Global Journal of Obesity, Diabetes and Metabolic Syndrome
A case of primary aldosteronism
Stephanie Zilberman*, Nawar Paracha and Daniel Rafii
Cite this as
Zilberman S, Paracha N, Rafii D (2022) A case of primary aldosteronism. Glob J Obes Diabetes Metab Syndr 9(2): 032-034. DOI: 10.17352/2455-8583.000059Copyright License
© 2022 Zilberman S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction
Primary aldosteronism, also known as Conn’s Syndrome, is one of the most common causes of secondary hypertension. It is a disorder of the adrenal glands that is caused by the hypersecretion of the hormone aldosterone from the adrenal glands. The mechanism of action is due to aldosterone’s role in increasing sodium reabsorption in the renal collecting ducts, which subsequently results in secretion of cellular potassium into the lumen of the cell, resulting in hypokalemia [1]. Therefore, the combination of hypertension and hypokalemia are characteristic features that lead to the screening of primary aldosteronism. There are three subtypes of primary hyperaldosteronism: aldosterone producing adenomas, bilateral adrenal hyperplasia, and familial hyperaldosteronism [2,3]. Here we will discuss a case of primary aldosteronism secondary to a suspected aldosterone producing adenoma.
Case
Medical history
A 60-year old male with a past medical history of hypertension (HTN), diabetes mellitus (DM) and cerebrovascular accident (CVA) with residual right sided weakness and mild aphasia, initially presented to the emergency room after suffering a fall. The patient denied head trauma, loss of consciousness, preceding chest pain, palpitations, or shortness of breath. He was found to have an acute to subacute lacunar infarct on magnetic resonance imaging (MRI), and was admitted to the telemetry unit for frequent neurological checks. The patient was initially observed in a permissively hypertensive state according to neurology recommendations. After a 48-hour period, his home blood pressure medications (Amlodipine 10 mg and Enalapril 20 mg) were restarted. However, his blood pressure was noted to be consistently elevated. He was then evaluated by cardiology. Enalapril was switched to Losartan 100mg daily, and Hydrochlorothiazide 12.5 mg and Spironolactone 12.5 mg were also added to his regimen.
Physical exam
On presentation the patient was seen resting comfortably, in no acute distress. He was alert and orientated. Cardiac, lung, and abdominal exam were all within normal limits. Neurological exam was significant for mild dysarthria. Right upper and lower extremity weakness was present with strength 4 out of 5. Left upper and lower extremities strength was completely intact.
Labs
Notable for hypokalemia with potassium levels consistently below 3.5 mmol/L. On admission, the potassium level was 3.1 mmol/L, with minimal improvement with oral replacement. Sodium levels ranged from 141 to 144 mmol/L. Serum bicarbonate level was in the normal range at 28 mmol/L. Potassium levels remained low despite starting Spironolactone and Losartan.
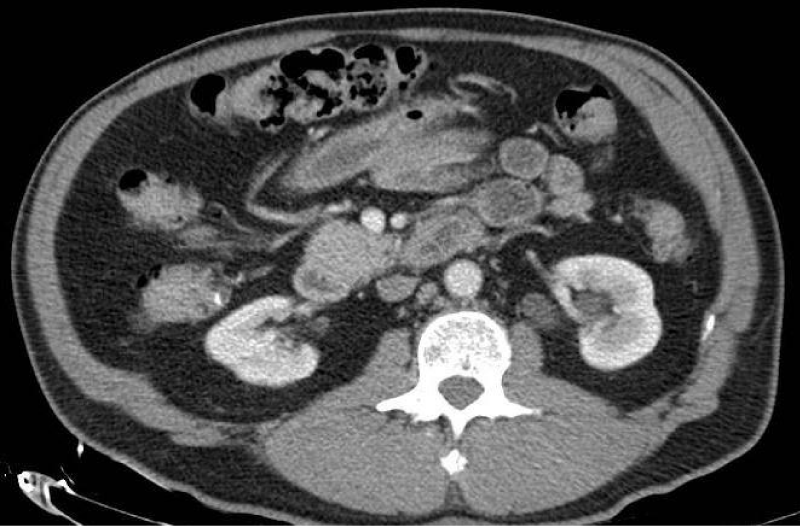
Due to persistent hypokalemia, hydrochlorothiazide was stopped and Spironolactone was increased to 25 mg. At this point, hyperaldosteronism became a top differential diagnosis in the setting of resistant hypertension with concomitant hypokalemia. Case-detection testing was performed with a plasma aldosterone concentration (PAC) and plasma renin activity. PAC was found to be elevated to 55.7 ng/dl, renin activity level was low at 0.3 ng/ml/hr, and aldosterone/renin activity ratio (ARR) was elevated to 166.3. Based on these findings, primary aldosteronism was suspected. Confirmatory testing was skipped in accordance with Endocrine Society practice guidelines. Our next thought was to obtain a computed tomography (CT) abdomen and pelvis with adrenal protocol to evaluate for a possible cause of the primary aldosteronism (subtype classification). CT was significant for 3.8 centimeter (cm) bilobed right adrenal nodule with calculated absolute washout of 67%, consistent with an adenoma (Figure 1). Due to the finding of an adrenal adenoma, pheochromocytoma was then ruled out as a cause of the patient’s hypertension with negative plasma metanephrines, making primary aldosteronism an even more likely diagnosis. A 1 milligram (mg) dexamethasone suppression test (DST) was also planned to screen for hypercortisolism, however, the patient was discharged prior to the completion of the test. The patient at this point was medically stable for discharge.
Treatment
The patient was sent home on Spironolactone 50 mg, Losartan 100 mg, and Amlodipine 10 mg. He followed up in endocrinology clinic a few weeks after discharge. Potassium levels remained stable on the current medication regimen and he was referred to interventional radiology for adrenal venous sampling and to endocrine surgery to determine if adrenalectomy would be an option to treat our patient’s primary aldosteronism.
Discussion
Primary aldosteronism is a prevalent cause of secondary hypertension, responsible for roughly 5-20% of cases of hypertension [4]. The most common causes of primary aldosteronism are unilateral adrenal producing adenomas or idiopathic hyperplasia of bilateral adrenal glands. Less common causes include unilateral hyperplasia of an adrenal gland, which clinically presents similarly to a unilateral adrenal adenoma [5], and familial hyperaldosteronism which is due to a mutation in the gene required to synthesize aldosterone [6]. The purpose of our case report is to highlight when a clinician should suspect primary aldosteronism, as it is a commonly underdiagnosed cause of hypertension. The classic textbook findings of hyperaldosteronism are hypertension and hypokalemia, which our patient exhibited. However, potassium levels may remain normal, as seen in a retrospective study that looked at data of primary aldosteronism from five different countries [7]. Other possible laboratory findings include metabolic alkalosis, mild hypernatremia, and hypomagnesemia. Metabolic alkalosis is a direct result of hypokalemia due to the exchange of hydrogen ions for potassium from the extracellular space to the intracellular space [8]. However, the aforementioned laboratory findings are not diagnostic criteria for primary aldosteronism. Instead, a clinician must rely on a patient’s history and clinical presentation to include primary aldosteronism in their differential diagnosis. This is not limited to patients with hypertension and spontaneous hypokalemia. Screening for primary aldosteronism should be considered in patients with severe hypertension, or drug-resistant hypertension when taking three or more medications including a diuretic, those with hypertension and sleep apnea, or hypertension and family history of early onset hypertension before age 40, or hypertension and atrial fibrillation, and, of course patients with an adrenal incidentaloma [9]. It has also been shown that patients with untreated primary aldosteronism have higher risks of cardiovascular morbidity and mortality [10], as well as increased risk of depression and anxiety [11]. This further stresses the importance of history taking to determine the need to screen for primary aldosteronism. Screening starts with a plasma aldosterone concentration and plasma renin activity level. A ratio (ARR) of greater than 20, with an absolute PAC greater or equal to 15ng/ml is considered a positive screen. In patient with a PAC >20 ng/ml and spontaneous hypokalemia, confirmatory testing with oral salt loading or saline infusion is recommended. Once the diagnosis of primary aldosteronism is made then subtype testing occurs with an adrenal CT scan to determine the etiology, followed by adrenal venous sampling in order to decide whether to treat surgically or pharmacologically with mineralocorticoid receptor antagonists.
Conclusion
We hope that our case study illustrates the significance of screening for primary aldosteronism in a patient with hypertension and associated risk factors.
Patient provided verbal consent for the CT imaging to be reproduced.
- Muto S. Action of aldosterone on renal collecting tubule cells. Curr Opin Nephrol Hypertens. 1995 Jan;4(1):31-40. doi: 10.1097/00041552-199501000-00005. PMID: 7743155.
- Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007 May;66(5):607-18. doi: 10.1111/j.1365-2265.2007.02775.x. PMID: 17492946.
- Mattsson C, Young WF Jr. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006 Apr;2(4):198-208; quiz, 1 p following 230. doi: 10.1038/ncpneph0151. PMID: 16932426.
- Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Ann Intern Med. 2020 Jul 7;173(1):10-20. doi: 10.7326/M20-0065. Epub 2020 May 26. PMID: 32449886; PMCID: PMC7459427.
- Weisbrod AB, Webb RC, Mathur A, Barak S, Abraham SB, Nilubol N, Quezado M, Stratakis CA, Kebebew E. Adrenal histologic findings show no difference in clinical presentation and outcome in primary hyperaldosteronism. Ann Surg Oncol. 2013 Mar;20(3):753-8. doi: 10.1245/s10434-012-2670-2. Epub 2012 Oct 23. PMID: 23090573; PMCID: PMC3556341.
- Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992 Jan 16;355(6357):262-5. doi: 10.1038/355262a0. PMID: 1731223.
- Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004 Mar;89(3):1045-50. doi: 10.1210/jc.2003-031337. PMID: 15001583.
- Dominguez A, Muppidi V, Gupta S. Hyperaldosteronism. [Updated 2022 Jul 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499983/
- John W. Funder, Robert M. Carey, Franco Mantero, M. Hassan Murad, Martin Reincke, Hirotaka Shibata, Michael Stowasser, William F. Young, Jr, The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 5, 1 May 2016, Pages 1889–1916, https://doi.org/10.1210/jc.2015-4061
- Shigematsu Y, Hamada M, Okayama H, Hara Y, Hayashi Y, Kodama K, Kohara K, Hiwada K. Left ventricular hypertrophy precedes other target-organ damage in primary aldosteronism. Hypertension. 1997 Mar;29(3):723-7. doi: 10.1161/01.hyp.29.3.723. PMID: 9052887.
- Velema MS, de Nooijer AH, Burgers VWG, Hermus ARMM, Timmers HJLM, Lenders JWM, Husson O, Deinum J. Health-Related Quality of Life and Mental Health in Primary Aldosteronism: A Systematic Review. Horm Metab Res. 2017 Dec;49(12):943-950. doi: 10.1055/s-0043-121706. Epub 2017 Dec 4. PMID: 29202493.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
