Studies on Stem Cells Research and Therapy
Differential expression of markers of pluripotency and neural/progenitor cells throughout embryonic brain development in mice
Cristiane Valverde Wenceslau1,2*, Diana Aparecida Dias Câmara1#, Damiana Pedro de Oliveira1,2#, Rodrigo Araldi Pinheiro1,3,4* and Irina Kerkis1*
2Cellavita Scientific Research Ltda, Valinhos, SP, Brazil
3Postgraduate Program in Biosciences, Federal University of Latin American Integration (UNILA), Foz do Iguaçu, PR, Brazil
4Biodecision Analytics Ltda, São Paulo, SP, Brazil
#These authors contributed equally to this work
Dr. Rodrigo Araldi Pinheiro, Laboratory of Genetics, Butantan Institute, Av. Vital Brazil 1500, ZIP 05503-900, São Paulo, SP, Brazil, Tel: + 5511 26279705; E-mail: rodrigo.araldi@biodecisionanalytics.com
Dr. Irina Kerkis, Professor, Laboratory of Genetics, Butantan Institute, Av. Vital Brazil 1500, ZIP 05503-900, São Paulo, SP, Brazil, Tel: + 5511 26279705; E-mail: irina.kerkis@butantan.gov.br
Cite this as
Wenceslau CV, Câmara DAD, Pedro de Oliveira D, Pinheiro RA, Kerkis I. (2023) Differential expression of markers of pluripotency and neural/progenitor cells throughout embryonic brain development in mice. Stud Stem Cells Res Ther 9(1): 001-010. DOI: 10.17352/sscrt.000020Copyright License
© 2023 Wenceslau CV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Knowledge regarding the spatiotemporal distribution of cells that express pluripotent and Progenitor-Neural Stem Cell Markers (PNSC) is vital for understanding their role in various stages of embryonic brain development. However, there are few data that connect these markers’ expression with the developmental stage in the mouse brain. We investigated the expression of pluripotent cell markers (Oct4, Nanog, and Sox2) and PNSC markers (Sox 1; nestin, vimentin, GFAP) in mice brains on Embryonic (E) days E9.5, E12.5, E15.5 and E18.5 and in the mature adult brain. We observed the expression of all studied markers in rostral and caudal neuropores at E9.5. The cells at E12.5 in primary brain vesicles showed only expression of four markers: Oct4, Sox2, vimentin and nestin. In addition, hindbrain cells express Sox1 and midbrain – Fragilis. The Ventricular Zone (VZ) at E15.5 and E18.5 shared the expression of Oct 4, Sox 2, Sox1, nestin, and GFAP, besides at E18.5 VZ expressed Fragilis. The olfactory bulb (OB) at E18.5 showed the expression of Sox2, Nanog, Fragilis, Nestin, and GFAP. In the adult brain, the sub-VZ (SVZ) showed expression of all studied markers, but not for Sox2 and Nanog; OB is positive for Nestin only, while cerebellum for Sox1 and Sox2. Neuropores in embryonic and the Subventricular Zone (SVZ) in adult brains express the most considerable number of studied markers, suggesting less cell specification. SVZ is a stem cell niche in the adult brain. Oct4, Sox2 and Nestin seem indispensable during brain development and in the adult brain in mice.
Introduction
Embryonic stem cells (ESCs) comprise a transient population of pluripotent cells derived from the inner cell mass of the blastocyst, that disappears after gastrulation when the three embryonic germ layers have been established [1-4]. These cells exhibit an unlimited capacity for self-renewal, being able to differentiate into a variety of tissues of all three germ layers. These capabilities are governed by the expression of transcription factors (TFs) that, by binding to specific DNA regions, regulate the differentiation-related gene expression [5].
Since 2006, when Kazutoshi Takahashi and Shinya Yamanaka demonstrated that the OCT3/4, SOX2, KLF4, and c-MYC gene insertion in mouse fibroblast promotes genetic reprogramming [6], resulting in the formation of induced pluripotent stem cells (iPSCs), these TFs have been extensively studied [7-9].
Since then, cumulative evidence showed that Oct-3/4 plays a key role in maintaining pluripotency [10,11], being crucial for neural differentiation in ESC [12]. This is because the Oct-3/4 suppresses neural ectodermal differentiation and promotes mesendodermal differentiation [13]. By contrast, Sox-2 inhibits mesendodermal differentiation and promotes neural ectodermal differentiation [13,14]. Along with the Nanog [15-17], Sox-2 also regulates cell proliferation, leading to inner cell mass formation during pre-implantation embryo development [18].
Thus, it is not surprising that the ESCs interact with each other during the formation of the whole organism [1-4]. However, there are few studies looking in-depth at the co-expression of these TFs during the early neurogenesis in the brain. For this reason, novel studies analyzing the co-expression of these TFs are crucial to better comprehend the role of these proteins in embryonic mouse brain development.
Based on this, herein we investigated the spatiotemporal expression pattern of pluripotency markers Oct-3/4, Sox-1, Sox2 and Nanog, as well as fragilis (protein associated with germ cell specification in mice [19]), nestin and vimentin (progenitor-neural stem cells (PNSCs) markers) and glial fibrillary acidic protein (GFAP, glial cells marker [20-22]) in mouse embryo brain with nine (E9.5), 12 (E12.5), 15 (E15.5) and 18 days (E18.5). Our data show that the expression of pluripotency and PNSC markers occur in a time- and brain-specific region-dependent manner.
Materials and methods
Ethical aspects
All procedures employed in this study were approved by the Ethics Committee on Animal Use of Butantan Institute (CEUA-IB, process number 888/12). Protocols concerning experimental animals’ maintenance, care, and handling were by all current Brazilian legislation and internationally recognized norms and protocols. All staff working with experimental animals was fully accredited as staff researchers/technicians and was adequately trained in the use of animals for experimental scientific purposes by current Brazilian regulations.
Animals
Embryo brain was obtained from 12 pregnant (n=12) 3-month-old female BALB/c mice at different embryonic developmental stages: With nine (E9.5), 12 (E12.5), 15 (E15.5), and 18 days (E18.5). The animals were obtained from the Central Bioterium of Butantan Institute (São Paulo-SP, Brazil) and, kept in the bioterium of Genetics Laboratory (Butantan Institute) under a constant temperature of 23±2 ºC, 48% humidity on a12/12-hour light-dark cycle. Food and water were available ad libitum. The animals were acclimated for seven days before the experiments.
Mating occurred between 18:00 PM and 8:00 AM. For purposes of assigning an age to the embryos, conception was assumed to have occurred at midnight, such that embryos collected at noon the next day were considered as being from embryonic day 0.5 (E 0.5). Adult brains were obtained from the same pregnant female from which embryos were collected.
Histology
Embryonic and adult brain tissues were dissected individually and fixed in buffered 4% paraformaldehyde (Sigma-Aldrich, St. Louis, USA) for 48 h. Tissues were t dehydrated, embedded in Paraplast (Sigma-Aldrich, St. Louis, USA), sectioned at 4 mm - 5 mm and stained with hematoxylin and eosin (Merck, Darmstadt, Germany).
Immunohistochemistry analysis
The expression levels of Oct-3/4, Nanog, Sox-1, Sox-2, fragilis, Pax-6, nestin, vimentin, and GFAP were assessed using immunohistochemistry (IHQ). For this, the tissue sections were deparaffinized using a routine technique. Then, the slides were incubated with ammonia hydroxide (Sigma-Aldrich, Saint Louis, USA) for 10 minutes and washed four times in distilled water for five minutes each. Next, the samples were subjected to antigen retrieval using a pH 6.0 buffer of 10 mM sodium citrate (Sigma-Aldrich, Saint Louis, USA), in a water bath set at 95°C for 35 minutes, followed by 20 minutes at room temperature. After this step, the endogenous peroxidase was blocked with hydrogen peroxide (Sigma-Aldrich, Saint Louis, USA) for 15 minutes. To inhibit nonspecific antigen binding, sections were incubated for 30 minutes with 5% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, USA) diluted in phosphate-buffered saline (PBS – 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4). Samples were permeabilized for 15 minutes with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, USA) diluted in PBS. Samples were incubated overnight at 4°C, in a humidity chamber, with primary antibodies (Table 1) diluted in 1% BSA. The slides were washed three times in PBS and, then, they were incubated for 30 minutes at room temperature (in a humidity chamber) with the secondary antibody rabbit or mouse anti-IgG-HRP contained in the Dako EnVisionTM + Dual Link System-HRP (Dako, Carpinteria, USA, reference code K4063). Alternatively, we used the rabbit anti-goat IgG-HRP secondary antibody (Abcan, UK, reference code ab6741) at a dilution of 1:100. Slides were washed three times with PSB. Next, the cuts were incubated for one minute with 3-diaminobenzidine (DAB, Dako, Carpinteria, USA). Slides were washed for 10 minutes and counterstained with Mayer’s hematoxylin solution (Sigma-Aldrich, St. Louis, USA) for five minutes. Slides were washed in PSB and mounted using Permount mounting medium (Sigma-Aldrich, St. Louis, USA), Sections were analyzed using the Axio Imager A1 microscopy (Carl Zeiss, Aalen, Germany) in different magnifications.
Results
In vivo analysis of the expression pattern of pluripotent and neural stem cell markers at different stages of mouse embryonic brain development
Expression of the pluripotency (Oct-4, Nanog, Sox-2), neural stem/progenitor (Sox-1), and primordial germ cells (fragilis) were assessed in different stages of mouse embryonic brain development (E9.5, E12.5, E15.5, and E18.5) using IHQ. Results showed that Oct-4 is widely expressed in the anterior neuropore (ANP, Figure 1A1 and A3) and posterior neuropore (PNP) at E9.5 (Figure 1A2), particularly in the ventricular zone (VZ) (also called the germinal, primitive ependymal or matrix layer), which contains mitotically divided cells (Figure 1F1). Oct-4-positive cells were also partly distributed in the intermediate zone (mantle zone), composed of migrant cells (Figure 1B1). At E12.5, positive immunostaining for Oct-4 was detected in the cells of the primary encephalic vesicles: forebrain (FB) in the lateral ventricle (LV) (Figure 1C1) and midbrain (MB) (Figure 1C2-C3). Note that, at E9.5 and E12.5, Oct-4 showed two distinct patterns of nuclear localization (Figures 1A4, 1B1, and 1C3). As shown in Figure 1A1-A2, this TF was tightly related to condensed chromatin in rapidly and apparently symmetrically diving cells of the germinal zone, while it had a diffuse distribution in the interphase nucleus of non-dividing cells, like that of PNSC (Figure 1A3). Both Oct-4 nuclear patterns are also observed in the FB (Figure 1C- C1) and MB (Figure 1C2-C3) at E12.5. At E15.5 and E18.5, positive diffuse nuclear Oct-4 immunostaining was observed in the SVZ and cells adjacent to the SVZ in the white matter (Figure 1D1 and 1E1, respectively). We also observed that Oct-4 (Figure 1F-F1), Sox2 (Figure 1G1), nestin (Figure 1H), and GFAP (Figure 1I1) are expressed in the SVZ of the adult brain. Nestin was also observed expressed in the olfactory epithelium (Figure 1H1) and, GFAP was detected in the cerebellum (Figure 1I) of the adult brain.
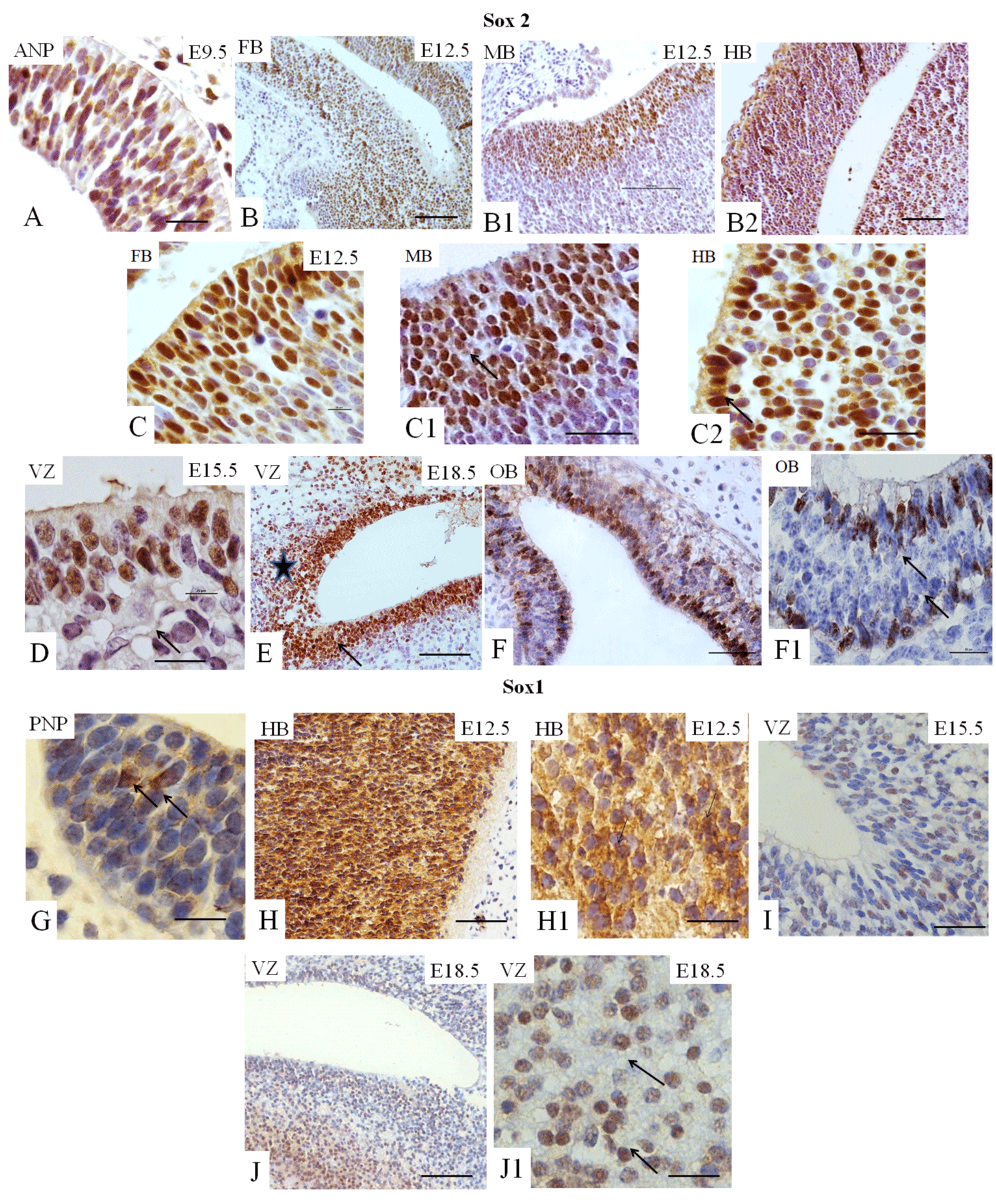
Sox2 and Sox1 play essential roles in neurogenesis [6,14]. At E12.5, these cells are widely distributed in all three primary encephalic vesicles (Figures 2B1-B2 and 2C-C2); at E15.5 and at E18.5, the expression of Sox2 is observed in the SVZ (Figure 2D and 2E), considered a source of NSC in the brain. At E18.5, cell immunopositive staining for Sox2 is identified in the region of white matter adjacent to the VZ (Figure 2E). At E18.5, Sox2-immunopositive cells are also present in the OB (Figure 2F-F1). Few cells immunopositive for Sox1 are identified in the early embryo (E9.5) in the ANP and the PNP (Figure 2G), while almost all cells of the hindbrain are positive for this TF (Figure 2H-H1). At E15.5 and E18.5, Sox1-immunopositive cells are identified in the VZ (Figures 2I, 3J–JI).
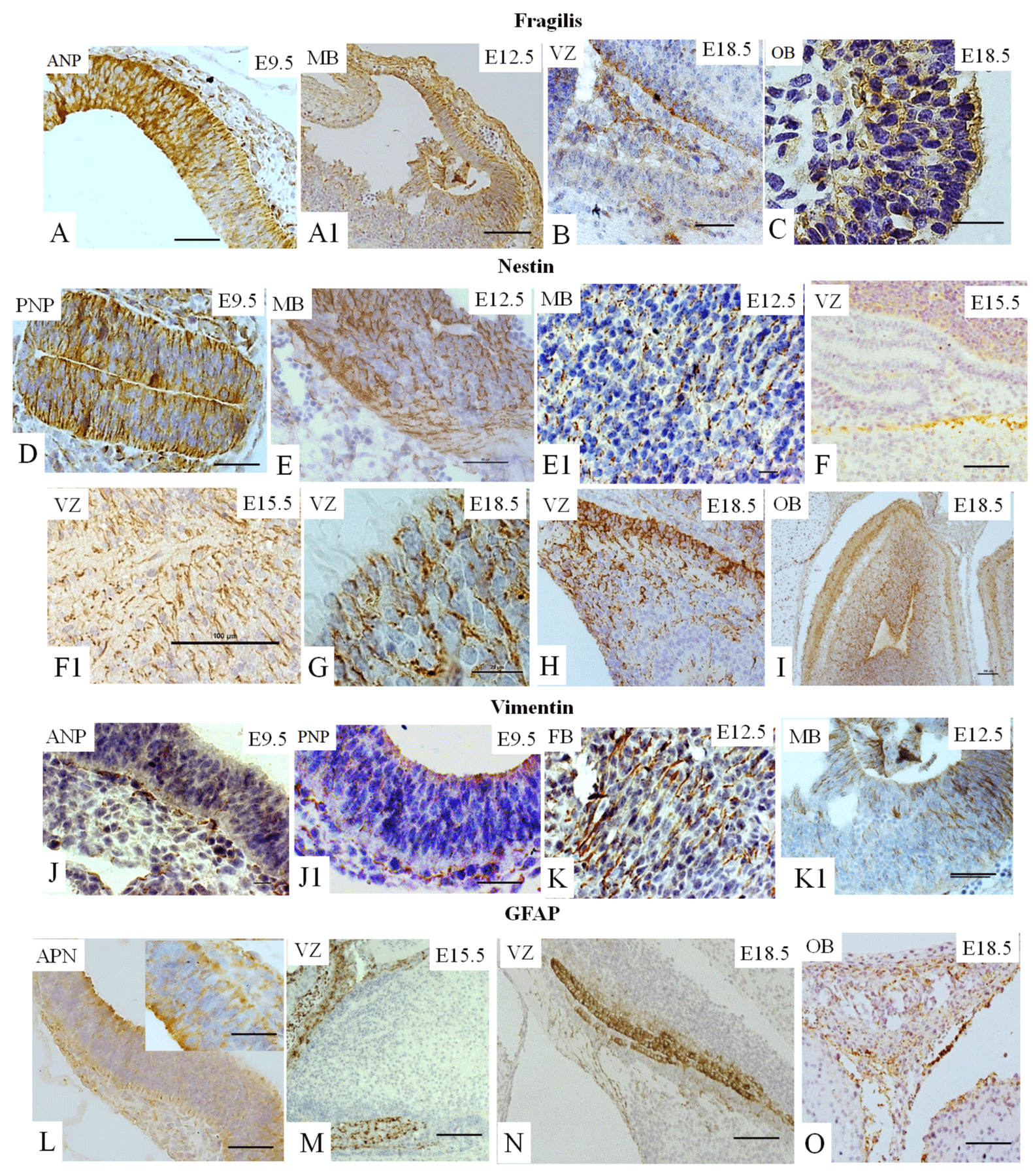
Fragilis is known to be expressed during early development [5,8,13-15]. However, a connection between Fragilis with neural cell fate has never been established. We showed that fragilis was found in the ANP (Figure 3A) and in the MB at E12.5.5 (Figure 3A1), demonstrating membrane localization, as expected. Fragilis is also observed at E18.5 in the VZ (Figure 3B) and in the OB (Figure 3C).
Nestin is known to be required for the self-renewal of neural stem cells [23]. Nestin expression is observed at E9.5 in the ANP (data not shown) and in the PNP (Figure 3D), At E12.5, nestin was found in all three cerebral vesicles. We observed expression in MB (Figures 4E- E1), and staining at other vesicles is not seen. At E15.5 and E18.5, nestin expression is observed in the VZ (Figures 3F-F1 and 3G, and 3H). Finally, nestin expression is found in the OB at E18.5 (Figure 3I).
Vimentin is a marker of mesenchymal stem cells (MSC) and of NSC [10,23]. The expression of vimentin is observed in the ANP (Figure 3J), the PNP (Figure 3J-41) and the PNP at E9.5. At E12.5, vimentin is expressed abundantly in all three subdivisions, forebrain (LV) and midbrain (Figure 3K–K1) and hindbrain (data not shown). Vimentin expression is not detectable in mouse brain compartments at E15.5 or E18.5 (data not shown).
GFAP is a marker of glial cells [10] weakly expressed in the ANP (Figure 3L) and the PNP (data not shown) at E9.5, with strongly positive immunostaining in the VZ at E1.5.5 and E18.5 (Figures 3M and 3N) and in the OB at E18.5 (Figure 3O).
In vivo analysis of the expression pattern of pluripotent and neural stem cells markers in mouse adult brain
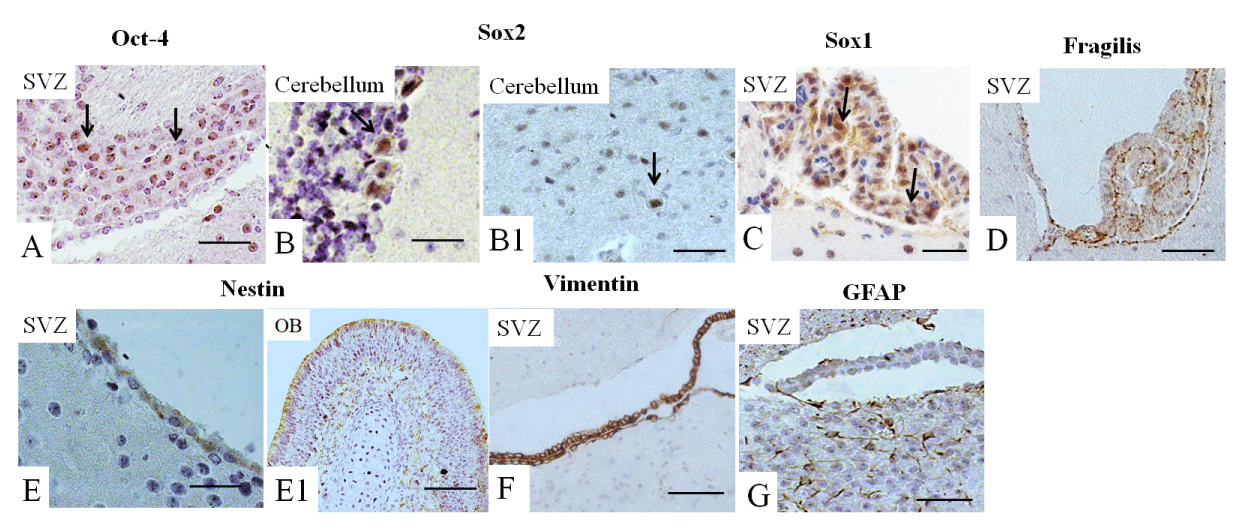
Next, we analyzed the expression of all these markers in the adult brain. Oct-4 expression was observed mainly in the SVZ (Figure 4A), Sox2 and Sox1 were detected mainly in the cerebellum (Figures 4B-B1,C) and fragilis was observed in the SVZ (Figure 4D), while the expression of Nanog was not detected (not shown). Nestin was highly expressed in the SVZ and the OB (Figures 4E-E1) of the adult brain, while the expression of vimentin was limited to the SVZ (Figure 4F). GFAP was expressed in the SVZ and in adjacent white matter (Figure 4G).
Discussion
Knowledge regarding the spatiotemporal distribution of cells that express pluripotent and neuronal stem cell (PNSC) markers is vital for understanding their roles in various stages of embryonic brain development and for determining neuronal stem cell fate. Although different biomarkers have been characterized and largely used in the investigation of ESC and P/NSC, there is no reliable data that connect cell-specific marker expression and the developmental stage of the mouse brain [24,25].
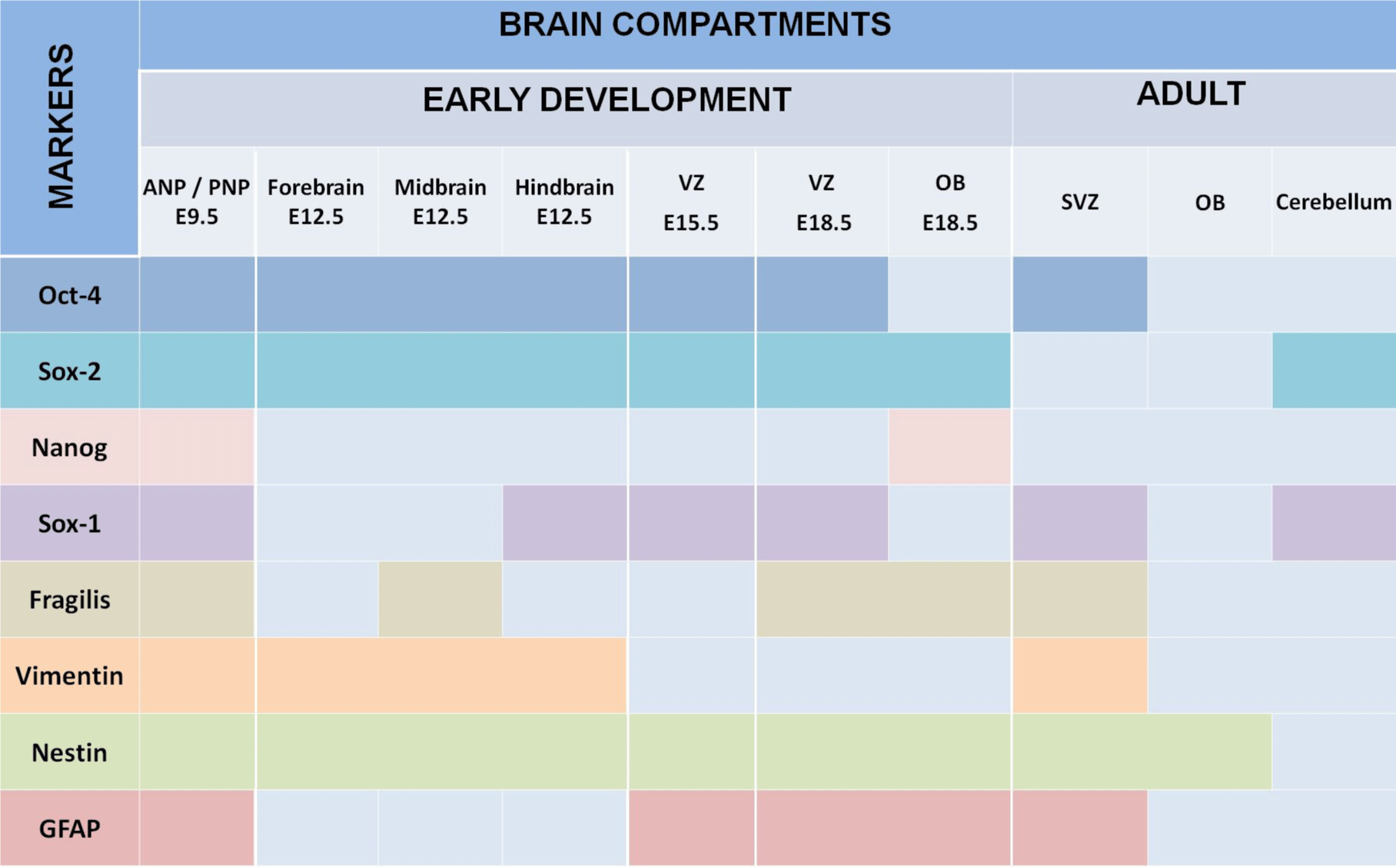
We summarized our findings in Figure 5, which demonstrated that expression of all studied markers Oct4, Nanog, Sox2, Sox1, GFAP, vimentin, nestin, and fragilis are essential at E9.5 in the ANP/PNP. Furthermore, we also showed that at E12.5, following cell lineage specification, three major parts of the brain forebrain, midbrain, and hindbrain showed the expression of Oct4 and Sox2, but not Nanog and GFAP. All these brain compartments additionally to Oct4 and Sox2 express nestin and vimentin. Expression of Sox1 is limited by the hindbrain , which corroborates with published data that explored the differentiation of human ESC to neural progenitor cells (NPC) and showed that SOX1 contributes to the specification of rostral hindbrain NPC [26]. Furthermore, we observed that VZ at E15.5 and E18.5 express Oct4, Sox2, Sox1, nestin, and GFAP. However, this region lacks Nanog and vimentin. VZ at E18.5 in addition to other positive markers shows the expression of Fragilis. OB markers expression analyses at E 18.5 revealed that OB lacks the expression of Oct4, Sox1, and vimentin while expressing Sox2, Nanog, nestin, and fragilis. Our findings correlate with current knowledge about the expression of the transcription factors Oct-4, Nanog, and Sox2. They are a part of the pluripotency network, working together and regulating their expression [2,22,27] and their expression we observed only at E9.5. In turn, Nanog is critical for inhibiting the differentiation of PNSC [19,10,20]. However, active Nanog showed nuclear localization [28]. We evidenced only cytoplasmic Nanog staining, which previously was observed in a variety of tumor and non-tumor cells [23]. Accordingly, its expression is inhibited at E12.5 in the forebrain, hindbrain, and midbrain. Sox1 and Sox2 are essential during early neurogenesis in mammals [29-33] and we observed their expression from E9.5 to E18.5. A previous study showed fragilis expression in mice during embryonic brain development in the PNP at E9.5 [34]. In addition, we showed fragilis expression in the MB at E12.5 and in the VZ and OB at E18.5. Our study suggests that fragilis could play a more critical role in early brain development and needs further investigation. Nestin has been a genuine marker of neural stem/progenitor cells for more than twenty years [23,35] and his expression begins with neurulation [36]. However, little has been studied on its spatiotemporal distribution in the mouse embryonic brain. For the first time, we demonstrated robust expression of nestin in the ANP and PNP at E9.5, in all three primary brain vesicles at E12.5, in the VZ at E15.5 and E18.5, and in the OB at E18.5 (Figure 5). The co-expression of nestin and vimentin has been reported, and both these proteins frequently share a common network [37–39]. Our results showed that this is only partially true since the spatial localization of nestin and vimentin differed in the neuropores at E9.5. Vimentin expression was limited to the mantle zone, whereas nestin is expressed mainly in the germinal zone (Figure 5). Like nestin, the expression of vimentin is detected in all three vesicles (forebrain, midbrain, and hindbrain) at E12.5. Such co-expression of nestin and vimentin corresponds to progenitor migratory cell types [40]. We observed that GFAP, together with nestin and vimentin, is expressed in mantle and germinal zones at E9.5. GFAP, together with vimentin and nestin, is expressed in radial glial cells [38-42] and GFAP expression occurs during development after E16.5 [40]. By contrast, to vimentin and nestin, GFAP expression was not detected at E12.5 in developing brain vesicles. However, strong GFAP expression was observed in the VZ at E15.5 and E18.8 and in the OB at E18.5.
Ventricular-SVZ and the subgranular zones within the hippocampus are regions where postnatal neurogenesis occurs. Therefore, we analyzed the expression of studied markers in SVZ and, in addition, in OB and cerebellum. We examined the expression of these markers in the three-month-old adult mouse brain, summarized in Figure 5. Interesting that we found Oct4-positive cells in the SVZ. According to a recent study, a rare population of primitive NSCs can be found in the adult brain [43]. The cerebellum is a derivative of the hindbrain, and we observed the expression of Sox1 and Sox2 in the hindbrain and the adult cerebellum [44].
The expression of Fragilis in the adult brain had not been reported previously; however, we also found the expression of this marker in the SVZ. Furthermore, we showed nestin, vimentin, and GFAP expression occur in the SVZ of the adult brain [45], as well; Nestin is expressed in the OB. Accordingly, in the adult rodent brain, NSCs are localized in the SVZ of the brain’s lateral ventricles and the subgranular zone of the hippocampus [40-42]. In the adult brain, nestin was found in the subependymal cells in the brain’s lateral ventricles and the subgranular cells of the dentate gyrus [46]. GFAP expression continues during adulthood, thereby replacing the expression of nestin in differentiated neural cells [36].
Conclusion
We demonstrated the expression of pluripotency and PNSC markers that occur in a time- and brain-specific region-dependent manner. Moreover, the marker of pluripotency Oct4 is active during brain development and in SVZ in mature mice brains, while active Nanog seems to be unnecessary at investigated stages. We also reinforced that Sox1, Sox2, and Nestin are specific markers of PNSC in embryonic and adult brains. Additionally, we showed the involvement of fragilis in embryonic (ANP/PNP, MB, VZ, and OB) and adult (SVZ) PNSC specification. Furthermore, our data serve as a “baseline” for further investigation of abnormal expression of studied markers.
We would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financing support (Grants number: 2010/51051-6 and 2012/14674-0), as well as Fundação Butantan for student fellowships.
Author’s contribution
Study design: CVW and IK; Data collection: DADC and DPDO; Study analysis: CVW, DADC and IK; manuscript preparation: CVW, RAP, and IK
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
- Kerkis I, Hayashi MAF, Lizier NF, Cassola AC, Pereira LV, Kerkis A. Pluripotent stem cells as an in vitro model of neuronal differentiation. In Embryonic Stem Cells - Differentiation and Pluripotent Alternatives. 2011; 81–98.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145-7. doi: 10.1126/science.282.5391.1145. Erratum in: Science 1998 Dec 4;282(5395):1827. PMID: 9804556.
- Wenceslau VC, Kerkis I, Lizier NF, Kerkis A. De-differentiation of somatic cells to a pluripotent state. In Pluripotent Stem Cells. 2013.
- Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res. 2002 Nov 15;91(10):866-76. doi: 10.1161/01.res.0000041435.95082.84. PMID: 12433831.
- Verneri P, Vazquez Echegaray C, Oses C, Stortz M, Guberman A, Levi V. Dynamical reorganization of the pluripotency transcription factors Oct4 and Sox2 during early differentiation of embryonic stem cells. Sci Rep. 2020 Mar 23;10(1):5195. doi: 10.1038/s41598-020-62235-0. PMID: 32251342; PMCID: PMC7089971.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663-76. doi: 10.1016/j.cell.2006.07.024. Epub 2006 Aug 10. PMID: 16904174.
- Rizzino A, Wuebben EL. Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis. Biochim Biophys Acta. 2016 Jun;1859(6):780-91. doi: 10.1016/j.bbagrm.2016.03.006. Epub 2016 Mar 23. PMID: 26992828.
- Tremble KC, Stirparo GG, Bates LE, Maskalenka K, Stuart HT, Jones K, Andersson-Rolf A, Radzisheuskaya A, Koo BK, Bertone P, Silva JCR. Sox2 modulation increases naïve pluripotency plasticity. iScience. 2021 Feb 6;24(3):102153. doi: 10.1016/j.isci.2021.102153. PMID: 33665571; PMCID: PMC7903329.
- Zeineddine D, Papadimou E, Chebli K, Gineste M, Liu J, Grey C, Thurig S, Behfar A, Wallace VA, Skerjanc IS, Pucéat M. Oct-3/4 dose dependently regulates specification of embryonic stem cells toward a cardiac lineage and early heart development. Dev Cell. 2006 Oct;11(4):535-46. doi: 10.1016/j.devcel.2006.07.013. PMID: 17011492.
- Wu G, Schöler HR. Role of Oct4 in the early embryo development. Cell Regen. 2014 Apr 29;3(1):7. doi: 10.1186/2045-9769-3-7. PMID: 25408886; PMCID: PMC4230828.
- Shi G, Jin Y. Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res Ther. 2010 Dec 14;1(5):39. doi: 10.1186/scrt39. PMID: 21156086; PMCID: PMC3025441.
- Chen SM, Lee MS, Chang CY, Lin SZ, Cheng EH, Liu YH, Pan HC, Lee HC, Su HL. Prerequisite OCT4 Maintenance Potentiates the Neural Induction of Differentiating Human Embryonic Stem Cells and Induced Pluripotent Stem Cells. Cell Transplant. 2015;24(5):829-44. doi: 10.3727/096368913X675179. Epub 2013 Nov 20. PMID: 24256943.
- Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011 Jun 10;145(6):875-89. doi: 10.1016/j.cell.2011.05.017. PMID: 21663792; PMCID: PMC5603300.
- Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012 Apr 6;10(4):440-54. doi: 10.1016/j.stem.2012.02.016. PMID: 22482508.
- Camp E, Sánchez-Sánchez AV, García-España A, Desalle R, Odqvist L, Enrique O'Connor J, Mullor JL. Nanog regulates proliferation during early fish development. Stem Cells. 2009 Sep;27(9):2081-91. doi: 10.1002/stem.133. PMID: 19544407.
- Yamaguchi S, Kimura H, Tada M, Nakatsuji N, Tada T. Nanog expression in mouse germ cell development. Gene Expr Patterns. 2005 Jun;5(5):639-46. doi: 10.1016/j.modgep.2005.03.001. Epub 2005 Apr 9. PMID: 15939376.
- Zhang X, Neganova I, Przyborski S, Yang C, Cooke M, Atkinson SP, Anyfantis G, Fenyk S, Keith WN, Hoare SF, Hughes O, Strachan T, Stojkovic M, Hinds PW, Armstrong L, Lako M. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J Cell Biol. 2009 Jan 12;184(1):67-82. doi: 10.1083/jcb.200801009. PMID: 19139263; PMCID: PMC2615089.
- Lee M, Choi KH, Oh JN, Kim SH, Lee DK, Choe GC, Jeong J, Lee CK. SOX2 plays a crucial role in cell proliferation and lineage segregation during porcine pre-implantation embryo development. Cell Prolif. 2021 Aug;54(8):e13097. doi: 10.1111/cpr.13097. Epub 2021 Jul 11. PMID: 34250657; PMCID: PMC8349655.
- Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012 Nov 1;4(11):a008375. doi: 10.1101/cshperspect.a008375. PMID: 23125014; PMCID: PMC3536339.
- Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013 Oct 30;80(3):588-601. doi: 10.1016/j.neuron.2013.10.037. PMID: 24183012.
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001 Dec;19(12):1134-40. doi: 10.1038/nbt1201-1134. PMID: 11731782.
- Zhang J, Jiao J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. Biomed Res Int. 2015;2015:727542. doi: 10.1155/2015/727542. Epub 2015 Sep 1. PMID: 26421301; PMCID: PMC4569757.
- Mikulenkova E, Neradil J, Vymazal O, Skoda J, Veselska R. NANOG/NANOGP8 Localizes at the Centrosome and is Spatiotemporally Associated with Centriole Maturation. Cells. 2020 Mar 11;9(3):692. doi: 10.3390/cells9030692. PMID: 32168958; PMCID: PMC7140602.
- Temple S. The development of neural stem cells. Nature. 2001 Nov 1;414(6859):112-7. doi: 10.1038/35102174. PMID: 11689956.
- Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008 May;135(9):1575-87. doi: 10.1242/dev.014977. Epub 2008 Mar 20. PMID: 18356248.
- Liu X, Fang Z, Wen J, Tang F, Liao B, Jing N, Lai D, Jin Y. SOX1 Is Required for the Specification of Rostral Hindbrain Neural Progenitor Cells from Human Embryonic Stem Cells. iScience. 2020 Aug 20;23(9):101475. doi: 10.1016/j.isci.2020.101475. Epub ahead of print. PMID: 32905879; PMCID: PMC7486433.
- Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009 Jul;136(14):2311-22. doi: 10.1242/dev.024398. PMID: 19542351; PMCID: PMC2729344.
- Do HJ, Lim HY, Kim JH, Song H, Chung HM, Kim JH. An intact homeobox domain is required for complete nuclear localization of human Nanog. Biochem Biophys Res Commun. 2007 Feb 16;353(3):770-5. doi: 10.1016/j.bbrc.2006.12.100. Epub 2006 Dec 22. PMID: 17196939.
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998 May;125(10):1967-78. doi: 10.1242/dev.125.10.1967. PMID: 9550729.
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004 Oct 7;369(1):24-7. doi: 10.1016/j.neulet.2004.07.035. PMID: 15380301.
- Kokovay E, Shen Q, Temple S. The incredible elastic brain: how neural stem cells expand our minds. Neuron. 2008 Nov 6;60(3):420-9. doi: 10.1016/j.neuron.2008.10.025. PMID: 18995816.
- Slater JL, Landman KA, Hughes BD, Shen Q, Temple S. Cell lineage tree models of neurogenesis. J Theor Biol. 2009 Jan 21;256(2):164-79. doi: 10.1016/j.jtbi.2008.09.034. Epub 2008 Oct 15. PMID: 18977364.
- Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep. 2014 Apr;15(4):351-64. doi: 10.1002/embr.201438447. Epub 2014 Mar 17. PMID: 24639559; PMCID: PMC3989667.
- Lange UC, Saitou M, Western PS, Barton SC, Surani MA. The fragilis interferon-inducible gene family of transmembrane proteins is associated with germ cell specification in mice. BMC Dev Biol. 2003 Mar 19;3:1. doi: 10.1186/1471-213x-3-1. Epub 2003 Mar 19. PMID: 12659663; PMCID: PMC153542.
- Rutka JT, Ivanchuk S, Mondal S, Taylor M, Sakai K, Dirks P, Jun P, Jung S, Becker LE, Ackerley C. Co-expression of nestin and vimentin intermediate filaments in invasive human astrocytoma cells. Int J Dev Neurosci. 1999 Aug-Oct;17(5-6):503-15. doi: 10.1016/s0736-5748(99)00049-0. PMID: 10571412.
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990 Feb 23;60(4):585-95. doi: 10.1016/0092-8674(90)90662-x. PMID: 1689217.
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994 Nov;13(5):1071-82. doi: 10.1016/0896-6273(94)90046-9. PMID: 7946346.
- Kornblum HI. Introduction to neural stem cells. Stroke. 2007 Feb;38(2 Suppl):810-6. doi: 10.1161/01.STR.0000255757.12198.0f. PMID: 17261745.
- Kornblum HI, Geschwind DH. Molecular markers in CNS stem cell research: hitting a moving target. Nat Rev Neurosci. 2001 Nov;2(11):843-6. doi: 10.1038/35097597. PMID: 11715062.
- McCarthy DM, Gioioso V, Zhang X, Sharma N, Bhide PG. Neurogenesis and neuronal migration in the forebrain of the TorsinA knockout mouse embryo. Dev Neurosci. 2012;34(4):366-78. doi: 10.1159/000342260. Epub 2012 Sep 26. PMID: 23018676; PMCID: PMC3712350.
- von Bohlen und Halbach O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011 Jul;345(1):1-19. doi: 10.1007/s00441-011-1196-4. Epub 2011 Jun 7. PMID: 21647561.
- Sachewsky N, Leeder R, Xu W, Rose KL, Yu F, van der Kooy D, Morshead CM. Primitive neural stem cells in the adult mammalian brain give rise to GFAP-expressing neural stem cells. Stem Cell Reports. 2014 May 22;2(6):810-24. doi: 10.1016/j.stemcr.2014.04.008. PMID: 24936468; PMCID: PMC4050350.
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007 Nov;1(5):515-28. doi: 10.1016/j.stem.2007.09.002. PMID: 18371391; PMCID: PMC2185820.
- Alcock J, Lowe J, England T, Bath P, Sottile V. Expression of Sox1, Sox2 and Sox9 is maintained in adult human cerebellar cortex. Neurosci Lett. 2009 Jan 30;450(2):114-6. doi: 10.1016/j.neulet.2008.11.047. Epub 2008 Nov 27. PMID: 19061938.
- Furube E, Ishii H, Nambu Y, Kurganov E, Nagaoka S, Morita M, Miyata S. Neural stem cell phenotype of tanycyte-like ependymal cells in the circumventricular organs and central canal of adult mouse brain. Sci Rep. 2020 Feb 18;10(1):2826. doi: 10.1038/s41598-020-59629-5. PMID: 32071335; PMCID: PMC7029029.
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003 Oct 15;23(28):9357-66. doi: 10.1523/JNEUROSCI.23-28-09357.2003. Erratum in: J Neurosci. 2004 Jan 7;24(1):24. PMID: 14561863; PMCID: PMC6740569.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
