultivated African ginger against the wild harvested African ginger of 2010which was used as a reference sample in this study. The LC-MS method was developed to aid in the detection of the chemical marker from the cultivated African ginger rhizomes and roots and compared to the wild harvested African ginger rhizomes and roots. The extracts were analyzed in ESI+ and ESI- modes to detect any chemical differences. The ESI+ analysis mode is the best to detect the known marker compound and similar compounds produced by African ginger.
The results demonstrated that the African ginger rhizomes and roots obtained from cultivated land in 2021 have the same marker compound as the wild-harvested African ginger chemical marker (see the study of Fouche et al., 2011), which belongs to a class of chemical compounds known as furanoterpenoids. However, over time, there was no evidence of the major compound detected in the wild harvested African ginger, due to the auto-oxidation process. The reason is that autoxidation of the African ginger powder occurs after and before extraction. Therefore the only compounds detected using LC-MS in the wild harvested African ginger samples were the oxidised derivatives of furanoterpenoid. The cultivated African ginger sample from a farm indicated the presence of the major chemical marker derivative, 4,4a,5,8a,9-tetrahydro-3,5,8a-trimethylnaptho[2,3-b]furan-8-one, as well as some significant concentrations of the oxidised derivatives (refer to Figure 3B), which has the potential to treat asthma through in vitro and in vivo studies as reported in the study of Fouche, et al. [6].
The use of LC-MS in the detection of chemical markers highlights how similar the cultivated and wild harvested African ginger is in relation to chemical market analysis. Since African ginger has become extinct in the wild due to overharvesting, the cultivated African ginger can be considered in the development of traditional remedies for the treatment of human diseases including treating pain, alleviating the perceptions among the traditional health practitioners that the cultivated African ginger is not as effective in health care from the indigenous knowledge systems perspective. The findings of this study have shed light on an underexplored aspect of African ginger, one that is significant on the African continent, and have both relevances for commercialisation and government policy.
- Makhuvha N, Van Wyk BE, Van Der Bank H, Van Der Bank M. Genetic polymorphism in wild and cultivated Siphonochilus aethiopicus (Zingiberaceae). Biochemical Systematics and Ecology. 1997; 25:343-351.
- Van Wyk, BE, Gericke N. People’s plants. Pretoria: Briza Publications. South Africa. 2000.
- Watt JM, Breyer-Brandwijk MG. The Medicinal and Poisonous Plants of Southern and Eastern Africa. Second Edition. Livingstone, London. 1962.
- Mander J, Mander M, Crouch N, McKean S, Nichols G. Catchment Action: Growing and Knowing Muthi Plants. Institute of Natural Resources. Pietermaritzburg and Share-Net. Howick. 1995.
- Igoli NP, Obanu ZA. The volatile components of wild ginger (Siphonochilus aethiopicus (Schweinf) B.l Burtt). African Journal of Food Science. 2011; 5:541-549. https://tinyurl.com/2s36fz78.
- Fouche G, Nieuwenhuizen N, Maharaj V, van Rooyen S, Harding N, Nthambeleni R, Jayakumar J, Kirstein F, Emedi B, Meoni P. Investigation of in vitro and in vivo anti-asthmatic properties of Siphonochilus aethiopicus. J Ethnopharmacol. 2011 Jan 27;133(2):843-9. doi: 10.1016/j.jep.2010.11.014. Epub 2010 Nov 11. PMID: 21075193.
- Lategan CA, Campbell WE, Seaman T, Smith PJ. The bioactivity of novel furanoterpenoids isolated from Siphonochilus aethiopicus. J Ethnopharmacol. 2009 Jan 12;121(1):92-7. doi: 10.1016/j.jep.2008.10.007. Epub 2008 Oct 17. PMID: 18996179.
- Al-Tannak NF, Anyam JV, Igoli NP, Gray AI, Alzharani MA, Igoli JO. A new sesquiterpene from South African wild ginger (Siphonochilus aethiopicus (Schweinf) B.L. Burtt). Nat Prod Res. 2021 May 20:1-6. doi: 10.1080/14786419.2021.1914029. Epub ahead of print. PMID: 34011227.
- Cunningham AB. An Investigation of the Herbal Medicine Trade in Natal/KwaZulu, ASIN: B0007BXC52. Institute of Natural Resources. University of Natal. Pietermaritzburg. South Africa. 1988.
- Mander M, The Marketing of Indigenous Medicinal Plants in South Africa: A Case Study in KwaZulu-Natal. Investigational Report 164. Institute of Natural Resources. Pietermaritzburg.1997.
- Moeng TE. An Investigation into the Trade of Medicinal Plants by Muthi Shops and Street Vendors in the Limpopo Province. South Africa. MSc. University of Limpopo. Polokwane. 2010.
- Coopoosamy RM, Naidoo KK. An ethnobotanical study of medicinal plants used by traditional healers in Durban. South Africa. African Journal of Ethnopharmacology. 2012;6: 818–823.
- Brueton VJ. Trade in Commonly Used Medicinal Bulbs: Value and Ecological. 2013.
- Lötter M, Burrows J, von Staden L. Siphonochilus aethiopicus (Schweinf.) B.L.Burtt. National Assessment: Red List of South African Plants. Pretoria: SANBI. Retrieved from SANBI. 2006.
- Manzini TZ. Production of African ginger (Siphonochilus aethiopicus) under protection and indigenous knowledge of the plant from traditional healers. University of Pretoria. Pretoria. South Africa. 2005.
- Ngwenya GL, Moodley N, Nemutanzhela ME, Crampton BG. A micropropagation protocol for Siphonochilus aethiopicus an endangered South African medicinal plant. South African journal of Botany. 2010; 76:414-414.
- Kunene E, Oseni T, Wahome P, Masarirambi M, McCubbin M, Dlamini P, Zwane M. Effects of plant growth regulators and explant type on the in vitro micro-propagation of wild ginger (Siphonochilus aethiopicus (Schweif.) B.L. Burt.). Advancement in Medical Research. 2018; 1-10.
- Keirungi J, Fabricius C. Selecting medicinal plants for cultivation at Nqabara on the Eastern Cape Wild Coast, South Africa. Research in action. South African Journal of Science. 2005; 101: 497-501.
- Mander M, Le Breton G. Overview of the medicinal plants industry in southern Africa. In: Diederichs, N (Ed), Commercialising Medicinal Plants: A Southern African Guide. Sun Press, Stellenbosch. 2006; 1-9.
- Ah Goo, DFS. The Contribution of the Trade in Medicinal Plants to Urban Livelihoods: A Case Study of the Informal Markets in the Nelson Mandela Bay Municipality, Eastern Cape. MSc dissertation. Nelson Mandela Metropolitan University, Port Elizabeth. 2012.
- Ndawonde, BG. Education for Sustainable Development of Medicinal Plant Sellers-Challenges in Relation to Marketing, Sales, Storage and Conservation. PhD thesis. University of Zululand, Richards Bay. 2015.
- Seile BP, Bareetseng S, Koitsiwe MT, Aremu AO. Indigenous knowledge on the uses sustainability and conservation of African ginger (Siphonochilus aethiopicus) among two communities in Mpumalanga province, South Africa diversity. 2022; 14. 1-14.
- Zongwe FK, Muya JT, Mutimana R, Varima M, Mayaliwa M, Chung H, Maharaj V. Autoxidation of Siphonochilone in Processed Rhizomes and Stored Powder of Siphonochilus aethiopicus (Schweinf.) B.L Burtt. Organic & Supramolecular Chemistry. 2018; 8569-8574.
- Van Wyk BE, van Oudtshoorn B, Gericke N. Medicinal Plants of South Africa (2nd edition). Briza Publications. Pretoria. South Africa. ISBN 978-1-875093-37-3. 2009.
- van Wyk BE. A broad review of commercially important southern African medicinal plants. J Ethnopharmacol. 2008 Oct 28;119(3):342-55. doi: 10.1016/j.jep.2008.05.029. Epub 2008 Jun 3. PMID: 18577439.
- Van Wyk BE. The potential of South African plants in the development of new medicinal products. South African journal of botany. 2011; 77:812-829.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
